Abstract
Molluscs are among the most diverse and widespread animal groups in freshwater habitats. Unfortunately, like most freshwater taxa, they are decreasing dramatically and are now among the most threatened animals on Earth, with many species already extinct or on the brink of extinction. Here, we review our current knowledge on the biodiversity and conservation of freshwater molluscs using the concept of knowledge shortfalls. We focus on seven previously proposed key shortfalls to review and analyse existing knowledge gaps relating to (1) taxonomy, the Linnean Shortfall; (2) distribution, the Wallacean Shortfall; (3) abundance and population dynamics, the Prestonian Shortfall: (4) evolution, the Darwinian Shortfall; (5) abiotic tolerances, the Hutchinsonian Shortfall; (6) traits, the Raunkiaeran Shortfall; and (7) biotic interactions, the Eltonian Shortfall. In addition, we address a new shortfall, which relates to the application and effectiveness of conservation measures, including assessments, methods, funding, and policies, the Ostromian Shortfall. Based on our review, we provide recommendations and suggest pathways to overcome these existing shortfalls. This work also introduces the articles in this special issue of Hydrobiologia, which represent key contributions to the First International Freshwater Mollusk Conservation Society Meeting held in Verbania, Italy, in 2018.



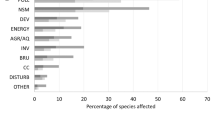
adapted from IPBES (2019)

adapted from IPBES (2019)

adapted from IPBES (2019)
Similar content being viewed by others
References
Ahlgren, J., X. Yang, L.-A. Hansson & C. Brönmark, 2013. Camouflaged or tanned: plasticity in freshwater snail pigmentation. Biology Letters. https://doi.org/10.1098/rsbl.2013.0464.
Aksenova, O. V., I. N. Bolotov, M. Y. Gofarov, A. V. Kondakov, M. V. Vinarski, Y. V. Bespalaya, Y. S. Kolosova, D. M. Palatov, S. E. Sokolova, V. M. Spitsyn, A. A. Tomilova, O. V. Travina & I. V. Vikhrev, 2018. Species richness, molecular taxonomy and biogeography of the radicine pond snails (Gastropoda: Lymnaeidae) in the Old World. Scientific Reports 8: 11199.
Albrecht, C., S. Trajanovski, K. Kuhn, B. Streit & T. Wilke, 2006. Rapid evolution of an ancient lake species flock: freshwater limpets (Gastropoda: Ancylidae) in the Balkan Lake Ohrid. Organisms Diversity & Evolution 6: 294–307.
Alexander, J. E. & A. P. Covich, 1991. Predation risk and avoidance behavior in two freshwater snails. The Biological Bulletin 180: 387–393.
Anderson, J. T. & L. M. Smith, 2000. Invertebrate response to moist-soil management of playa wetlands. Ecological Applications 10: 550–558.
Andree, K. B. & M. A. López Robles, 2013. Species identification from archived snail shells via genetic analysis: a method for DNA extraction from empty shells. Molluscan Research 33: 1–5.
Araujo, R. & M. A. Ramos, 2001. Action plans for Margaritifera auricularia and Margaritifera margaritifera in Europe (No. 18-117). Council of Europe.
Archambault, J. M., W. G. Cope & T. J. Kwak, 2014. Influence of sediment presence on freshwater mussel thermal tolerance. Freshwater Science 33: 56–65.
Augspurger, T., A. E. Keller, M. C. Black, W. Gregory Cope & F. J. Dwyer, 2003. Water quality guidance for protection of freshwater mussels (Unionidae) from ammonia exposure. Environmental Toxicology and Chemistry 22: 2569.
Bachman, S. P., R. Field, T. Reader, D. Raimondo, J. Donaldson, G. E. Schatz & E. N. Lughadha, 2019. Progress, challenges and opportunities for Red Listing. Biological Conservation 234: 45–55.
Baker, S. M. & J. S. Levinton, 2003. Selective feeding by three native North American freshwater mussels implies food competition with zebra mussels. Hydrobiologia 505: 97–105.
Barnes, M. A. & C. R. Turner, 2016. The ecology of environmental DNA and implications for conservation genetics. Conservation Genetics 17: 1–17.
Bauer, G., 1987. Reproductive strategy of the Freshwater Pearl Mussel Margaritifera margaritifera. The Journal of Animal Ecology 56: 691.
Bauer, G., 1994. The adaptive value of offspring size among freshwater mussels (Bivalvia; Unionoidea). Journal of Animal Ecology 63: 933–944.
Bauer, G., 2001. Framework and driving forces for the evolution of Naiad life histories. In Bauer, G. & K. Wächtler (eds), Ecology and Evolution of the Freshwater Mussels Unionoida. Springer, Berlin: 233–255.
Bespalaya, Y. V., O. V. Aksenova, S. E. Sokolova, A. R. Shevchenko, A. A. Tomilova & N. A. Zubrii, 2020. Biodiversity and distributions of freshwater mollusks in relation to chemical and physical factors in the thermokarst lakes of the Gydan Peninsula, Russia. Hydrobiologia. https://doi.org/10.1007/s10750-020-04227-9.
Bieler, R. & P. M. Mikkelsen, 2019. Cyrenidae. In Lydeard, C. & K. S. Cummings (eds), Freshwater mollusks of the world: A Distribution Atlas. John Hopkins University Press, Baltimore: 187–192.
Bílý, M., O. Simon, V. Barák & V. Jahelková, 2020. Occurrence depth of juvenile freshwater pearl mussels (Margaritifera margaritifera) in a river bed tested by experimental mesh tubes. Hydrobiologia. https://doi.org/10.1007/s10750-020-04298-8.
Bódis, E., B. Tóth, J. Szekeres, P. Borza & R. Sousa, 2014. Empty native and invasive bivalve shells as benthic habitat modifiers in a large river. Limnologica 49: 1–9.
Böhm, M., N. I. Dewhurst-Richman, M. Seddon, S. E. H. Ledger, C. Albrecht, D. Allen, A. E. Bogan, J. Cordeiro, K. S. Cummings, A. Cuttelod, G. Darrigran, W. Darwall, Z. Fehér, C. Gibson, D. L. Graf, F. Köhler, M. Lopes-Lima, G. Pastorino, K. E. Perez, K. Smith, D. van Damme, M. V. Vinarski, T. von Proschwitz, T. von Rintelen, D. C. Aldridge, N. A. Aravind, P. B. Budha, C. Clavijo, D. Van Tu, O. Gargominy, M. Ghamizi, M. Haase, C. Hilton-Taylor, P. D. Johnson, Ü. Kebapçı, J. Lajtner, C. N. Lange, D. A. W. Lepitzki, A. Martínez-Ortí, E. A. Moorkens, E. Neubert, C. M. Pollock, V. Prié, C. Radea, R. Ramirez, M. A. Ramos, S. B. Santos, R. Slapnik, M. O. Son, A.-S. Stensgaard & B. Collen, 2020. The conservation status of the world’s freshwater molluscs. Hydrobiologia. https://doi.org/10.1007/s10750-020-04385-w.
Bolotov, I. N., A. L. Klass, A. V. Kondakov, I. V. Vikhrev, Y. V. Bespalaya, M. Y. Gofarov, B. Y. Filippov, A. E. Bogan, M. Lopes-Lima, Z. Lunn, N. Chan, O. V. Aksenova, G. A. Dvoryankin, Y. E. Chapurina, S. K. Kim, Y. S. Kolosova, E. S. Konopleva, J. H. Lee, A. A. Makhrov, D. M. Palatov, E. M. Sayenko, V. M. Spitsyn, S. E. Sokolova, A. A. Tomilova, T. Win, N. A. Zubrii & M. V. Vinarski, 2019. Freshwater mussels house a diverse mussel-associated leech assemblage. Scientific Reports 9: 16449.
Bolotov, I. N., A. V. Kondakov, E. S. Konopleva, I. V. Vikhrev, O. V. Aksenova, A. S. Aksenov, Y. V. Bespalaya, A. V. Borovskoy, P. P. Danilov, G. A. Dvoryankin, M. Y. Gofarov, M. B. Kabakov, O. K. Klishko, Y. S. Kolosova, A. A. Lyubas, A. P. Novoselov, D. M. Palatov, G. N. Savvinov, N. M. Solomonov, V. M. Spitsyn, S. E. Sokolova, A. A. Tomilova, E. Froufe, A. E. Bogan, M. Lopes-Lima, A. A. Makhrov & M. V. Vinarski, 2020a. Integrative taxonomy, biogeography and conservation of freshwater mussels (Unionidae) in Russia. Scientific Reports 10: 3072.
Bolotov, I. N., E. S. Konopleva, I. V. Vikhrev, M. Y. Gofarov, M. Lopes-Lima, A. E. Bogan, Z. Lunn, N. Chan, T. Win, O. V. Aksenova, A. A. Tomilova, K. Tanmuangpak, S. Tumpeesuwan & A. V. Kondakov, 2020b. New freshwater mussel taxa discoveries clarify biogeographic division of Southeast Asia. Scientific Reports 10: 6616.
Boss, K. J., 1971. Critical estimate of the number of Recent Mollusca. Occasional Papers on Mollusks 3: 81–135.
Bosso, L., C. De Conno & D. Russo, 2017. Modelling the Risk Posed by the Zebra Mussel Dreissena polymorpha: Italy as a Case Study. Environmental Management 60: 304–313.
Bouchet, P., G. Falkner & M. B. Seddon, 1999. Lists of protected land and freshwater molluscs in the Bern Convention and European Habitats Directive: are they relevant to conservation? Biological Conservation 90: 21–31.
Box, J. B. & J. Mossa, 1999. Sediment, Land Use, and Freshwater Mussels: prospects and Problems. Journal of the North American Benthological Society 18: 99–117.
Box, B. J., J. K. Howard, D. Wolf, C. O’Brien, D. Nez, D. Close, J. C. Brim Box, J. K. Howard, D. Wolf, C. O. Brien, D. Nez & D. Close, 2006. Freshwater mussels (Bivalvia: Unionoida) of the Umatilla and Middle Fork John Day Rivers in Eastern Oregon. Northwest Science 80: 95–107.
Brandt, R. A. M., 1974. The non-marine aquatic Mollusca of Thailand. Archiv für Molluskenkunde 105: 1–423.
Brian, J. I., I. S. Ollard, & D. C. Aldridge, 2021. Don’t move a mussel? Parasite and disease risk in conservation action. Conservation Letters e12799. https://doi.org/10.1111/conl.12799
Brown, D. S., 1994. Freshwater snails of Africa and their medical importance. Taylor & Francis, London.
Brown, R. J., 2007. Freshwater mollusks survive fish gut passage. Arctic 60: 124–128.
Brönmark, Ch, 1992. Leech predation on juvenile freshwater snails: effects of size, species and substrate. Oecologia 91(4): 526–529.
Brönmark, C., S. D. Rundle & A. Erlandsson, 1991. Interactions between freshwater snails and tadpoles: competition and facilitation. Oecologia 87: 8–18.
Bunchom, N., W. Saijuntha, W. Pilap, W. Suksavate, K. Vaisusuk, N. Suganuma, T. Agatsuma, T. N. Petney & C. Tantrawatpan, 2019. Genetic variation of a freshwater snail Hydrobioides nassa (Gastropoda: Bithyniidae) in Thailand examined by mitochondrial DNA sequences. Hydrobiologia. https://doi.org/10.1007/s10750-019-04013-2.
Burns, C. W., M. Schallenberg & P. Verburg, 2014. Potential use of classical biomanipulation to improve water quality in New Zealand lakes: a re-evaluation. New Zealand Journal of Marine and Freshwater Research 48: 127–138.
Butcher, B. A., M. A. Smith, M. J. Sharkey & D. L. J. Quicke, 2012. A turbo-taxonomic study of Thai Aleiodes (Aleiodes) and Aleiodes (Arcaleiodes) (Hymenoptera: Braconidae: Rogadinae) based largely on COI barcoded specimens, with rapid descriptions of 179 new species. Zootaxa 3457: 1–232.
Byers, J. E., W. G. McDowell, S. R. Dodd, R. S. Haynie, L. M. Pintor & S. B. Wilde, 2013. Climate and pH predict the potential range of the invasive apple snail (Pomacea insularum) in the southeastern United States. PLoS ONE 8:
Carmignani, J. R., A. H. Roy, P. D. Hazelton & H. Giard, 2019. Annual winter water level drawdowns limit shallow-water mussel densities in small lakes. Freshwater Biology. https://doi.org/10.1111/fwb.13324.
Carpenter, S. R., J. J. Cole, J. F. Kitchell, M. L. Pace, 2009. Trophic cascades in lakes: lessons and prospects. In Terborgh, J. & J. A. Estes (eds), Trophic Cascades. Island Press: Washington, DC: 55–69.
Cardoso, P., P. Borges, K. Triantis, M.-A. Ferrández & J. Martín Esquivel, 2011. Adapting the IUCN Red List criteria for invertebrates. Biological Conservation. https://doi.org/10.1016/j.biocon.2011.06.020.
CE, 1992. Council of Europe Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Council of Europe, Brussels.
Cecala, K. K., S. J. Price, & M. E. Dorcas, 2007. Diet of Larval Red Salamanders (Pseudotriton Ruber) Examined Using a Nonlethal Technique. Journal of Herpetology 41: 741–745. https://doi.org/10.1670/07-019.1.Chambers, E. A. & D. M. Hillis, 2020. The multispecies coalescent over-splits species in the case of geographically widespread taxa. Systematic Biology 69: 184–193.
Chape, S., J. Harrison, M. Spalding & I. Lysenko, 2005. Measuring the extent and effectiveness of protected areas as an indicator for meeting global biodiversity targets. Philosophical Transactions of the Royal Society B: Biological Sciences 360: 443–455.
Charlier, J., K. Soenen, E. De Roeck, W. Hantson, E. Ducheyne, F. Van Coillie, R. De Wulf, G. Hendrickx & J. Vercruysse, 2014. Longitudinal study on the temporal and micro-spatial distribution of Galba truncatula in four farms in Belgium as a base for small-scale risk mapping of Fasciola hepatica. Parasites & Vectors 7: 528.
Chen, L. Y., A. G. Heath & R. J. Neves, 2001. Comparison of oxygen consumption in freshwater mussels (Unionidae) from different habitats during declining dissolved oxygen concentration. Hydrobiologia 450: 209–214.
Chowdhury, M. M. R., T. J. Marjomäki & J. Taskinen, 2019. Effect of glochidia infection on growth of fish: freshwater pearl mussel Margaritifera margaritifera and brown trout Salmo trutta. Hydrobiologia. https://doi.org/10.1007/s10750-019-03994-4.
Ćmiel, A. M., T. Zając, K. Zając, A. M. Lipińska & K. Najberek, 2019. Single or multiple spawning? Comparison of breeding strategies of freshwater Unionidae mussels under stochastic environmental conditions. Hydrobiologia. https://doi.org/10.1007/s10750-019-04045-8
Collado, G. A., M. A. Vidal, K. P. Aguayo, M. A. Méndez, M. A. Valladares, F. J. Cabrera, L. Pastenes, D. E. Gutiérrez Gregoric & N. Puillandre, 2019. Morphological and molecular analysis of cryptic native and invasive freshwater snails in Chile. Scientific Reports 9: 7846.
Cope, J. N. & M. J. Winterbourn, 2004. Competitive interactions between two successful molluscan invaders of freshwaters: an experimental study. Aquatic Ecology 38: 83–91.
Covich, A. P., 2010. Winning the biodiversity arms race among freshwater gastropods: competition and coexistence through shell variability and predator avoidance. Hydrobiologia 653: 191–215.
Cowie R. H., Régnier C., Fontaine R. & Ph. Bouchet, 2017. Measuring the Sixth Extinction: What do mollusks tell us? The Nautilus 131: 3–41. https://www.biodiversitylibrary.org/page/59337187
Cuezzo, M. G., D. E. Gutiérrez Gregoric, J.-P. Pointier, A. A. Vázquez, C. Ituarte, M. C. Dreher Mansur, J. O. Arruda, G. M. Barker, S. B. dos Santos, X. M. C. Ovando, L. E. M. de Lacerda, M. A. Fernandez, S. C. Thiengo, A. C. de Mattos, E. F. da Silva, M. I. Berning, G. A. Collado, I. C. Miyahira, T. N. Antoniazzi, D. M. Pimpão & C. Damborenea, 2020. Thorp and Covich's Freshwater Invertebrates (Fourth Edition) Volume 5: Keys to Neotropical and Antarctic Fauna, 261–430. Academic Press.
Cummings, K. S., H. A. Jones & M. Lopes-Lima, 2016. Rapid bioassessment methods for freshwater molluscs. In Larsen, T. H. (ed.), Core standardized methods for rapid biological field assessment: 185–207. Conservation International, Arlington.
Currier, C. A., T. J. Morris, C. C. Wilson & J. R. Freeland, 2018. Validation of environmental DNA (eDNA) as a detection tool for at-risk freshwater pearly mussel species (Bivalvia: Unionidae). Aquatic Conservation: Marine and Freshwater Ecosystems 28: 545–558.
Cyr, H., 2020. Site exposure, substrate, depth, and the thermocline affect the growth of native unionid mussels in a stratified lake. Freshwater Science 39: 773–790.
Cyr, H., D. M. Storisteanu & M. S. Ridgway, 2012. Sediment accumulation predicts the distribution of a unionid mussel (Elliptio complanata) in nearshore areas of a Canadian Shield lake. Freshwater Biology 57: 2125–2140.
Darwall, W. R. T., R. A. Holland, K. G. Smith, D. Allen, E. G. E. Brooks, V. Katarya, C. M. Pollock, Y. Shi, V. Clausnitzer, N. Cumberlidge, A. Cuttelod, K.-D. B. Dijkstra, M. D. Diop, N. García, M. B. Seddon, P. H. Skelton, J. Snoeks, D. Tweddle & J.-C. Vié, 2011. Implications of bias in conservation research and investment for freshwater species. Conservation Letters 4: 474–482.
Darwall, W., V. Bremerich, A. De Wever, A. I. Dell, J. Freyhof, M. O. Gessner, H.-P. Grossart, I. Harrison, K. Irvine, S. C. Jähnig, J. M. Jeschke, J. J. Lee, C. Lu, A. M. Lewandowska, M. T. Monaghan, J. C. Nejstgaard, H. Patricio, A. Schmidt-Kloiber, S. N. Stuart, M. Thieme, K. Tockner, E. Turak & O. Weyl, 2018. The Alliance for Freshwater Life: a global call to unite efforts for freshwater biodiversity science and conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 28: 1015–1022.
Darwin, C., 1882. On the Dispersal of Freshwater Bivalves. Nature 25: 529–530.
de Andrade, J. T. M., N. I. S. Cordeiro, L. C. Montresor, D. M. R. da Luz, E. M. de F. Viana, C. B. Martinez & T. H. D. A. Vidigal, 2020. Tolerance of Limnoperna fortunei (Dunker, 1857) (Bivalvia: Mytilidae) to aerial exposure at different temperatures. Hydrobiologia. https://doi.org/10.1007/s10750-020-04191-4.
de Bello, F., S. Lavorel, S. Díaz, R. Harrington, J. H. C. Cornelissen, R. D. Bardgett, M. P. Berg, P. Cipriotti, C. K. Feld, D. Hering, P. Martins da Silva, S. G. Potts, L. Sandin, J. P. Sousa, J. Storkey, D. A. Wardle & P. A. Harrison, 2010. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodiversity and Conservation 19: 2873–2893.
De Queiroz, K., 2005. Different species problems and their resolution. BioEssays 27: 1263–1269.
De Queiroz, K., 2007. Species Concepts and Species Delimitation. Systematic Biology 56: 879–886.
De Queiroz, K. & M. J. Donoghue, 1988. Phylogenetic systematics and the species problem. Cladistics 4: 317–338.
Der Sarkissian, C., V. Pichereau, C. Dupont, P. C. Ilsøe, M. Perrigault, P. Butler, L. Chauvaud, J. Eiríksson, J. Scourse, C. Paillard & L. Orlando, 2017. Ancient DNA analysis identifies marine mollusc shells as new metagenomic archives of the past. Molecular Ecology Resources 17: 835–853.
Dias, A. R., A. Teixeira, M. Lopes-Lima, S. Varandas & R. Sousa, 2020. From the lab to the river: determination of ecological hosts of Anodonta anatina. Aquatic Conservation: Marine and Freshwater Ecosystems 30: 988–999.
Dillon, R.T., 2000. The Ecology of Freshwater Mollusks. New York: Cambridge University Press.
Dimock, R. & A. Wright, 1993. Sensitivity of juvenile freshwater mussels to hypoxic, thermal and acid stress. Journal of the Elisha Mitchell Scientific Society 109: 183–192.
Donegal County Council, 2013. Maggie my story. A publication of the Freshwater Pearl Mussel Practical Measures Project Interreg IVa, Donegal County Council, Ireland. http://www.donegalcoco.ie/media/donegalcountyc/environment/pdfs/Maggie%20the%20Mussel.pdf
Donrovich, S. W., K. Douda, V. Plechingerová, K. Rylková, P. Horký, O. Slavík, H.-Z. Liu, M. Reichard, M. Lopes-Lima & R. Sousa, 2017. Invasive Chinese pond mussel Sinanodonta woodiana threatens native mussel reproduction by inducing cross-resistance of host fish. Aquatic Conservation: Marine and Freshwater Ecosystems 27: 1325–1333.
Douda, K., M. Lopes-Lima, M. Hinzmann, J. Machado, S. Varandas, A. Teixeira & R. Sousa, 2013. Biotic homogenization as a threat to native affiliate species: fish introductions dilute freshwater mussel’s host resources. Diversity and Distributions 19: 933–942.
Douda, K. & Z. Čadková, 2018. Water clearance efficiency indicates potential filter-feeding interactions between invasive Sinanodonta woodiana and native freshwater mussels. Biological Invasions 20: 1093–1098.
Downing, J. A., Y. Rochon, M. Pérusse, H. Harvey, M. Perusse & H. Harvey, 1993. Spatial aggregation, body size, and reproductive success in the freshwater mussel Elliptio complanata. Journal of the North American Benthological Society 12: 148.
Downing, J. A., P. Van Meter & D. A. Woolnough, 2010. Suspects and evidence: a review of the causes of extirpation and decline in freshwater mussels. Animal Biodiversity and Conservation 33: 151–185.
Du, L.-N., F. Köhler, G.-H. Yu, X.-Y. Chen & J.-X. Yang, 2019. Comparative morpho-anatomy and mitochondrial phylogeny of Semisulcospiridae in Yunnan, south-western China, with description of four new species (Gastropoda: Cerithioidea). Invertebrate Systematics. https://doi.org/10.1071/IS18084.
Dudley, N., I. J. Harrison, M. Kettunen, J. Madgwick & V. Mauerhofer, 2016. Natural solutions for water management of the future: freshwater protected areas at the 6th World Parks Congress. Aquatic Conservation: Marine and Freshwater Ecosystems 26: 121–132.
Duggan, I. C., A. A. C. Pearson & I. A. Kusabs, 2020. Effects of a native New Zealand freshwater mussel on zooplankton assemblages, including non-native Daphnia: a mesocosm experiment. Marine and Freshwater Research. https://doi.org/10.1071/MF20116.
Dupuis, J. R., A. D. Roe & F. A. H. Sperling, 2012. Multi-locus species delimitation in closely related animals and fungi: one marker is not enough. Molecular Ecology 21: 4422–4436.
Dysthe, J. C., T. Rodgers, T. W. Franklin, K. J. Carim, M. K. Young, K. S. McKelvey, K. E. Mock & M. K. Schwartz, 2018. Repurposing environmental DNA samples-detecting the western pearlshell (Margaritifera falcata) as a proof of concept. Ecology and Evolution 8: 2659–2670.
Dzierżyńska-Białończyk, A., Ł. Jermacz, J. Zielska & J. Kobak, 2019. What scares a mussel? Changes in valve movement pattern as an immediate response of a byssate bivalve to biotic factors. Hydrobiologia 841: 65–77.
Edwards, D. D. & M. F. Vidrine, 2013. Mites of freshwater mollusks. Malcolm F, Vidrine, Eunice, Louisiana.
Eken, G., L. Bennun, T. M. Brooks, W. Darwall, L. D. C. Fishpool, M. Foster, D. Knox, P. Langhammer, P. Matiku, E. Radford, P. Salaman, W. Sechrest, M. L. Smith, S. Spector & A. Tordoff, 2004. Key Biodiversity Areas as site conservation targets. BioScience 54: 1110–1118.
Farrington, S. J., R. W. King, J. A. Baker & J. G. Gibbons, 2020. Population genetics of freshwater pearl mussel (Margaritifera margaritifera) in central Massachusetts and implications for conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 30: 1945–1958.
Ferreira-Rodríguez, N., R. Sousa & I. Pardo, 2018. Negative effects of Corbicula fluminea over native freshwater mussels. Hydrobiologia 810: 85–95.
FMCS, 2016. A national strategy for the conservation of native freshwater mollusks*. Freshwater Mollusk Biology and Conservation 19: 1–21. https://doi.org/10.31931/fmbc.v19i1.2016.1-21
Froufe, E., V. Prié, J. Faria, M. Ghamizi, D. V. Gonçalves, M. E. Gürlek, I. Karaouzas, Ü. Kebapçi, H. Şereflişan, C. Sobral, R. Sousa, A. Teixeira, S. Varandas, S. Zogaris & M. Lopes-Lima, 2016. Phylogeny, phylogeography, and evolution in the Mediterranean region: news from a freshwater mussel (Potomida, Unionida). Molecular Phylogenetics and Evolution 100: 322–332.
Fuentes-Pardo, A. P. & D. E. Ruzzante, 2017. Whole-genome sequencing approaches for conservation biology: advantages, limitations and practical recommendations. Molecular Ecology 26: 5369–5406.
Gallardo, B., A. E. Bogan, S. Harun, L. Jainih, M. Lopes-Lima, M. Pizarro, K. A. Rahim, R. Sousa, S. G. P. Virdis & A. Zieritz, 2018. Current and future effects of global change on a hotspot’s freshwater diversity. Science of The Total Environment 635: 750–760.
Gama, M., D. Crespo, M. Dolbeth & P. M. Anastácio, 2017. Ensemble forecasting of Corbicula fluminea worldwide distribution: projections of the impact of climate change. Aquatic Conservation: Marine and Freshwater Ecosystems 27: 675–684.
Gangloff, M. M., K. K. Lenertz & J. W. Feminella, 2008. Parasitic mite and trematode abundance are associated with reduced reproductive output and physiological condition of freshwater mussels. Hydrobiologia 610: 25–31.
Garner, J. T., M. L. Buntin, T. B. Fobian, J. T. Holifield, T. A. Tarpley & P. D. Johnson, 2016. Use of Side-Scan Sonar to Locate Tulotoma magnifica (Conrad, 1834) (Gastropoda: Viviparidae) in the Alabama River. Freshwater Mollusk Biology and Conservation 19: 51.
Garrick, D. E., J. W. Hall, A. Dobson, R. Damania, R. Q. Grafton, R. Hope, C. Hepburn, R. Bark, F. Boltz, L. De Stefano, E. O’Donnell, N. Matthews & A. Money, 2017. Valuing water for sustainable development. Science 358: 1003–1005.
Gates, K. K., C. C. Vaughn & J. P. Julian, 2015. Developing environmental flow recommendations for freshwater mussels using the biological traits of species guilds. Freshwater Biology 60: 620–635.
Gauffre-Autelin, P., B. Stelbrink, T. von Rintelen & C. Albrecht, 2021. Miocene geologic dynamics of the Australian Sahul Shelf determined the biogeographic patterns of freshwater planorbid snails (Miratestinae) in the Indo-Australian Archipelago. Molecular Phylogenetics and Evolution. https://doi.org/10.1016/j.ympev.2020.107004.
Gilioli, G., G. Schrader, N. Carlsson, E. van Donk, C. H. A. van Leeuwen, P. R. Martín, S. Pasquali, M. Vilà & S. Vos, 2017. Environmental risk assessment for invasive alien species: a case study of apple snails affecting ecosystem services in Europe. Environmental Impact Assessment Review 65: 1–11.
Glaubrecht, M., 2009. On “Darwinian Mysteries” or molluscs as models in evolutionary biology: from local speciation to global radiation. American Malacological Bulletin 27: 3–23.
Glaubrecht, M. & T. von Rintelen, 2008. The species flocks of lacustrine gastropods: Tylomelania on Sulawesi as models in speciation and adaptive radiation. Hydrobiologia 615: 181–199.
Goldberg, C. S., A. Sepulveda, A. Ray, J. Baumgardt & L. P. Waits, 2013. Environmental DNA as a new method for early detection of New Zealand mudsnails (Potamopyrgus antipodarum). Freshwater Science 32: 792–800.
Gomes-dos-Santos, A., E. Froufe, D. V. Gonçalves, R. Sousa, V. Prié, M. Ghamizi, H. Benaissa, S. Varandas, A. Teixeira & M. Lopes-Lima, 2019. Freshwater conservation assessments in (semi-)arid regions: testing river intermittence and buffer strategies using freshwater mussels (Bivalvia, Unionida) in Morocco. Biological Conservation 236: 420–434.
Gomes-dos-Santos, A., M. Lopes-Lima, L. F. C. Castro & E. Froufe, 2020. Molluscan genomics: the road so far and the way forward. Hydrobiologia 847: 1705–1726.
Graf, D. L., 2001. The Cleansing of the Augean Stables, or a lexicon of the nominal species of the Pleuroceridae (Gastropoda: Prosobranchia) of Recent North America, north of Mexico. Walkerana 12: 1–124.
Graf, D. L., 2007. Palearctic freshwater mussel (Mollusca: Bivalvia: Unionoida) diversity and the Comparatory Method as a species concept. Proceedings of the Natural Sciences of Philadelphia 156: 71–88.
Graf, D. L., 2010. Funeral for the Nouvelle École -iana Generic Names Introduced for Freshwater Mussels (Mollusca: Bivalvia: Unionoida). Proceedings of the Academy of Natural Sciences of Philadelphia 159: 1–23.
Graf, D. L., 2013. Patterns of freshwater bivalve global diversity and the state of phylogenetic studies on the Unionoida, Sphaeriidae, and Cyrenidae. American Malacological Bulletin 31: 135–153.
Graf, D. L., A. Jørgensen, D. Van Damme & T. K. Kristensen, 2011. The status and distribution of freshwater molluscs (Mollusca). In Brooks, E., D. Allen & W. R. T. Darwall (eds), The Status and Distribution of Freshwater Biodiversity in Central Africa. IUCN, Cambridge.
Graham, C. H. & P. V. A. Fine, 2008. Phylogenetic beta diversity: linking ecological and evolutionary processes across space in time. Ecology Letters 11: 1265–1277.
Grego, J., L. Mumladze, A. Falniowski, A. Osikowski, A. Rysiewska, D. M. Palatov & S. Hofman, 2020. Revealing the stygobiotic and crenobiotic molluscan biodiversity hotspot in Caucasus: part I. The phylogeny of stygobiotic Sadlerianinae Szarowska, 2006 (Mollusca, Gastropoda, Hydrobiidae) from Georgia with descriptions of five new genera and twenty-one new species. ZooKeys 955: 1–77.
Grizzle, J. M. & C. J. Brunner, 2009. Infectious diseases of freshwater mussels and other freshwater bivalve mollusks. Reviews in Fisheries Science 17: 425–467.
Guerra, D., F. Plazzi, D. T. Stewart, A. E. Bogan, W. R. Hoeh & S. Breton, 2017. Evolution of sex-dependent mtDNA transmission in freshwater mussels (Bivalvia: Unionida). Scientific Reports 7: 1–13.
Guerra, D., M. Lopes-Lima, E. Froufe, H. M. Gan, P. Ondina, R. Amaro, M. W. Klunzinger, C. Callil, V. Prié, A. E. Bogan, D. T. Stewart & S. Breton, 2019. Variability of mitochondrial ORFans hints at possible differences in the system of doubly uniparental inheritance of mitochondria among families of freshwater mussels (Bivalvia: Unionida). BMC Evolutionary Biology 19: 1–22.
Guillera-Arroita, G., 2017. Modelling of species distributions, range dynamics and communities under imperfect detection: advances, challenges and opportunities. Ecography 40: 281–295.
Guillera-Arroita, G., J. J. Lahoz-Monfort, M. A. McCarthy & B. A. Wintle, 2015. Threatened species impact assessments: survey effort requirements based on criteria for cumulative impacts. Diversity and Distributions 21: 620–630.
Guisan, A. & W. Thuiller, 2005. Predicting species distribution: offering more than simple habitat models. Ecology Letters 8: 993–1009.
Gulati, R. D., L. M. Dionisio Pires & E. Van Donk, 2008. Lake restoration studies: failures, bottlenecks and prospects of new ecotechnological measures. Limnologica 38: 233–247.
Gutiérrez, J. L., C. G. Jones, D. L. Strayer & O. O. Iribarne, 2003. Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos 101: 79–90.
Haag, W. R., 2012. North American freshwater mussels. Natural history, ecology, and conservation. Cambridge University Press, Cambridge, UK
Haag, W. R., 2013. The role of fecundity and reproductive effort in defining life-history strategies of North American freshwater mussels. Biological Reviews of the Cambridge Philosophical Society 88: 745–766.
Haag, W. R. & J. D. Williams, 2014. Biodiversity on the brink: an assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 735: 45–60.
Haag, W. R., J. Culp, A. N. Drayer, M. A. McGregor, D. E. J. White & S. J. Price, 2020. Abundance of an invasive bivalve, Corbicula fluminea, is negatively related to growth of freshwater mussels in the wild. Freshwater Biology. https://doi.org/10.1111/fwb.13651.
Haase, M., T. Wilke & P. Mildner, 2007. Identifying species of Bythinella (Caenogastropoda: Rissooidea): A plea for an integrative approach. Zootaxa 1563: 1–16.
Habdija, I., J. Lajtner & I. Belinić, 1995. The Contribution of Gastropod Biomass in Macrobenthic Communities of a Karstic River. Internationale Revue der gesamten Hydrobiologie und Hydrographie 80: 103–110.
Haberl, W., 2002. Food storage, prey remains and notes on occasional vertebrates in the diet of the Eurasian water shrew, Neomys fodiens. Folia Zoologica 51: 93–102.
Hall Jr, R. O., J. L. Tank & M. F. Dybdahl, 2003. Exotic snails dominate nitrogen and carbon cycling in a highly productive stream. Frontiers in Ecology and the Environment 1: 407–411.
Hastie, L. C., P. J. Boon, M. R. Young & S. Way, 2001. The effects of a major flood on an endangered freshwater mussel population. Biological Conservation 98: 107–115.
Hastie, T. & W. Fithian, 2013. Inference from presence-only data; the ongoing controversy. Ecography 36: 864–867.
Hayward, M. W., M. F. Child, G. I. H. Kerley, P. A. Lindsey, M. J. Somers & B. Burns, 2015. Ambiguity in guideline definitions introduces assessor bias and influences consistency in IUCN Red List status assessments. Frontiers in Ecology and Evolution. https://doi.org/10.3389/fevo.2015.00087.
He, J. & Z. Zhuang, 2013. The Freshwater Bivalves of China. ConchBooks, Germany, Harxheim.
Hegeman, E. E., S. W. Miller & K. E. Mock, 2014. Modeling freshwater mussel distribution in relation to biotic and abiotic habitat variables at multiple spatial scales. Canadian Journal of Fisheries and Aquatic Sciences 71: 1483–1497.
Heifrich, L. A., M. Zimmerman & D. L. Weigmann, 1995. Control of suspended solids and phytoplankton with fishes and a mussel. Journal of the American Water Resources Association 31: 307–316.
Hermoso, V., A. F. Filipe, P. Segurado & P. Beja, 2015. Filling gaps in a large reserve network to address freshwater conservation needs. Journal of Environmental Management 161: 358–365.
Hermoso, V., R. Abell, S. Linke & P. Boon, 2016. The role of protected areas for freshwater biodiversity conservation: challenges and opportunities in a rapidly changing world. Aquatic Conservation: Marine and Freshwater Ecosystems 26: 3–11.
Hinzmann, M., M. Lopes-Lima, A. Teixeira, S. Varandas, R. Sousa, A. Lopes, E. Froufe & J. Machado, 2013. Reproductive cycle and strategy of Anodonta anatina (L., 1758): notes on hermaphroditism. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 319: 378–390.
Hirano, T., T. Saito, Y. Tsunamoto, J. Koseki, B. Ye, V. T. Do, O. Miura, Y. Suyama & S. Chiba, 2019. Enigmatic incongruence between mtDNA and nDNA revealed by multi-locus phylogenomic analyses in freshwater snails. Scientific Reports 9: 6223.
Hoeh, W. R., D. T. Stewart & S. I. Guttman, 2002. High fidelity of mitochondrial genome transmission under the doubly uniparental mode of inheritance in freshwater mussels (Bivalvia: Unionoidea). Evolution 56: 2252–2261.
Hoellein, T. J., C. B. Zarnoch, D. A. Bruesewitz & J. DeMartini, 2017. Contributions of freshwater mussels (Unionidae) to nutrient cycling in an urban river: filtration, recycling, storage, and removal. Biogeochemistry 135: 307–324.
Hornbach, D. J., H. N. Stutzman, M. C. Hove, J. L. Kozarek, K. R. MacGregor, T. J. Newton & P. R. Ries, 2019. Influence of surrounding land-use on mussel growth and glycogen levels in the St. Croix and Minnesota River Basins. Hydrobiologia. https://doi.org/10.1007/s10750-019-04016-z->.
Horák, P. & L. Kolářová, 2011. Snails, waterfowl and cercarial dermatitis. Freshwater Biology 56: 779–790.
Hortal, J., F. de Bello, J. A. F. Diniz-Filho, T. M. Lewinsohn, J. M. Lobo & R. J. Ladle, 2015. Seven Shortfalls that Beset Large-Scale Knowledge of Biodiversity. Annual Review of Ecology, Evolution, and Systematics 46: 523–549.
Hubendick, B., 1951a. Recent Lymnaeidae. Their variation, morphology, taxonomy, nomenclature and distribution. Kunglike Svenska Vetenskapsakademiens Handlingar, series 4 3(1): 1–223.
Hyvärinen, H. S. H., M. M. R. Chowdhury & J. Taskinen, 2020. Pulsed flow-through cultivation of Margaritifera margaritifera: effects of water source and food quantity on the survival and growth of juveniles. Hydrobiologia. https://doi.org/10.1007/s10750-020-04225-x.
Ibrahim, M. M., 2007. Population dynamics of Chaetogaster limnaei (Oligochaeta: Naididae) in the field populations of freshwater snails and its implications as a potential regulator of trematode larvae community. Parasitology Research 101: 25–33.
Ilarri, M. I., A. T. Souza, V. Modesto, L. Guilhermino & R. Sousa, 2015. Differences in the macrozoobenthic fauna colonising empty bivalve shells before and after invasion by Corbicula fluminea. Marine and Freshwater Research 66: 549.
Ilarri, M. I., L. Amorim, A. T. Souza & R. Sousa, 2018. Physical legacy of freshwater bivalves: effects of habitat complexity on the taxonomical and functional diversity of invertebrates. Science of the Total Environment 634: 1398–1405.
IPBES (Intergovernmental Science-Policy Platform on Biodiversity) 2019. Summary for Policymakers of the Global Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. IPBES Secretariat Bonn.
Jacobs, S. J., C. Kristofferson, S. Uribe-Convers, M. Latvis & D. C. Tank, 2018. Incongruence in molecular species delimitation schemes: what to do when adding more data is difficult. Molecular Ecology 27: 2397–2413.
Johnson, N. A., C. H. Smith, J. M. Pfeiffer, C. R. Randklev, J. D. Williams & J. D. Austin, 2018. Integrative taxonomy resolves taxonomic uncertainty for freshwater mussels being considered for protection under the U.S. Endangered Species Act. Scientific Reports 8: 15892.
Jokela, J., L. Uotila & J. Taskinen, 1993. Effect of the Castrating Trematode Parasite Rhipidocotyle fennica on Energy Allocation of Fresh-Water Clam Anodonta piscinalis. Functional Ecology 7: 332.
Kappes, H. & P. Haase, 2012. Slow, but steady: dispersal of freshwater molluscs. Aquatic Sciences 74: 1–14.
Karatayev, A. Y., K. Mehler, L. E. Burlakova, E. K. Hinchey & G. J. Warren, 2018. Benthic video image analysis facilitates monitoring of Dreissena populations across spatial scales. Journal of Great Lakes Research 44: 629–638.
Keith, D. A., J. P. Rodríguez, K. M. Rodríguez-Clark, E. Nicholson, K. Aapala, A. Alonso, M. Asmussen, S. Bachman, A. Basset, E. G. Barrow, J. S. Benson, M. J. Bishop, R. Bonifacio, T. M. Brooks, M. A. Burgman, P. Comer, F. A. Comín, F. Essl, D. Faber-Langendoen, P. G. Fairweather, R. J. Holdaway, M. Jennings, R. T. Kingsford, R. E. Lester, R. Mac Nally, M. A. McCarthy, J. Moat, M. A. Oliveira-Miranda, P. Pisanu, B. Poulin, T. J. Regan, U. Riecken, M. D. Spalding & S. Zambrano-Martínez, 2013. Scientific foundations for an IUCN red list of ecosystems. PLoS ONE 8: e62111.
Kerstes, N. A. G., T. Breeschoten, V. J. Kalkman & M. Schilthuizen, 2019. Snail shell colour evolution in urban heat islands detected via citizen science. Communications Biology 2: 264.
Khan, J. M., M. Hart, J. Dudding, C. R. Robertson, R. Lopez & C. R. Randklev, 2019. Evaluating the upper thermal limits of glochidia for selected freshwater mussel species (Bivalvia: Unionidae) in central and east Texas, and the implications for their conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 29: 1202–1215.
Klunzinger, M. W., M. Lopes-Lima, A. Gomes-dos-Santos, E. Froufe, A. J. Lymbery & L. Kirkendale, 2020. Phylogeographic study of the West Australian freshwater mussel, Westralunio carteri, uncovers evolutionarily significant units that raise new conservation concerns. Hydrobiologia. https://doi.org/10.1007/s10750-020-04200-6.
Knutson, L. V. & J. C. Vala, 2011. Biology of Snail-Killing Sciomyzidae Flies. Cambridge University Press, Cambridge.
Kobak, J., Ł. Jermacz & D. Płąchocki, 2014. Effectiveness of zebra mussels to act as shelters from fish predators differs between native and invasive amphipod prey. Aquatic Ecology 48: 397–408.
Köhler, F., 2016. Rampant taxonomic incongruence in a mitochondrial phylogeny of Semisulcospira freshwater snails from Japan (Cerithioidea: Semisulcospiridae). Journal of Molluscan Studies 82: 268–281.
Köhler, F., 2017. Corrigendum to: against the odds of unusual mtDNA inheritance, introgressive hybridisation and phenotypic plasticity: systematic revision of Korean freshwater gastropods (Semisulcospiridae, Cerithioidea). Invertebrate Systematics 31: 361.
Köhler, F. & M. Glaubrecht, 2010. Uncovering an overlooked radiation: molecular phylogeny and biogeography of Madagascar’s endemic river snails (Caenogastropoda: Pachychilidae: Madagasikara gen. nov.). Biological Journal of the Linnean Society 99: 867–894.
Köhler, F., N. Brinkmann & M. Glaubrecht, 2008. Convergence Caused Confusion: on the Systematics Of the Freshwater Gastropod Sulcospira pisum (Brot, 1868) (Cerithioidea, Pachychilidae). Malacologia 50: 331–339.
Köhler, F., M. Seddon, A. E. Bogan, D. V. Tu, P. Sri-Aroon & D. Allen, 2012. The status and distribution of freshwater molluscs of the Indo-Burma region. In Allen, D. J., K. G. Smith & W. R. T. Darwall (eds), The status and distribution of freshwater biodiversity in IndoBurma. IUCN, Gland, Switzerland and Cambridge, UK: 66–89.
Kołodziejczyk, A., 1984. Occurrence of Gastropoda in the lake littoral and their role in the production and transformation of detritus, II. Ecological activity of snails. Ekologia Polska 32: 469–492.
Konopleva, E. S., J. M. Pfeiffer, I. V. Vikhrev, A. V. Kondakov, M. Y. Gofarov, O. V. Aksenova, Z. Lunn, N. Chan & I. N. Bolotov, 2019. A new genus and two new species of freshwater mussels (Unionidae) from western Indochina. Scientific Reports 9: 4106.
Kristensen, T. K., C. C. Appleton, B. Curtis & A.-S. Stensgaard, 2009. The status and distribution of freshwater molluscs. In Darwall, W. R. T., K. G. Smith, D. Tweddle & P. Skelton (eds), The Status and Distribution of Freshwater Biodiversity in Southern Africa. IUCN and SAIAB, Gland, Switzerland and Grahmstown, South Africa: 38–47.
Kristensen, T. K., A.-S. Stensgaard, M. B. Seddon & A. McIvor, 2010. The status and distribution of freshwater molluscs (Mollusca). In Smith, K. G., M. D. Diop, M. Niane & W. R. T. Darwall (eds), The Status and Distribution of Freshwater Biodiversity in Western Africa. IUCN, Gland, Switzerland and Cambridge, UK: 33–40.
Kwak, M. L., A. C. G. Heath & P. Cardoso, 2020. Methods for the assessment and conservation of threatened animal parasites. Biological Conservation. https://doi.org/10.1016/j.biocon.2020.108696.
Labecka, A. M. & M. Czarnoleski, 2019. Patterns of growth, brooding and offspring size in the invasive mussel Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae) from an anthropogenic heat island. Hydrobiologia. https://doi.org/10.1007/s10750-019-04141-9.
Layzer, J. B. & L. M. Madison, 1995. Microhabitat use by freshwater mussels and recommendations for determining their instream flow needs. Regulated Rivers: Research & Management 10: 329–345.
Lee, T. & D. Ó. Foighil, 2003. Phylogenetic structure of the Sphaeriinae, a global clade of freshwater bivalve molluscs, inferred from nuclear (ITS-1) and mitochondrial (16S) ribosomal gene sequences. Zoological Journal of the Linnean Society 137: 245–260.
Lymbery, A. J., L. Ma, S. J. Lymbery, M. W. Klunzinger, S. J. Beatty & D. L. Morgan, 2020. Burrowing behavior protects a threatened freshwater mussel in drying rivers. Hydrobiologia. https://doi.org/10.1007/s10750-020-04268-0.
Linke, S., V. Hermoso & S. Januchowski-Hartley, 2019. Toward process-based conservation prioritizations for freshwater ecosystems. Aquatic Conservation: Marine and Freshwater Ecosystems 29: 1149–1160.
Liquin, F., L. A. Hünicken, F. Arrighetti, D. Davies, E. M. Paolucci & F. Sylvester, 2020. Parasitism and fitness of invaders: oligochaete Chaetogaster limnaei produces gill damage and increased respiration rates in freshwater Asian clams. Hydrobiologia. https://doi.org/10.1007/s10750-020-04424-6.
Lopes-Lima, M., A. Teixeira, E. Froufe, A. Lopes, S. Varandas & R. Sousa, 2014a. Biology and conservation of freshwater bivalves: past, present and future perspectives. Hydrobiologia 735: 1–13.
Lopes-Lima, M., P. Lima, M. Hinzmann, A. Rocha & J. Machado, 2014b. Selective feeding by Anodonta cygnea (Linnaeus, 1771): the effects of seasonal changes and nutritional demands. Limnologica 44: 18–22.
Lopes-Lima, M., R. Sousa, A. Teixeira, S. Varandas, N. Riccardi, D. C. Aldridge & E. Froufe, 2016. Newly developed microsatellite markers for the pan-European duck mussel, Anodonta anatina: revisiting the main mitochondrial lineages. Aquatic Conservation: Marine and Freshwater Ecosystems 26: 307–318.
Lopes-Lima, M., E. Froufe, V. T. Do, M. Ghamizi, K. E. Mock, Ü. Kebapçı, O. Klishko, S. Kovitvadhi, U. Kovitvadhi, O. S. Paulo, J. M. Pfeiffer III, M. Raley, N. Riccardi, H. Şereflişan, R. Sousa, A. Teixeira, S. Varandas, X. Wu, D. T. Zanatta, A. Zieritz & A. E. Bogan, 2017a. Phylogeny of the most species-rich freshwater bivalve family (Bivalvia: Unionida: Unionidae): Defining modern subfamilies and tribes. Molecular Phylogenetics and Evolution 106: 174–191.
Lopes-Lima, M., R. Sousa, J. Geist, D. C. Aldridge, R. Araujo, J. Bergengren, Y. Bespalaya, E. Bódis, L. Burlakova, D. Van Damme, K. Douda, E. Froufe, D. Georgiev, C. Gumpinger, A. Karatayev, Ü. Kebapçi, I. Killeen, J. Lajtner, B. M. Larsen, R. Lauceri, A. Legakis, S. Lois, S. Lundberg, E. Moorkens, G. Motte, K. O. Nagel, P. Ondina, A. Outeiro, M. Paunovic, V. Prié, T. von Proschwitz, N. Riccardi, M. Rudzīte, M. Rudzītis, C. Scheder, M. Seddon, H. Şereflişan, V. Simić, S. Sokolova, K. Stoeckl, J. Taskinen, A. Teixeira, F. Thielen, T. Trichkova, S. Varandas, H. Vicentini, K. Zajac, T. Zajac & S. Zogaris, 2017b. Conservation status of freshwater mussels in Europe: state of the art and future challenges. Biological Reviews 92: 572–607.
Lopes-Lima, M., L. E. Burlakova, A. Y. Karatayev, K. Mehler, M. Seddon & R. Sousa, 2018a. Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia 810: 1–14.
Lopes-Lima, M., I. N. Bolotov, V. T. Do, D. C. Aldridge, M. M. Fonseca, H. M. Gan, M. Y. Gofarov, A. V. Kondakov, V. Prié, R. Sousa, S. Varandas, I. V. Vikhrev, A. Teixeira, R.-W. Wu, X. Wu, A. Zieritz, E. Froufe & A. E. Bogan, 2018b. Expansion and systematics redefinition of the most threatened freshwater mussel family, the Margaritiferidae. Molecular Phylogenetics and Evolution 127: 98–118.
Lopes-Lima, M., L. Burlakova, A. Karatayev, A. Gomes-dos-Santos, A. Zieritz, E. Froufe & A. E. Bogan, 2019. Revisiting the North American freshwater mussel genus Quadrula sensu lato (Bivalvia Unionidae): phylogeny, taxonomy and species delineation. Zoologica Scripta 48: 313–336.
Lopes-Lima, M., A. Hattori, T. Kondo, J. Hee Lee, S. Ki Kim, A. Shirai, H. Hayashi, T. Usui, K. Sakuma, T. Toriya, Y. Sunamura, H. Ishikawa, N. Hoshino, Y. Kusano, H. Kumaki, Y. Utsugi, S. Yabe, Y. Yoshinari, H. Hiruma, A. Tanaka, K. Sao, T. Ueda, I. Sano, J.-I. Miyazaki, D. V. Gonçalves, O. K. Klishko, E. S. Konopleva, I. V. Vikhrev, A. V. Kondakov, M Yu Gofarov, I. N. Bolotov, E. M. Sayenko, M. Soroka, A. Zieritz, A. E. Bogan & E. Froufe, 2020. Freshwater mussels (Bivalvia: Unionidae) from the rising sun (Far East Asia): phylogeny, systematics, and distribution. Molecular Phylogenetics and Evolution. https://doi.org/10.1016/j.ympev.2020.106755.
Lounnas, M., A. A. Vázquez, P. Alda, K. Sartori, J.-P. Pointier, P. David & S. Hurtrez-Boussès, 2017. Isolation, characterization and population-genetic analysis of microsatellite loci in the freshwater snail Galba cubensis (Lymnaeidae). Journal of Molluscan Studies 83: 63–68.
Lydeard, C. & K. S. Cummings (eds), 2019. Freshwater mollusks of the world: A Distribution Atlas. John Hopkins University Press, Baltimore.
Lydeard, C., R. H. Cowie, W. F. Ponder, A. E. Bogan, P. Bouchet, S. A. Clark, K. S. Cummings, T. J. Frest, O. Gargominy, D. G. Herbert, R. Hershler, K. E. Perez, B. Roth, M. Seddon, E. E. Strong & F. G. Thompson, 2004. The global decline of nonmarine mollusks. BioScience 54: 321–330.
Macher, J. N., M. Weiss, A. J. Beermann & F. Leese, 2016. Cryptic diversity and population structure at small scales: the freshwater snail Ancylus (Planorbidae, Pulmonata) in the Montseny mountain range. Annales de Limnologie - International Journal of Limnology 52: 387–399.
Machida, Y. & Y. B. Akiyama, 2013. Impacts of invasive crayfish (Pacifastacus leniusculus) on endangered freshwater pearl mussels (Margaritifera laevis and M. togakushiensis) in Japan. Hydrobiologia 720: 145–151.
Magoulick, D. D. & L. C. Lewis, 2002. Predation on exotic zebra mussels by native fishes: effects on predator and prey. Freshwater Biology 47: 1908–1918.
Mammola, S., N. Riccardi, V. Prié, R. Correia, P. Cardoso, M. Lopes-Lima & R. Sousa, 2020. Towards a taxonomically unbiased European Union biodiversity strategy for 2030. Proceedings of the Royal Society B: Biological Sciences. https://doi.org/10.1098/rspb.2020.2166.
Manguin, S., & J.-C. Vala, 1989. Prey Consumption by Larvae of Tetanocera ferruginea (Diptera: Sciomyzidae) in Relation to Number of Snail Prey Species Available. Annals of the Entomological Society of America 82: 588–592. https://doi.org/10.1093/aesa/82.5.588.
Margules, C. R. & R. L. Pressey, 2000. Systematic conservation planning. Nature 405: 243–253. https://doi.org/10.1038/35012251.
Markovic, D., S. Carrizo, J. Freyhof, N. Cid, S. Lengyel, M. Scholz, H. Kasperdius & W. Darwall, 2014. Europe’s freshwater biodiversity under climate change: distribution shifts and conservation needs. Diversity and Distributions 20: 1097–1107.
Marroni, S., N. Mazzeo, J. P. Pacheco, J. Clemente & C. Iglesias, 2017. Interactions between bivalves and zooplankton: competition or intraguild predation? Implications for biomanipulation in subtropical shallow lakes. Marine and Freshwater Research 68: 1036.
McGill, B., B. Enquist, E. Weiher & M. Westoby, 2006. Rebuilding community ecology from functional traits. Trends in Ecology & Evolution 21: 178–185.
McDowell, W. G. & R. Sousa, 2019. Mass mortality events of invasive freshwater bivalves: current understanding and potential directions for future research. Frontiers in Ecology and Evolution 7: 331.
Mehler, K., L. E. Burlakova, A. Y. Karatayev, Z. Biesinger, A. Valle-Levinson, C. Castiglione & D. Gorsky, 2018. Sonar technology and underwater imagery analysis can enhance invasive Dreissena distribution assessment in large rivers. Hydrobiologia 810: 119–131.
Meier-Brook, C., 1993. Artaufassungen in Bereich der limnischen Molluscen und ihr Wandel im 20. Jahrhundert. Archiv für Molluskenkunde 122: 133–147.
Meira, A., M. Lopes-Lima, S. Varandas, A. Teixeira, F. Arenas & R. Sousa, 2019. Invasive crayfishes as a threat to freshwater bivalves: interspecific differences and conservation implications. Science of The Total Environment 649: 938–948.
Melchior, M., K. J. Collier & S. J. Clearwater, 2019. First record of complex release strategies and morphometry of glochidia in sympatric Echyridella species (Bivalvia: Unionida: Hyriidae). Hydrobiologia. https://doi.org/10.1007/s10750-019-03995-3.
Metcalfe-Smith, J. L., S. K. Staton, G. L. Mackie & N. M. Lane, 1998. Changes in the biodiversity of freshwater mussels in the Canadian waters of the Lower Great Lakes Drainage Basin over the past 140 years. Journal of Great Lakes Research 24: 845–858.
Michelson, E. H., 1964. The protective action of Chaetogaster limnaei on snails exposed to Schistosoma mansoni. The Journal of Parasitology 50: 441.
Mitchell, Z. A., L. E. Burlakova, A. Y. Karatayev & A. N. Schwalb, 2019. Changes in community composition of riverine mussels after a severe drought depend on local conditions: a comparative study in four tributaries of a subtropical river. Hydrobiologia. https://doi.org/10.1007/s10750-019-04058-3.
Miura, O., F. Köhler, T. Lee, J. Li & D. Ó. Foighil, 2013. Rare, divergent Korean Semisulcospira spp. mitochondrial haplotypes have Japanese sister lineages. Journal of Molluscan Studies 79: 86–89.
Miura, O., M. Urabe, T. Nishimura, K. Nakai & S. Chiba, 2019. Recent lake expansion triggered the adaptive radiation of freshwater snails in the ancient Lake Biwa. Evolution Letters 3: 43–54.
Mock, K. E., J. C. Brim Box, J. P. Chong, J. K. Howard, D. A. Nez, D. Wolf & R. S. Gardner, 2010. Genetic structuring in the freshwater mussel Anodonta corresponds with major hydrologic basins in the western United States. Molecular Ecology 19: 569–591.
Modesto, V., M. Ilarri, A. T. Souza, M. Lopes-Lima, K. Douda, M. Clavero & R. Sousa, 2018. Fish and mussels: importance of fish for freshwater mussel conservation. Fish and Fisheries 19: 244–259.
Modesto, V., P. Castro, M. Lopes-Lima, C. Antunes, M. Ilarri & R. Sousa, 2019. Potential impacts of the invasive species Corbicula fluminea on the survival of glochidia. Science of The Total Environment 673: 157–164.
Moles, J. & G. Giribet, 2021. A polyvalent and universal tool for genomic studies in gastropod molluscs (Heterobranchia). Molecular Phylogenetics and Evolution 155: 106996. https://doi.org/10.1016/j.ympev.2020.106996.
Molina, F. R., S. J. de Paggi & D. Boltovskoy, 2011. Vulnerability of microcrustaceans to predation by the invasive filter-feeding mussel Limnoperna fortunei (Dunker). Marine and Freshwater Behaviour and Physiology 44: 329–338.
Molina, F. R., S. J. De Paggi & D. Frau, 2012. Impacts of the invading golden mussel Limnoperna fortunei on zooplankton: a mesocosm experiment. Zoological Studies 51: 733–744.
MolluscaBase eds. (2021). MolluscaBase. Available at http://www.molluscabase.org. Accessed 11 Jan 2021
Monnet, C., C. Zollikofer, H. Bucher & N. Goudemand, 2009. Three-dimensional morphometric ontogeny of mollusc shells by micro-computed tomography and geometric analysis. Palaeontologia electronica 12: 1–13.
Moore, T. P. & S. J. Clearwater, 2019. Non-native fish as glochidial sinks: elucidating disruption pathways for Echyridella menziesii recruitment. Hydrobiologia. https://doi.org/10.1007/s10750-019-04035-w.
Müller, O. & B. Baur, 2011. Survival of the Invasive Clam Corbicula fluminea (Müller) in Response to Winter Water Temperature. Malacologia 53: 367–371.
Naddafi, R. & L. G. Rudstam, 2013. Predator-induced behavioural defences in two competitive invasive species: the zebra mussel and the quagga mussel. Animal Behaviour 86: 1275–1284.
Nakamura, K., J. Cañete, D. Vijuesca, N. Guillén, C. Sosa, F. Mesquita-Joanes, R. Sousa, E. Ginés & V. Sorribas, 2020. Sensitivity of Pseudunio auricularius to metals and ammonia: first evaluation. Hydrobiologia. https://doi.org/10.1007/s10750-020-04277-z.
Negus, C. L., 1966. A quantitative study of growth and production of unionid mussels in the River Thames at Reading. The Journal of Animal Ecology 35: 513.
Nichols, S. J. & D. Garling, 2000. Food-web dynamics and trophic-level interactions in a multispecies community of freshwater unionids. Can. J. Zool. 78:
Nogueira, J. G., M. Lopes-Lima, S. Varandas, A. Teixeira & R. Sousa, 2020. Effects of an extreme drought on the endangered pearl mussel Margaritifera margaritifera: a before/after assessment. Hydrobiologia. https://doi.org/10.1007/s10750-019-04103-1.
Nogueira, J. G., A. Teixeira, S. Varandas, M. Lopes-Lima & R. Sousa, 2021. Assessment of a terrestrial protected area for the conservation of freshwater biodiversity. Marine and Freshwater Ecosystems, Aquatic Conservation. https://doi.org/10.1002/aqc.3502.
Noss, R. F., M. O’Connell & D. D. Murphy, 1997. The science of conservation planning: habitat conservation under the Endangered Species Act. Island Press, Washington, DC.
Novais, A., A. T. Souza, M. Ilarri, C. Pascoal & R. Sousa, 2015. From water to land: how an invasive clam may function as a resource pulse to terrestrial invertebrates. Science of the Total Environment 538: 664–671.
Novais, A., E. Dias & R. Sousa, 2016. Inter- and intraspecific variation of carbon and nitrogen stable isotope ratios in freshwater bivalves. Hydrobiologia 765: 149–158.
Novais, A., C. Pascoal & R. Sousa, 2017. Effects of invasive aquatic carrion on soil chemistry and terrestrial microbial communities. Biological Invasions 19: 2491–2502.
Oheimb, P. V., C. Albrecht, F. Riedel, L. Du, J. Yang, D. C. Aldridge, U. Bößneck, H. Zhang & T. Wilke, 2011. Freshwater biogeography and limnological evolution of the Tibetan Plateau - insights from a plateau-wide distributed gastropod taxon (Radix spp.). PLoS ONE 6: e26307.
Ostrovsky, A. N. & I. Y. Popov, 2011. Rediscovery of the largest population of the freshwater pearl mussel (Margaritifera margaritifera) in the Leningrad oblast (north-west Russia). Aquatic Conservation: Marine and Freshwater Ecosystems 21: 113–121.
Ożgo, M., M. Urbańska, P. Hoos, H. K. Imhof, M. Kirschenstein, J. Mayr, F. Michl, R. Tobiasz, M. Wesendonk, S. Zimmermann & J. Geist, 2020. Invasive zebra mussel (Dreissena polymorpha) threatens an exceptionally large population of the depressed river mussel (Pseudanodonta complanata) in a postglacial lake. Ecology and Evolution 10: 4918–4927.
Ożgo, M., M. Urbańska, M. Marzec, A. Kamocki, W. Andrzejewski, J. Golski K. Lewandowski & J. Geist, 2021. Lake-stream transition zones support hotspots of freshwater ecosystem services: evidence from a 35-year study on unionid mussels. Science of the Total Environment
Pacific Northwest Freshwater Mussel Workgroup, 2020. Freshwater mussels of the Western U. S. Citizen Science Project. https://www.inaturalist.org/projects/freshwater-mussels-of-the-western-u-s
Pandolfo, T. J., T. J. Kwak & W. G. Cope, 2012. Thermal tolerances of freshwater mussels and their host fishes: Species interactions in a changing climate. Freshwater Mollusk Biology and Conservation 15: 69.
Pearson, A. A. C. & I. C. Duggan, 2020. Dividing the algal soup: is there niche separation between native bivalves (Echyridella menziesii) and non-native Daphnia pulex in New Zealand? New Zealand Journal of Marine and Freshwater Research 54: 45–59.
Pedraza-Marrón, C. del R., R. Silva, J. Deeds, S. M. Van Belleghem, A. Mastretta-Yanes, O. Domínguez-Domínguez, R. A. Rivero-Vega, L. Lutackas, D. Murie, D. Parkyn, L. H. Bullock, K. Foss, H. Ortiz-Zuazaga, J. Narváez-Barandica, A. Acero, G. Gomes, & R. Betancur-R, 2019. Genomics overrules mitochondrial DNA, siding with morphology on a controversial case of species delimitation. Proceedings of the Royal Society B: Biological Sciences 286: 20182924.
Pfeiffer, J. M., D. L. Graf, K. S. Cummings & L. M. Page, 2021. Taxonomic revision of a radiation of South-east Asian freshwater mussels (Unionidae: Gonideinae: Contradentini+ Rectidentini). Invertebrate Systematics 35: 394–470. https://doi.org/10.1071/IS20044.
Pfeiffer, J. M., A. E. Sharpe, N. A. Johnson, K. F. Emery & L. M. Page, 2018. Molecular phylogeny of the Nearctic and Mesoamerican freshwater mussel genus Megalonaias. Hydrobiologia 811: 139–151.
Pfeiffer, J. M., J. W. Breinholt & L. M. Page, 2019. Unioverse: a phylogenomic resource for reconstructing the evolution of freshwater mussels (Bivalvia, Unionoida). Molecular Phylogenetics and Evolution 137: 114–126.
Pilotto, F., R. Sousa & D. C. Aldridge, 2016. Is the body condition of the invasive zebra mussel (Dreissena polymorpha) enhanced through attachment to native freshwater mussels (Bivalvia, Unionidae)? Science of The Total Environment 553: 243–249.
Ponder, W. F., A. Hallan, M. Shea & S. A. Clark, 2016. Australian freshwater molluscs. http://keys.lucidcentral.org/keys/v3/freshwater_molluscs/
Prashad, B., 1932. Some Noteworthy Examples of Parallel Evolution in the Molluscan Faunas of South-eastern Asia and South America. Proceedings of the Royal Society of Edinburgh 51: 42–53.
Prié, V., N. Puillandre & P. Bouchet, 2013. Bad taxonomy can kill: molecular reevaluation of Unio mancus Lamarck, 1819 (Bivalvia: Unionidae) and its accepted subspecies. Knowledge and Management of Aquatic Ecosystems. https://doi.org/10.1051/kmae/2013071.
Prié, V., Q. Molina & B. Gamboa, 2014. French naiad (Bivalvia: Margaritiferidae, Unionidae) species distribution models: prediction maps as tools for conservation. Hydrobiologia 735: 81–94.
Prié, V., A. Valentini, M. Lopes-Lima, E. Froufe, M. Rocle, N. Poulet, P. Taberlet & T. Dejean, 2020. Environmental DNA metabarcoding for freshwater bivalves biodiversity assessment: methods and results for the Western Palearctic (European sub-region). Hydrobiologia. https://doi.org/10.1007/s10750-020-04260-8.
Prins, T. & V. Escaravage, 2005. Can Bivalve Suspension-Feeders Affect Pelagic Food Web Structure? The Comparative Roles of Suspension-Feeders in Ecosystems. Springer, Berlin. https://doi.org/10.1007/1-4020-3030-4_3.
Ravera, O., G. M. Beone, P. R. Trincherini, & N. Riccardi, 2007. Seasonal variations in metal content of two Unio pictorum mancus (Mollusca Unionidae) populations from two lakes of different trophic state. Journal of Limnology 66: 28–39. https://doi.org/10.4081/jlimnol.2007.28
Ravera, O., Sprocati, A.R. 1997. Population dynamics, production, assimilation and respiration of two freshwater mussels: Unio mancus, Zhadin and Anodonta cygnea Lam. Memorie-Istituto Italiano di Idrobiologia 56: 113–130.
Régnier, C., B. Fontaine & Ph Bouchet, 2009. Not knowing, not recording, not listing: numerous unnoticed mollusk extinctions. Conservation Biology 23: 1214–1221.
Reichard, M., H. Liu & C. Smith, 2007. The co-evolutionary relationship between bitterling fishes and freshwater mussels: insights from interspecific comparisons. Evolutionary Ecology Research 9: 239–259.
Reid, W. V., 1998. Biodiversity hotspots. Trends in Ecology & Evolution 13: 275–280.
Relf, V., B. Good, J. P. Hanrahan, E. McCarthy, A. B. Forbes & T. DeWaal, 2011. Temporal studies on Fasciola hepatica in Galba truncatula in the west of Ireland. Veterinary Parasitology 175: 287–292.
Reynolds, J. D., V. J. Debuse & D. C. Aldridge, 1997. Host specialisation in an unusual symbiosis: European bitterlings spawning in freshwater mussels. Oikos 78: 539.
Riccardi, A., F. G. Whoriskey & J. B. Rasmussen, 1997. The role of the zebra mussel (Dreissena polymorpha) in structuring macroinvertebrate communities on hard substrata. Canadian Journal of Fisheries and Aquatic Sciences 54: 2596–2608.
Richard, J. C., E. Leis, C. D. Dunn, R. Agbalog, D. Waller, S. Knowles, J. Putnam & T. L. Goldberg, 2020. Mass mortality in freshwater mussels (Actinonaias pectorosa) in the Clinch River, USA, linked to a novel densovirus. Scientific Reports 10: 14498.
Riedel, A., K. Sagata, Y. R. Suhardjono, R. Tänzler & M. Balke, 2013. Integrative taxonomy on the fast track - towards more sustainability in biodiversity research. Frontiers in Zoology 10: 15.
Rintelen, T. von, A. B. Wilson, A. Meyer & M. Glaubrecht, 2004. Escalation and trophic specialization drive adaptive radiation of freshwater gastropods in ancient lakes on Sulawesi, Indonesia. Proceedings of the Royal Society of London. Series B: Biological Sciences 271: 2541–2549.
Rosa, I. C., J. L. Pereira, R. Costa, F. Gonçalves & R. Prezant, 2012. Effects of upper-limit water temperatures on the dispersal of the Asian clam Corbicula fluminea. PLoS ONE 7:
Rosauer, D., S. W. Laffan, M. D. Crisp, S. C. Donnellan & L. G. Cook, 2009. Phylogenetic endemism: a new approach for identifying geographical concentrations of evolutionary history. Molecular Ecology 18: 4061–4072.
Rondelaud, D., 1977. Results and problems set by the introduction of Zonitidae snails in some biotopes of Lymnaea truncatula Müller in Indre and Haute-Vienne, France. Annales de Parasitologie Humaine et Comparee 52: 521–530.
Rondelaud, D., 1978. The effects of an association of predatory snails (Zonitidea-Physidea) in biological control of Lymnaea (Galba) truncatula Müller. Annales de Parasitologie Humaine et Comparee 53: 511–517.
Ruhi, A., M. L. Messager & J. D. Olden, 2018. Tracking the pulse of the Earth’s fresh waters. Nature Sustainability 1: 198–203.
Rychlik, L., 2002. Prey size, prey nutrition, and food handling by shrews of different body sizes. Behavioral Ecology 13: 216–223.
Ryo, M., B. Angelov, S. Mammola, J. M. Kass, B. M. Benito & F. Hartig, 2020. Explainable artificial intelligence enhances the ecological interpretability of black‐box species distribution models. Ecography. https://doi.org/10.1111/ecog.05360
Sagehashi, M., A. Sakoda & M. Suzuki, 2000. A predictive model of long-term stability after biomanipulation of shallow lakes. Water Research 34: 4014–4028.
Sanchez, B. & A. N. Schwalb, 2019. Detectability affects the performance of survey methods: a comparison of sampling methods of freshwater mussels in Central Texas. Hydrobiologia. https://doi.org/10.1007/s10750-019-04017-y.
Sagorny, C., C. Wesseler, D. Krämer & J. von Döhren, 2019. Assessing the diversity and distribution of Cephalothrix species (Nemertea: Palaeonemertea) in European waters by comparing different species delimitation methods. Journal of Zoological Systematics and Evolutionary Research 57: 497–519.
Sands, A. F., T. A. Neubauer, S. Nasibi, M. F. Harandi, V. V. Anistratenko, T. Wilke & C. Albrecht, 2019. Old lake versus young taxa: a comparative phylogeographic perspective on the evolution of Caspian Sea gastropods (Neritidae: Theodoxus). Royal Society Open Science. https://doi.org/10.1098/rsos.190965.
Sarremejane, R., N. Cid, R. Stubbington, T. Datry, M. Alp, M. Cañedo-Argüelles, A. Cordero-Rivera, Z. Csabai, C. Gutiérrez-Cánovas, J. Heino, M. Forcellini, A. Millán, A. Paillex, P. Pařil, M. Polášek, J. M. Tierno de Figueroa, P. Usseglio-Polatera, C. Zamora-Muñoz & N. Bonada, 2020. DISPERSE, a trait database to assess the dispersal potential of European aquatic macroinvertebrates. Scientific Data 7: 386.
Schermer, M. & L. Hogeweg, 2018. Supporting citizen scientists with automatic species identification using deep learning image recognition models. Biodiversity Information Science and Standards 2: e25268. https://doi.org/10.3897/biss.2.25268
Schultheiß, R., B. Van Bocxlaer, T. Wilke & C. Albrecht, 2009. Old fossils–young species: evolutionary history of an endemic gastropod assemblage in Lake Malawi. Proceedings of the Royal Society B: Biological Sciences 276: 2837–2846.
Seddon, M. B., Ü. Kebapçı, M. Lopes-Lima, D. van Damme & K. G. Smith, 2014. Freshwater mollusks. In Smith, K. G., V. Barrios, W. R. T. Darwall & C. Numa (eds), The Status and Distribution of Freshwater Biodiversity in the Eastern Mediterranean. Malaga, Spain and Gland, Switzerland, Cambridge: 43–56.
Shapiro, J., 1980. The Importance of Trophic-Level Interactions to the Abundance and Species Composition of Algae in Lakes Hypertrophic Ecosystems. Springer, Netherlands, Dordrecht. https://doi.org/10.1007/978-94-009-9203-0_12.
Shu, F., F. Köhler, C. Fu & H. Wang, 2013. A new species of Gyraulus (Gastropoda: Planorbidae) from Ancient Lake Lugu, Yunnan-Guizhou Plateau, Southwest China. Molluscan Research 33: 34–39. https://doi.org/10.1080/13235818.2012.754146.
Simone, L. R. L., 2006. Land and freshwater molluscs of Brazil. Editora Grafíca Bernardi, FAPESP, São Paulo, Brasil.
Simeone, D., C. H. Tagliaro & C. R. Beasley, 2021. Amazonian freshwater mussel density: a useful indicator of macroinvertebrate assemblage and habitat quality. Ecological Indicators 122: https://doi.org/10.1016/j.ecolind.2020.107300.
Sitnikova, T. Y., Y. R. Tulupova, I. V. Khaev & L. A. Prozorova, 2013. Novel spirochetes in the crystalline style of freshwater gastropods. Biology Bulletin 40: 107–110. https://doi.org/10.1134/S1062359012060131.
Smit, R. & A. Kaeser, 2016. Defining freshwater mussel mesohabitat associations in an alluvial, Coastal Plain river. Freshwater Science 35: 1276–1290. https://doi.org/10.1086/688928.
Smith, C. H., N. A. Johnson, K. Inoue, R. D. Doyle & C. R. Randklev, 2019. Integrative taxonomy reveals a new species of freshwater mussel, Potamilus streckersoni sp. nov. (Bivalvia: Unionidae): implications for conservation and management. Systematics and Biodiversity 17: 331–348. https://doi.org/10.1080/14772000.2019.1607615.
Smith, C. H., J. M. Pfeiffer & N. A. Johnson, 2020. Comparative phylogenomics reveal complex evolution of life history strategies in a clade of bivalves with parasitic larvae (Bivalvia: Unionoida: Ambleminae). Cladistics 36: 505–520. https://doi.org/10.1111/cla.12423.
Soga, M. & K. J. Gaston, 2018. Shifting baseline syndrome: causes, consequences, and implications. Frontiers in Ecology and the Environment 16: 222–230. https://doi.org/10.1002/fee.1794.
Sokolow, S. H., E. Huttinger, N. Jouanard, M. H. Hsieh, K. D. Lafferty, A. M. Kuris, G. Riveau, S. Senghor, C. Thiam, A. N’Diaye, D. S. Faye & G. A. De Leo, 2015. Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proceedings of the National Academy of Sciences 112: 9650–9655. https://doi.org/10.1073/pnas.1502651112.
Søndergaard, M., E. Jeppesen, T. L. Lauridsen, C. Skov, E. H. Van Nes, R. Roijackers, E. Lammens & R. Portielje, 2007. Lake restoration: successes, failures and long-term effects. Journal of Applied Ecology 44: 1095–1105. https://doi.org/10.1111/j.1365-2664.2007.01363.x.
Sousa, R., L. Guilhermino & C. Antunes, 2005. Molluscan fauna in the freshwater tidal area of the River Minho estuary, NW of Iberian Peninsula. Annales de Limnologie - International Journal of Limnology 41: 141–147. https://doi.org/10.1051/limn/2005009.
Sousa, R., C. Antunes & L. Guilhermino, 2007. Species composition and monthly variation of the Molluscan fauna in the freshwater subtidal area of the River Minho estuary. Estuarine, Coastal and Shelf Science 75: 90–100. https://doi.org/10.1016/j.ecss.2007.02.020.
Sousa, R., A. J. A. Nogueira, M. B. Gaspar, C. Antunes & L. Guilhermino, 2008a. Growth and extremely high production of the non-indigenous invasive species Corbicula fluminea (Müller, 1774): possible implications for ecosystem functioning. Estuarine, Coastal and Shelf Science 80: 289–295. https://doi.org/10.1016/j.ecss.2008.08.006.
Sousa, R., A. J. A. Nogueira, C. Antunes & L. Guilhermino, 2008b. Growth and production of Pisidium amnicum in the freshwater tidal area of the River Minho estuary. Estuarine, Coastal and Shelf Science 79: 467–474. https://doi.org/10.1016/j.ecss.2008.04.023.
Sousa, R., P. Morais, C. Antunes & L. Guilhermino, 2008c. Factors affecting Pisidium amnicum (Müller, 1774; Bivalvia: Sphaeriidae) distribution in the River Minho Estuary: consequences for its conservation. Estuaries and Coasts 31: 1198–1207. https://doi.org/10.1007/s12237-008-9090-3.
Sousa, R., F. Pilotto & D. C. Aldridge, 2011. Fouling of European freshwater bivalves (Unionidae) by the invasive zebra mussel (Dreissena polymorpha). Freshwater Biology 56: 867–876. https://doi.org/10.1111/j.1365-2427.2010.02532.x.
Sousa, R., S. Varandas, R. Cortes, A. Teixeira, M. Lopes-Lima, J. Machado & L. Guilhermino, 2012. Massive die-offs of freshwater bivalves as resource pulses. Annales de Limnologie - International Journal of Limnology 48: 105–112. https://doi.org/10.1051/limn/2012003.
Sousa, R., A. Novais, R. Costa & D. L. Strayer, 2014. Invasive bivalves in fresh waters: impacts from individuals to ecosystems and possible control strategies. Hydrobiologia 735: 233–251. https://doi.org/10.1007/s10750-012-1409-1.
Sousa, R., Â. Amorim, E. Froufe, S. Varandas, A. Teixeira & M. Lopes-Lima, 2015. Conservation status of the freshwater pearl mussel Margaritifera margaritifera in Portugal. Limnologica 50: 4–10. https://doi.org/10.1016/j.limno.2014.07.004.
Sousa, R., J. G. Nogueira, A. Ferreira, F. Carvalho, M. Lopes-Lima, S. Varandas & A. Teixeira, 2019. A tale of shells and claws: the signal crayfish as a threat to the pearl mussel Margaritifera margaritifera in Europe. Science of the Total Environment 665: 329–337. https://doi.org/10.1016/j.scitotenv.2019.02.094.
Sousa, R., J. G. Nogueira, F. Miranda & A. Teixeira, 2020. Time travelling through local ecological knowledge regarding an endangered species. Science of The Total Environment 739: https://doi.org/10.1016/j.scitotenv.2020.140047.
Standley, C. J., L. Prepelitchi, S. M. Pietrokovsky, L. Issia, J. R. Stothard & C. Wisnivesky-Colli, 2013. Molecular characterization of cryptic and sympatric lymnaeid species from the Galba/Fossaria group in Mendoza Province, Northern Patagonia, Argentina. Parasites & Vectors 6: 304. https://doi.org/10.1186/1756-3305-6-304.
Stelbrink, B., A. A. Shirokaya, C. Clewing, T. Y. Sitnikova, L. A. Prozorova & C. Albrecht, 2015. Conquest of the deep, old and cold: an exceptional limpet radiation in Lake Baikal. Biology Letters 11: 20150321. https://doi.org/10.1098/rsbl.2015.0321.
Stelbrink, B., R. Richter, F. Köhler, F. Riedel, E. E. Strong, B. Van Bocxlaer, C. Albrecht, T. Hauffe, T. J. Page, D. C. Aldridge, A. E. Bogan, L.-N. Du, M. R. Manuel-Santos, R. M. Marwoto, A. A. Shirokaya & T. Von Rintelen, 2020. Global diversification dynamics since the Jurassic: low dispersal and habitat-dependent evolution explain hotspots of diversity and shell disparity in river snails (Viviparidae). Systematic Biology 69: 944–961.
Stoeckle, B. C., R. Kuehn & J. Geist, 2016. Environmental DNA as a monitoring tool for the endangered freshwater pearl mussel (Margaritifera margaritifera L.): a substitute for classical monitoring approaches? Aquatic Conservation: Marine and Freshwater Ecosystems 26: 1120–1129.
Stoll, S., D. Früh, B. Westerwald, N. Hormel & P. Haase, 2013. Density-dependent relationship between Chaetogaster limnaei limnaei (Oligochaeta) and the freshwater snail Physa acuta (Pulmonata). Freshwater Science 32: 642–649.
Strayer, D. L., 2006. Challenges for freshwater invertebrate conservation. Journal of the North American Benthological Society 25: 271–287.
Strayer, D. L., 2008. Freshwater mussel ecology: a multifactor approach to distribution and abundance. University of California Press, Berkeley.
Strayer, D. L., 2014. Understanding how nutrient cycles and freshwater mussels (Unionoida) affect one another. Hydrobiologia 735: 277–292. https://doi.org/10.1007/s10750-013-1461-5.
Strayer, D. L. & D. R. Smith, 2003. A guide to sampling freshwater mussel populations. Monograph No. 8. American Fisheries Society, Bethesda, Maryland.
Strayer, D. L., N. Cid & H. M. Malcom, 2011. Long-term changes in a population of an invasive bivalve and its effects. Oecologia 165: 1063–1072. https://doi.org/10.1007/s00442-010-1792-0.
Strayer, D. L. & H. M. Malcom, 2018. Long-term responses of native bivalves (Unionidae and Sphaeriidae) to a Dreissena invasion. Freshwater Science 37: 697–711. https://doi.org/10.1086/700571.
Strong, E. E., O. Gargominy, W. F. Ponder & P. Bouchet, 2008. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 595: 149–166. https://doi.org/10.1007/s10750-007-9012-6.
Subba Rao, M. V., 1989. Handbook: Freshwater Molluscs of India. Zoological Survey of India, Calcutta.
Taskinen, J., E. T. Valtonen & D. I. Gibson, 1991. Studies on bucephalid digeneans parasitising molluscs and fishes in Finland I. Ecological data and experimental studies. Systematic Parasitology 19: 81–94. https://doi.org/10.1007/BF00009906.
Taskinen, J., M. Urbańska, F. Ercoli, W. Andrzejewski, M. Ożgo, B. Deng, J. M. Choo & N. Riccardi, 2020. Parasites in sympatric populations of native and invasive freshwater bivalves. Hydrobiologia. https://doi.org/10.1007/s10750-020-04284-0.
Tedesco, P. A., R. Bigorne, A. E. Bogan, X. Giam, C. Jézéquel & B. Hugueny, 2014. Estimating how many undescribed species have gone extinct. Conservation Biology 28: 1360–1370. https://doi.org/10.1111/cobi.12285.
Terui, A., Y. Miyazaki, A. Yoshioka, K. Kaifu, S. I. S. Matsuzaki & I. Washitani, 2014. Asymmetric dispersal structures a riverine metapopulation of the freshwater pearl mussel Margaritifera laevis. Ecology and Evolution 4: 3004–3014. https://doi.org/10.1002/ece3.1135.
Tudorancea, C., 1972. Studies on Unionidae populations from the Crapina-Jijila complex of pools (Danube zone liable to inundation). Hydrobiologia 39: 527–561. https://doi.org/10.1007/BF00046744.
Turner, A. M., 1996. Freshwater snails alter habitat use in response to predation. Animal Behaviour 51: 747–756. https://doi.org/10.1006/anbe.1996.0079.
Urbańska, M., M. Kirschenstein, K. Obolewski & M. Ożgo, 2019. Silent invasion: Sinanodonta woodiana successfully reproduces and possibly endangers native mussels in the north of its invasive range in Europe. International Review of Hydrobiology 104: 127–136. https://doi.org/10.1002/iroh.201801971.
van Ee, B. C., Z. L. Nickerson & C. L. Atkinson, 2020. Picky pigs prefer pigtoes: Evidence for species-selective feral pig predation on freshwater mussels. Freshwater Mollusk Biology and Conservation. https://doi.org/10.31931/fmbc.v23i2.2020.92-98
van Leeuwen, C. H. A., G. van der Velde, B. van Lith & M. Klaassen, 2012. Experimental quantification of long distance dispersal potential of aquatic snails in the gut of migratory birds. PLoS ONE 7: https://doi.org/10.1371/journal.pone.0032292.
Vaughn, C. C., 2012. Life history traits and abundance can predict local colonisation and extinction rates of freshwater mussels. Freshwater Biology 57: 982–992. https://doi.org/10.1111/j.1365-2427.2012.02759.x.
Vinarski, M. V., 2018. The species question in freshwater malacology: from Linnaeus to the present day. Folia Malacologica 26: 39–52.
Vinarski, M. V. & S. S. Kramarenko, 2015. How does the discrepancies among taxonomists affect macroecological patterns? A case study of freshwater snails of the Western Siberia. Biodiversity and Conservation 24(8): 2079–2091. https://doi.org/10.1007/s10531-015-0934-4.
Vinarski, M. V. & D. M. Palatov, 2019. A survey of the Belgrandiella -like gastropods of the Northern Black Sea region (Mollusca, Gastropoda, Hydrobiidae s. l.): Morphological variability and morphospecies. Zoologicheskii Zhurnal 98: 988–1002. https://doi.org/10.1134/S0044513419070122.
Vinarski, M. V., I. N. Bolotov, O. V. Aksenova, E. S. Babushkin, Y. V. Bespalaya, A. A. Makhrov, I. O. Nekhaev & I. V. Vikhrev, 2020. Freshwater Mollusca of the circumpolar arctic: a review on their taxonomy, diversity and biogeography. Hydrobiologia. https://doi.org/10.1007/s10750-020-04270-6.
Wada, S., K. Kawakami & S. Chiba, 2012. Snails can survive passage through a bird’s digestive system. Journal of Biogeography 39: 69–73. https://doi.org/10.1111/j.1365-2699.2011.02559.x.
Waller, D. L., & W. G. Cope, 2019. The status of mussel health assessment and a path forward. Freshwater Mollusk Biology and Conservation 22: 26
Walters, A. D., M. A. Brown, G. M. Cerbie, M. G. Williams, J. A. Banta, L. R. Williams, N. B. Ford & D. J. Berg, 2019. Do hotspots fall within protected areas? A geographic approach to planning analysis of regional freshwater biodiversity. Freshwater Biology 64: 2046–2056. https://doi.org/10.1111/fwb.13394.
Weiss, M., H. Weigand, A. M. Weigand & F. Leese, 2017. Genome-wide single-nucleotide polymorphism data reveal cryptic species within cryptic freshwater snail species-The case of the Ancylus fluviatilis species complex. Ecology and Evolution 8: 1063–1072. https://doi.org/10.1002/ece3.3706.
van der Welde, S., T. A. Yanina, T. A. Neubaier & F. P. Wesselingh, 2020. The Late Pleistocene mollusk fauna of Selitrennoye (Astrakhan province, Russia): a natural baseline for endemic Caspian Sea faunas. Journal of Great Lakes Research 46: 1227–1239. https://doi.org/10.1016/j.jglr.2019.04.001.
von Proschwitz, T. & N. Wengström, 2020. Zoogeography, ecology, and conservation status of the large freshwater mussels in Sweden. Hydrobiologia. https://doi.org/10.1007/s10750-020-04351-6.
Wethington, A. R., C. R. Jackson & C. Albritton, 2018. Assessing predator risk: how leeches affect life history and behaviour of the freshwater snail Physa acuta. Journal of Molluscan Studies 84: 379–385. https://doi.org/10.1093/mollus/eyy030.
Wheeler, Q. D., 2007. Invertebrate systematics or spineless taxonomy? Zootaxa 1668: 11–18
Wilson, R. A. & J. Denison, 1980. The parasitic castration and gigantism of Lymnaea truncatula infected with the larval stages of Fasciola hepatica. Zeitschrift für Parasitenkunde 61: 109–119. https://doi.org/10.1007/BF00925458.
Wilson, A. B., M. Glaubrecht & A. Meyer, 2004. Ancient lakes as evolutionary reservoirs: evidence from the thalassoid gastropods of Lake Tanganyika. Proceedings of the Royal Society of London. Series B: Biological Sciences 271: 529–536.
Wilson, C. D., D. Roberts & N. Reid, 2011. Applying species distribution modelling to identify areas of high conservation value for endangered species: a case study using Margaritifera margaritifera (L.). Biological Conservation 144: 821–829. https://doi.org/10.1016/j.biocon.2010.11.014.
Wiese, R., C. Clewing, C. Albrecht, C. Rabethge, H. Zhang & F. Riedel, 2020. How ancient is Lake Lugu (Yunnan, China)? The gastropods’ viewpoint with focus on Radix (Lymnaeidae). Journal of Great Lakes Research 46: 1099–1112. https://doi.org/10.1016/j.jglr.2020.06.003.
Wood, L., R. Griffiths, K. Groh, E. Engel & L. Schley, 2008. Interactions between freshwater mussels and newts: a novel form of parasitism? Amphibia-Reptilia 29: 457–462. https://doi.org/10.1163/156853808786230433.
Wolff, C., C. Albrecht & T. Wilke, 2020. Recovery from interglacial-related bottleneck likely triggered diversification of Lake Titicaca gastropod species flock. Journal of Great Lakes Research 46: 1199–1206. https://doi.org/10.1016/j.jglr.2019.08.006.
Yoshida, K., K. Matsukura, N. J. Cazzaniga & T. Wada, 2014. Tolerance to low temperature and desiccation in two invasive apple snails, Pomacea canaliculata and P. maculata (Caenogastropoda: Ampullariidae), collected in their original distribution area (northern and central Argentina). Journal of Molluscan Studies 80: 62–66. https://doi.org/10.1093/mollus/eyt042.
Zapitis, C., M. Huck & A. Ramsey, 2021. Oxygen consumption during digestion in Anodonta anatina and Unio pictorum in response to algal concentration. Hydrobiologia. https://doi.org/10.1007/s10750-021-04559-0.
Zając, K., 2014. Size-dependent predation by the Otter Lutra lutra on Swan Mussels Anodonta cygnea (Linnaeus 1758): observations and radiotelemetry experiment. Journal of Conchology 41: 559–564.
Zając, K. & T. A. Zając, 2020. Seasonal patterns in the developmental rate of glochidia in the endangered thick-shelled river mussel, Unio crassus Philipsson, 1788. Hydrobiologia. https://doi.org/10.1007/s10750-020-04240-y.
Zając, K., J. Florek, T. Zając, P. Adamski, W. Bielański, A. M. Ćmiel, M. Klich & A. M. Lipińska, 2018. On the reintroduction of the endangered thick-shelled river mussel Unio crassus: the importance of the river’s longitudinal profile. Science of the Total Environment 624: 273–282.
Zdelar, M., F. Mullin, C. Cheung, M. Yousif, B. Baltaretu & J. R. Stone, 2018. Pollution-, temperature- and predator-induced responses in phenotypically plastic gastropod shell traits. Molluscan Research 38: 34–40. https://doi.org/10.1080/13235818.2017.1358587.
Zelaya, D. G. & M. C. Marinone, 2012. A case of phoresis of sphaeriids by corixids: first report for the Americas. Malacologia 55: 363–367. https://doi.org/10.4002/040.055.0209.
Zhang, L.-J., S.-C. Chen, L.-T. Yang, L. Jin & F. Köhler, 2015. Systematic revision of the freshwater snail Margarya Nevill, 1877 (Mollusca: Viviparidae) endemic to the ancient lakes of Yunnan, China, with description of new taxa. Zoological Journal of the Linnean Society 174: 760–800.
Zieritz, A., A. E. Bogan, E. Froufe, O. Klishko, T. Kondo, U. Kovitvadhi, S. Kovitvadhi, J. H. Lee, M. Lopes-Lima, J. M. Pfeiffer, R. Sousa, T. Van Do, I. Vikhrev & D. T. Zanatta, 2018. Diversity, biogeography and conservation of freshwater mussels (Bivalvia: Unionida) in East and Southeast Asia. Hydrobiologia 810: 29–44. https://doi.org/10.1007/s10750-017-3104-8.
Zieritz, A., W. N. Chan, S. McGowan & C. Gibbins, 2020. High rates of biodeposition and N-excretion indicate strong functional effects of mussels (Bivalvia: Unionida) in certain anthropogenic tropical freshwater habitats. Hydrobiologia. https://doi.org/10.1007/s10750-020-04464-y.
Zukowski, S. & K. F. Walker, 2009. Freshwater snails in competition: alien Physa acuta (Physidae) and native Glyptophysa gibbosa (Planorbidae) in the River Murray, South Australia. Marine and Freshwater Research 60: 999. https://doi.org/10.1071/MF08183.
Funding
This work was supported by the COST Action [CA18239].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known conflicts or competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Manuel P. M. Lopes-Lima, Nicoletta Riccardi, Maria Urbanska & Ronaldo G. Sousa / Biology and Conservation of Freshwater Molluscs
Rights and permissions
About this article
Cite this article
Lopes-Lima, M., Riccardi, N., Urbanska, M. et al. Major shortfalls impairing knowledge and conservation of freshwater molluscs. Hydrobiologia 848, 2831–2867 (2021). https://doi.org/10.1007/s10750-021-04622-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04622-w