Abstract
Patient-specific cardiovascular simulation has become a paradigm in cardiovascular research and is emerging as a powerful tool in basic, translational and clinical research. In this paper we discuss the recent development of a fully open-source SimVascular software package, which provides a complete pipeline from medical image data segmentation to patient-specific blood flow simulation and analysis. This package serves as a research tool for cardiovascular modeling and simulation, and has contributed to numerous advances in personalized medicine, surgical planning and medical device design. The SimVascular software has recently been refactored and expanded to enhance functionality, usability, efficiency and accuracy of image-based patient-specific modeling tools. Moreover, SimVascular previously required several licensed components that hindered new user adoption and code management and our recent developments have replaced these commercial components to create a fully open source pipeline. These developments foster advances in cardiovascular modeling research, increased collaboration, standardization of methods, and a growing developer community.


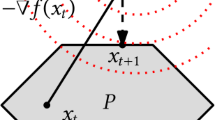
Adapted from Ref. 29.







Figures adapted from Ref. 1.





Adapted from Ref. 49.
Similar content being viewed by others
References
Arzani, A., P. Dyverfeldt, T. Ebbers, and S. C. Shadden (2012) In vivo validation of numerical prediction for turbulence intensity in an aortic coarctation. Ann. Biomed. Eng. 40(4):860–870.
Arzani, A., A. M. Gambaruto, G. Chen, and S. C. Shadden. Lagrangian wall shear stress structures and near wall transport in high schmidt aneurysmal flows. J. Fluid Mech. 790:158–172, 2016.
Arzani, A., A. S Les, R. L. Dalman, and S. C. Shadden. Effect of exercise on patient specific abdominal aortic aneurysm flow topology and mixing. Int. J. Numer. Methods Biomed. Eng. 30(2):280–295, 2014.
Arzani A., and S. C. Shadden. Characterization of the transport topology in patient-specific abdominal aortic aneurysm models. Phys. Fluids (1994-present) (1994), 24(8):081901, 2012.
Arzani, A., G. Y. Suh, R. L. Dalman, and S. C. Shadden. A longitudinal comparison of hemodynamics and intraluminal thrombus deposition in abdominal aortic aneurysms. Am. J. Physiol. Heart Circ. Physiol. 307(12):H1786–H1795, 2014.
Astorino, M., J. Hamers, S. C. Shadden, and J. Gerbeau. A robust and efficient valve model based on resistive immersed surfaces. Int. J. Numer. Methods Biomed. Eng. 28(9):937–959, 2012.
Bezdek, J. C., L. O. Hall, and L. P. Clarke. Review of MR image segmentation techniques using pattern recognition. Med. Phys. 20(4):1033–1048, 1992.
Bockman, M. D., A. P. Kansagra, S. C. Shadden, E. C. Wong, and A. L. Marsden. Fluid mechanics of mixing in the vertebrobasilar system: Cardiovasc. Eng. Technol. 3(4):450–461, 2012.
Bogren, H. G., R. H. Klipstein, D. N. Firmin, R. H. Mohiaddin, S. R. Underwood, R. S. O. Rees, and D. B. Longmore. Quantitation of antegrade and retrograde blood flow in the human aorta by magnetic resonance velocity mapping. Am. Heart J. 117(6):1214–1222, 1989.
Braunwald, E., E. M. Antman, J. W. Beasley, R. M. Califf, M. D. Cheitlin, J. S. Hochman, R. H. Jones, D. Kereiakes, J. Kupersmith, T. N. Levin, et al. Acc/aha 2002 guideline update for the management of patients with unstable angina and non-st-segment elevation myocardial infarction–summary article: a report of the american college of cardiology/american heart association task force on practice guidelines (committee on the management of patients with unstable angina). J. Am. Coll. Cardiol. 40(7):1366–1374, 2002.
Carr, I. A., N. Nemoto, R. S. Schwartz, and S. C. Shadden. Size-dependent predilections of cardiogenic embolic transport. Am. J. Physiol. Heart Circ. Physiol. 305(5):H732–H739, 2013.
Cheng, C. P., R. J. Herfkens, C. A. Taylor, and J. A. Feinstein. Proximal pulmonary artery blood flow characteristics in healthy subjects measured in an upright posture using MRI: the effects of exercise and age. J. Magn. Resonance Imaging 21(6):752–758, 2005.
Coogan, J. S., J. D. Humphrey, and C. A. Figueroa. Computational simulations of hemodynamic changes within thoracic, coronary, and cerebral arteries following early wall remodeling in response to distal aortic coarctation. Biomech. Model. Mechanobiol. 12(1):79–93, 2013.
Davies, P. F., M. Civelek, Y. Fang, and I. Fleming. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc. Res. 99(2):315–327, 2013.
Esmaily-Moghadam, M., Y. Bazilevs, T.-Y. Hsia, I. E. Vignon-Clementel, and A. L. Marsden. A comparison of outlet boundary treatments for prevention of backflow divergence with relevance to blood flow simulations. Comput. Mech. 48:277–291, 2011.
Esmaily-Moghadam, M., Y. Bazilevs, and A. L. Marsden. A new preconditioning technique for implicitly coupled multidomain simulations with applications to hemodynamics. Comput. Mech. 52:1141–1152, 2013.
Esmaily-Moghadam, M., T.-Y. Hsia, and A. L. Marsden. The Assisted Bidirectional Glenn: a novel surgical approach for first stage single ventricle heart palliation. J. Thorac. Cardiovasc. Surg. 149(3):699–705, 2015.
Esmaily-Moghadam, M., I. E. Vignon-Clementel, R. Figliola, and A. L. Marsden. A modular numerical method for implicit 0D/3D coupling in cardiovascular finite element simulations. J. Comput. Phys. 244:63–79, 2013.
Figueroa, C. A., I. E. Vignon-Clementel, K. E. Jansen, T. J.R. Hughes, and C. A. Taylor. A coupled momentum method for modeling blood flow in three-dimensional deformable arteries. Comput. Methods Appl. Mech. Eng. 195:5685–5706, 2006.
L. P. Franca and S. L. Frey. Stabilized finite element methods: II. The incompressible navier-stokes equations. Comput. Methods Appl. Mech. Eng. 99(2–3):209–233, 1992.
Hansen K. B., and S. C. Shadden. A reduced-dimensional model for near-wall transport in cardiovascular flows. Biomech. Model. Mechanobiol. 15(3):713–722, 2016.
Kim, H. J., I. E. Vignon-Clementel, J. S. Coogan, C. A. Figueroa, K. E. Jansen, and C.A. Taylor. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann. Biomed. Eng. 38(10):3195–3209, 2010.
Krams, R., J. J. Wentzel, J. A.F. Oomen, R. Vinke, J. C.H. Schuurbiers, P. J. De Feyter, P. W. Serruys, and C. J. Slager. Evaluation of endothelial shear stress and 3D geometry as factors determining the development of atherosclerosis and remodeling in human coronary arteries in vivo Combining 3D reconstruction from angiography and IVUS (ANGUS) with computational fluid dynamics. Arterioscler. Thromb. Vasc. Biol. 17(10):2061–2065, 1997.
Ku, D. N., D. P. Giddens, C. K. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation. positive correlation between plaque location and low oscillating shear stress. Arterioscler. Thromb. Vasc. Biol. 5(3):293–302, 1985.
Kung, E. O., A. Baretta, C. Baker, G. Arbia, G. Biglino, C. Corsini, S. Schievano, I. E. Vignon-Clementel, G. Dubini, G. Pennati, et al. Predictive modeling of the virtual Hemi-Fontan operation for second stage single ventricle palliation: two patient-specific cases. J. Biomech. 46(2):423–429, 2013.
Kung, E. O., A. M. Kahn, J. C. Burns, and A. L. Marsden. In vitro validation of patient-specific hemodynamic simulations in coronary aneurysms caused by Kawasaki disease. Cardiovasc. Eng. Technol. 5(2):189–201, 2014.
Kung, E. O., A. S. Les, C. A. Figueroa, F. Medina, K. Arcaute, R. B. Wicker, M. V. McConnell, and C. A. Taylor. In vitro validation of finite element analysis of blood flow in deformable models. Ann. Biomed. Eng. 39(7):1947–1960, 2011.
Kwak, B. R., M. Bäck, M.-L. Bochaton-Piallat, G. Caligiuri, M. J.A.P. Daemen, P. F. Davies, I. E. Hoefer, P. Holvoet, H. Jo, R. Krams, et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur. Heart J. 35(43):3013-3020, 2014.
Les, A. S., S. C. Shadden, C. A. Figueroa, J. M. Park, M. M. Tedesco, R. J. Herfkens, R. L. Dalman, and C. A. Taylor. Quantification of Hemodynamics in Abdominal Aortic Aneurysms During Rest and Exercise Using Magnetic Resonance Imaging and Computational Fluid DynamicsQuantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics. Ann. Biomed. Eng. 38(4):1288–1313, 2010.
Li, C., R. Huang, Z. Ding, J. C. Gatenby, D. N. Metaxas, and J. C. Gore. A level set method for image segmentation in the presence of intensity inhomogeneities with application to mri. IEEE Trans. Image Process. 20(7):2007–2016, 2011.
Lonyai, A., A. M. Dubin, J. A. Feinstein, C. A. Taylor, and S. C. Shadden. New insights into pacemaker lead-induced venous occlusion: Simulation-based investigation of alterations in venous biomechanics. Cardiovasc. Eng. 10(2):84–90, 2010.
Lorigo, L. M., O. D. Faugeras, W. E. L. Grimson, R. Keriven, R. Kikinis, A. Nabavi, and C.-F. Westin. Curves: Curve evolution for vessel segmentation. Med. Image Anal. 5(3):195–206, 2001.
Malek, A. M., S. L. Alper, and S. Izumo. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282(21):2035–2042, 1999.
Marsden, A. L., A. J. Bernstein, V. M. Reddy, S. C. Shadden, R. L. Spilker, F. P. Chan, C. A. Taylor, and J. A. Feinstein. Evaluation of a novel Y-shaped extracardiac Fontan baffle using computational fluid dynamics. J. Thorac. Cardiovasc. Surg. 137(2):394–U187, 2009.
Marsden A. L., and M. Esmaily-Moghadam. Multiscale modeling of cardiovascular flows for clinical decision support. Appl. Mech. Rev. 67(3):030804, 2015.
Marsden, A. L., V. M. Reddy, S. C. Shadden, F. P. Chan, C. A. Taylor, and J. A. Feinstein. A new multiparameter approach to computational simulation for Fontan assessment and redesign. Congenit. Heart Dis. 5(2):104–117, 2010.
Martin, M. H., J. A. Feinstein, F. P. Chan, A. L. Marsden, W. Yang, and V. M. Reddy. Technical feasibility and intermediate outcomes of a hand-crafted, area-preserving, bifurcated “Y-Graft” Fontan. Thorac. Cardiovasc. Surg. 149(1):247–255, 2015.
Merkow, J., Z. Tu, D. Kriegman, and A. L. Marsden. Structural edge detection for cardiovascular modeling. In: International Conference on Medical Image Computing and Computer-Assisted Intervention, pp. 735–742. Springer, New York, 2015.
Milner, J. S., J. A. Moore, B. K. Rutt, and D. A. Steinman. Hemodynamics of human carotid artery bifurcations: computational studies with models reconstructed from magnetic resonance imaging of normal subjects. J. Vasc. Surg. 28(1):143–156, 1998.
Moore, J. A., D. A. Steinman, D. W. Holdsworth, and C. R. Ethier. Accuracy of computational hemodynamics in complex arterial geometries reconstructed from magnetic resonance imaging. Ann. Viomedical Eng. 27(1):32–41, 1999.
Morbiducci, U., A. M. Kok, B. R. Kwak, P. H. Stone, D. A. Steinman, J. J. Wentzel, et al. Atherosclerosis at arterial bifurcations: evidence for the role of haemodynamics and geometry. Thromb. Haemost. 115(3):484–492, 2016.
Morgan, V. L., R. J. Roselli, and C. H. Lorenz. Normal three-dimensional pulmonary artery flow determined by phase contrast magnetic resonance imaging. Ann. Biomed. Eng. 26(4):557–566, 1998.
Mukherjee, D., N.D. Jani, K. Selvaganesan, C.L. Weng, and S.C. Shadden. Computational assessment of the relation between embolism source and embolus distribution to the circle of Willis for improved understanding of stroke etiology. J. Biomech. Eng. 138(8):081008-1–081008-13, 2016.
Mukherjee, D., J. Padilla, and S. C. Shadden. Numerical investigation of fluid-particle interactions for embolic stroke. Theor. Comput. Fluid Dyn. 30(1):23–39, 2016.
Nichols, W., M. O’Rourke, and C. Vlachopoulos. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. CRC Press, Boca Raton, 2011.
Oakes, J. M., A. L. Marsden, C. Grandmont, S. C. Shadden, C. Darquenne, and I. E. Vignon-Clementel. Airflow and particle deposition simulations in health and emphysema: from in vivo to in silico animal experiments. Ann. Biomed. Eng. 42(4):899–914, 2014.
Olufsen, M. S., C. S. Peskin, W. Y. Kim, E. M. Pedersen, A. Nadim, and J. Larsen. Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions. Ann. Biomed. Eng. 28(11):1281–1299, 2000.
Perktold, K., M. Hofer, G. Karner, W. Trubel, and H. Schima. Computer simulation of vascular fluid dynamics and mass transport: optimal design of arterial bypass anastomoses. Proc. ECCOMAS 98:484–489, 1998.
Ramachandra, A. B., A. M. Kahn, and A. L. Marsden. Patient-specific simulations reveal significant differences in mechanical stimuli in venous and arterial coronary grafts. J. Cardiovasc. Transl. Res. 9(4):279–290, 2016.
Roccabianca, S., C.A. Figueroa, G. Tellides, and J.D. Humphrey. Quantification of regional differences in aortic stiffness in the aging human. J. Mech. Behav. Biomed. Mater. 29:618–634, 2014.
Sahni, O., J. Müller, K. E. Jansen, M. S. Shephard, and C. A. Taylor. Efficient anisotropic adaptive discretization of the cardiovascular system. Comput. Methods Appl. Mech. Eng. 195(41–43):5634–5655, August 2006.
Sankaran, S., M. Esmaily-Moghadam, A. M. Kahn, J. Guccione, E. Tseng, and A. L. Marsden. Patient-specific multiscale modeling of blood flow for coronary artery bypass graft surgery. Ann. Biomed. Eng. 40(1):2228–2242, 2012.
Schiavazzi, D. E., G. Arbia, C. Baker, A. M. Hlavacek, T. Y. Hsia, A. L. Marsden, I. E. Vignon-Clementel, and The Modeling of Congenital Hearts Alliance (MOCHA) Investigators. Uncertainty quantification in virtual surgery hemodynamics predictions for single ventricle palliation. Int. J. Numer. Methods Biomed. Eng. 32(3), 2016.
Schiavazzi, D. E., E. O. Kung, A. L. Marsden, C. Baker, G. Pennati, T.-Y. Hsia, A. Hlavacek, A. L. Dorfman and Modeling of Congenital Hearts Alliance (MOCHA) Investigators et al. Hemodynamic effects of left pulmonary artery stenosis after superior cavopulmonary connection: a patient-specific multiscale modeling study. J. Thorac. Cardiovasc. Surg. 149(3):689–696, 2015.
Shadden S. C., and C. A. Taylor. Characterization of coherent structures in the cardiovascular system. Ann. Biomed. Eng. 36:1152–1162, 2008.
Steinman, D. A., Y. Hoi, P. Fahy, L. Morris, M. T. Walsh, N. Aristokleous, A. S. Anayiotos, Y. Papaharilaou, A. Arzani, S. C. Shadden, et al. Variability of computational fluid dynamics solutions for pressure and flow in a giant aneurysm: the ASME 2012 Summer Bioengineering Conference CFD Challenge. J. Biomech. Eng. 135(2):021016, 2013.
Suh, G. Y., A. S. Les, A. S. Tenforde, S. C. Shadden, R. L. Spilker, J. J. Yeung, C. P. Cheng, R. J. Herfkens, R. L. Dalman, and C. A. Taylor. Quantification of particle residence time in abdominal aortic aneurysms using magnetic resonance imaging and computational fluid dynamics. Ann. Biomed. Eng. 39:864–883, 2011.
Suh, G. Y., A. S. Tenforde, S. C. Shadden, R. L. Spilker, C. P. Cheng, R. J. Herfkens, R. L. Dalman, and C. A. Taylor. Hemodynamic changes in abdominal aortic aneurysms with increasing exercise intensity using MR exercise imaging and image-based computational fluid dynamics. Ann. Biomed. Eng. 39:2186–2202, 2011.
Tang, B. T., S. S. Pickard, F. P. Chan, P. S. Tsao, C. A. Taylor, and J. A. Feinstein. Wall shear stress is decreased in the pulmonary arteries of patients with pulmonary arterial hypertension: an image-based, computational fluid dynamics study. Pulm. Circ. 2(4):470–476, 2012.
Taylor, C. A., M. T. Draney, J. P. Ku, D. Parker, B. N. Steele, K. Wang, and C. K. Zarins. Predictive medicine: computational techniques in therapeutic decision-making. Comput. Aided Surg. 4(5):231–247, 1999.
Taylor, C. A., T. A. Fonte, and J. K. Min. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J. Am. Coll. Cardiol. 61(22):2233–2241, 2013.
Taylor, C. A., T. J.R. Hughes, and C. K. Zarins. Computational investigations in vascular disease. Comput. Phys. 10(3):224–232, 1996.
Taylor, C. A, T. J.R. Hughes, and C. K. Zarins. Finite element modeling of blood flow in arteries. Comput. Methods Appl. Mech. Eng. 158(1):155–196, 1998.
Tran, J. S., D. E. Schiavazzi, A. B. Ramachandra, A. M. Kahn, and A. L. Marsden. Automated tuning for parameter identification and uncertainty quantification in multi-scale coronary simulations. Comput. Fluids, 2016.
Updegrove, A., N. M. Wilson, and S. C. Shadden. Boolean and smoothing of discrete polygonal surfaces. Adv. Eng. Softw. 95:16–27, 2016.
Vignon-Clementel, I. E., C. A. Figueroa, K. E. Jansen, and C. A. Taylor. Outflow boundary conditions for three-dimensional finite element modeling of blood flow and pressure in arteries. Comput. Methods Appl. Mech. Eng. 195(29–32):3776–3796, 2006.
Wang K. C.Y. Level set methods for computational prototyping with application to hemodynamic modeling. PhD thesis, Stanford University, 2001.
Wentzel, J. J., E. Janssen, J. Vos, J. C.H. Schuurbiers, R. Krams, P. W. Serruys, P. J. de Feyter, and C. J. Slager. Extension of increased atherosclerotic wall thickness into high shear stress regions is associated with loss of compensatory remodeling. Circulation 108(1):17–23, 2003.
Whiting C. H., and K. E. Jansen. A stabilized finite element method for the incompressible Navier-Stokes equations using a hierarchical basis. Int. J. Numer. Methods Fluids 35(1):93–116, 2001.
Wilson, N. M., F. R. Arko, and C. A. Taylor. Predicting changes in blood flow in patient-specific operative plans for treating aortoiliac occlusive disease. Comput. Aided Surg. 10(4):257–277, 2005.
Yang, W., F. P. Chan, V. M. Reddy, A. L. Marsden, and J. A. Feinstein. Flow simulations and validation for the first cohort of patients undergoing the Y-graft Fontan procedure. J. Thorac. Cardiovascu. Surg. 149(1):247–255, 2015.
Zhou, M., O. Sahni, H. J. Kim, C. A. Figueroa, C. A. Taylor, M. S. Shephard, and K. E. Jansen. Cardiovascular flow simulation at extreme scale. Comput. Mech., 46(1):71–82, 2010.
Acknowledgments
This work was supported by the National Science Foundation SI2 program (Award No. 1407834 and 1562450) and in part by the NIH (Contract HHSN268201100035C).
Conflicts of Interest
The authors do not have conflicts of interest relevant to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ender A. Finol.
Rights and permissions
About this article
Cite this article
Updegrove, A., Wilson, N.M., Merkow, J. et al. SimVascular: An Open Source Pipeline for Cardiovascular Simulation. Ann Biomed Eng 45, 525–541 (2017). https://doi.org/10.1007/s10439-016-1762-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1762-8