Abstract
We investigated the landscape epidemiology of a globally distributed mammal, the wild pig (Sus scrofa), in Florida (U.S.), where it is considered an invasive species and reservoir to pathogens that impact the health of people, domestic animals, and wildlife. Specifically, we tested the hypothesis that two commonly cited factors in disease transmission, connectivity among populations and abundant resources, would increase the likelihood of exposure to both pseudorabies virus (PrV) and Brucella spp. (bacterial agent of brucellosis) in wild pigs across the Kissimmee Valley of Florida. Using DNA from 348 wild pigs and sera from 320 individuals at 24 sites, we employed population genetic techniques to infer individual dispersal, and an Akaike information criterion framework to compare candidate logistic regression models that incorporated both dispersal and land cover composition. Our findings suggested that recent dispersal conferred higher odds of exposure to PrV, but not Brucella spp., among wild pigs throughout the Kissimmee Valley region. Odds of exposure also increased in association with agriculture and open canopy pine, prairie, and scrub habitats, likely because of highly localized resources within those land cover types. Because the effect of open canopy on PrV exposure reversed when agricultural cover was available, we suggest that small-scale resource distribution may be more important than overall resource abundance. Our results underscore the importance of studying and managing disease dynamics through multiple processes and spatial scales, particularly for non-native pathogens that threaten wildlife conservation, economy, and public health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Host ecology is a major contributing factor to the patterns of pathogen emergence across a landscape. The movement of individual animals, particularly dispersal or migration of infected hosts, drives the spread of directly transmitted diseases by introducing a pathogen to naïve populations (Russell et al. 2004; Hosseini et al. 2006; Macdonald and Laurenson 2006; Altizer et al. 2011). As infected hosts disperse across the landscape, they affect the rate of pathogen spread, spatial distribution of infection, and the likelihood of new exposures (Cullingham et al. 2008). Landscape composition influences a host’s exposure to pathogens either by facilitating or hindering contact between individuals or groups (Blanchong et al. 2008; Rees et al. 2008; Root et al. 2009; Barton et al. 2010; Tardy et al. 2018). For example, high rates of dispersal and contact among individual white-tailed deer (Odocoileus virginianus) have been suggested as a potential mechanism for long-distance spread of chronic wasting disease (CWD), a prion disease of cervids (Kelly et al. 2010; Cullingham et al. 2011), and of bovine tuberculosis (bTB) among sympatric elk (Cervus canadensis) subpopulations (Vander Wal et al. 2013).
Because patterns of host dispersal influence that of pathogens, the identification of environmental variables that influence host movement also contribute to spatial distribution and occurrence of disease agents, vectors, and reservoirs (Ostfeld and LoGiudice 2003; Collinge et al. 2005; Storm et al. 2013; McAlpine et al. 2017), and determine pathogen exposure (Langlois et al. 2001; Riley 2007; Cullingham et al. 2008; Biek and Real 2010; Meentemeyer et al. 2012). Identifying land cover features correlated with the distribution of invasive species carrying non-native pathogens may therefore facilitate protection of both biodiversity and human health via habitat and land use management.
Wild pigs (Sus scrofa) are one of the most widely distributed mammals in the world and are considered invasive species on multiple continents, including North America. In the USA, a recent and rapid range expansion has led to the establishment of free-ranging populations in as many as 44 states (Barrios-Garcia and Ballari 2012; Bevins et al. 2014). The rapid spread of wild pigs has been related to both intrinsic (e.g., ability to adapt to diverse habitat types) and extrinsic causes (e.g., human-mediated movement) throughout the whole country (Seward et al. 2004; Bevins et al. 2014). Regionally, a long and continuous history of anthropogenic movement has become the principal source of wild pig introductions and dispersal in places like Florida, which is evident in the high intermixing of wild pigs from different genetic backgrounds (Hernández et al. 2018). However, despite their broad geographic distribution and adaptability, wild pigs have physiological and resource limitations that may influence individual movement patterns and fine-scale distribution (McClure et al. 2015; Snow et al. 2017). Wild pigs depend on habitats with suitable natural or artificial forage resources, and water and cover to thermoregulate during periods of high temperatures (Choquenot and Ruscoe 2003; Mayer and Brisbin Jr 2009). Specifically, hardwood forests provide hard mast (Geisser and Reyer 2005) and shade cover (Choquenot and Ruscoe 2003), and wetland-riparian systems provide important wallowing, cooling, and feeding opportunities (Gaston et al. 2008). Cropland and pastures also concentrate high densities of wild pigs due to their attraction to abundant artificial food resources (Schley and Roper 2003; Herrero et al. 2006). By contrast, open canopy habitats such as Gulf Coast pine forests, wet, and dry prairies, Florida scrubland, and human settlement areas are less frequently utilized by the species, which is likely due to limited cover and resource availability (Mayer and Brisbin Jr 2009; Saito et al. 2012; Keiter and Beasley 2017). Previous studies have explored how the combination of large-scale patterns of dispersal and local patterns of land cover composition affects disease exposure rates on wild pigs (e.g., Cowled et al. 2012; Pearson et al. 2014), contributing to address the growing concern surrounding the spread of pathogens by the species, some of which can severely impact public health, domestic animals, and wildlife (Seward et al. 2004; Meng et al. 2009).
Two infectious pathogens harbored by wild pig populations throughout their global distribution are pseudorabies virus (PrV or Aujeszky’s disease—caused by Suid alphaherpesvirus) and Brucella spp. (bacterial agent of brucellosis). Both pathogens are directly transmitted by exposure to oro-nasal fluids or sexual contact, and exposure typically leads to lifelong infection accompanied by neutralizing antibodies in wild pigs (Müller et al. 2011; Leiser et al. 2013). Although mortality is rarely associated with either PrV or Brucella spp. in adult wild pigs (Müller et al. 2011; Leiser et al. 2013), both pathogens can be lethal to other non-suid species. For example, PrV is lethal to mammalian carnivores and is an emerging health threat to the endangered Florida panther (Puma concolor coryi), which preys on wild pigs (Glass et al. 1994). Brucella spp. is also a major zoonotic pathogen globally, and Brucella suis is one of the most prevalent zoonotic pathogens affecting Floridians (Florida Department of Health 2017). The pathogen produces serious, lifelong health complications in humans if untreated (Franco et al. 2007). Commercial livestock in the USA are considered free of PrV and Brucella spp., yet both pathogens are widespread in free-living wild pig populations throughout the country, which increases the risk of reintroduction of the diseases into commercial herds (Pedersen et al. 2012, 2013). Although both PrV and Brucella spp. can severely impact wildlife conservation, public health and the livestock industry, little is known about how host-dependent and environmental factors could predict the risk of pathogen exposure in wild pigs. In this regard, further studies are warranted to inform efficient management and control decisions on the spread of wild pigs and diseases at the landscape level.
This study tested two main hypotheses concerning the effect of movement and land cover composition on PrV and Brucella spp. exposure among wild pigs across the Kissimmee Valley of Florida (USA). First, because wild pig migration may enhance contact rates between pathogen-exposed and susceptible individuals, we hypothesized that recent dispersal would be predictive of a higher likelihood of PrV and Brucella spp. exposure in individual wild pigs. Second, because habitats with high resource availability, like hardwood forests, freshwater wetlands and agriculture, would theoretically support a higher occurrence and density of wild pigs, we hypothesized that animals would exhibit a higher likelihood of pathogen exposure in high resource habitats than in limited resource habitats.
Methods
Sample Collection
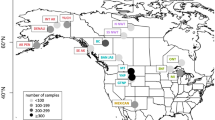
Between January 2014 and March 2016, we collected blood and/or hair samples from 348 wild pigs at 24 sites across the Kissimmee Valley of Florida (U.S.) (Fig. 1). We sampled animals opportunistically as part of a national wild pig disease monitoring effort led by the United States Department of Agriculture (USDA), Animal Plant and Health Inspection Service, Wildlife Services, National Wildlife Disease Program. Sampled pigs were either trapped and euthanized during animal control efforts conducted by USDA, or legally harvested by hunters at hunter check stations on federal and state wildlife management areas, military bases, and private properties. We recorded demographic data for each animal, which included sex, age, and sampling location. Specifically, we used body size, reproductive traits, and tooth eruption patterns (Matschke, 1967) to classify animals as adults (≥ 1 year), sub-adults (2 months–1 year), or juveniles (< 2 months). For PrV and Brucella spp. serological analyses, we collected up to 35 ml blood from 320 of the 348 wild pigs using 9 ml Covidien® serum separator tubes (Covidien AG, Dublin, Ireland). Samples were immediately refrigerated at 4 °C and centrifuged within 12 h of collection. Serum from each wild pig was aliquoted into 2 ml Corning® cryovials (Corning Incorporated, Lowell, Massachusetts, USA) and refrigerated for up to 4 days prior to shipment on ice packs to a designated National Animal Health Laboratory Network facility (see serological analyses subsection). For population genetic analyses, we collected an additional 0.5 ml whole blood from 301 of the 348 animals by cardiac puncture or orbital draw. The sample was stored immediately in 1 ml mammalian lysis buffer (Qiagen, Valencia, CA, USA) on ice packs prior to refrigeration at 4 °C. Due to logistic constraints, we collected additional whole blood from only a subset of the individuals. From the remaining 47 animals, we collected hair, which was stored in paper envelopes in the field. Both whole blood and hair samples were transported to the University of Florida and stored at − 80 °C until DNA could be extracted. The University of Florida’s Institutional Animal Care and Use Committee approved the protocol for this study.
Serological Analyses
Serological tests indicated the presence or absence of host antibodies to a pathogen. Because both PrV and Brucella spp. induce a lifelong infection and antibody production, a positive antibody test indicated that an animal was either previously exposed and infected but not currently infected, or exposed and infected. Seroprevalence data for PrV and Brucella spp. have previously been used to determine disease prevalence and risk of transmission in wildlife populations (Cross et al. 2007; Pannwitz et al. 2012). We included serological data for wild pigs if the sample was unequivocally determined to be either seropositive or seronegative for PrV and/or Brucella spp., and the sex and age of the animal were known. Serological tests were performed at the Kentucky Federal Brucellosis Laboratory (KY-FBL). Sera were screened for PrV using the PrV-gB enzyme-linked immunosorbent assay per the manufacturer’s recommendations (ELISA; Idexx Laboratories, Westbrook, Maine, USA). Samples with S/N ratios ≤ 0.6 were determined as PrV-seropositive, while samples with values > 0.7 were considered as PrV-seronegative. Sera were screened for Brucella spp. using the fluorescence polarization assay (FPA), as described by Nielsen et al. (1999). Samples with a result of 20 millipolarization units or above were determined as Brucella spp.-seropositive, while samples with values < 20 were considered as Brucella spp.-seronegative.
DNA Isolation and Microsatellite Genotyping
We extracted DNA from blood or hair using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA) or the QIAamp DNA Micro Kit (Qiagen, Valencia, CA, USA), respectively. For both procedures, we followed the manufacturer’s protocol, with slight modifications reported previously (see Hernández et al. 2018). We stored isolated DNA at − 20 °C. Sixty-one microsatellite markers were initially selected for multilocus genotyping and have been previously described (Ellegren et al. 1993; Robic et al. 1994; Alexander et al. 1996; Rohrer et al. 1996; Hernández et al. 2018). Ultimately, 52 markers were multiplexed for PCR (Hernández et al. 2018). We analyzed PCR products by capillary electrophoresis on an ABI 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) and scored using GeneMarker version 2.6.2 (SoftGenetics, State College, PA, USA) at the University of Florida. Validation of genotypes, comparison of genotypes from different biosamples, and calculation of genotyping error have been previously reported (Hernández et al. 2018).
Population Genetic and Dispersal Analyses
To quantify the level of genetic differentiation (i.e., genetic distance) across wild pig sampling locations, we calculated the overall FST across all genotypes and loci, and their statistical significance tested by 999 permutations using the G-statistic Monte Carlo test implemented in the R package hierfstat 0.04-26 (Goudet 2005). We also calculated pairwise FST values between sampling locations (Weir and Cockerham 1984) and their statistical significance determined by 999 permutation using GenAlEx version 6.5 (Peakall and Smouse 2012).
We estimated population-wide dispersal among locations in the Kissimmee River Valley by estimating the mean posterior proportion of individuals that migrated between each pair of locations. We calculated 95% credible intervals (CI) for pairwise migration estimates between sampling locations, considering credible intervals that did not include zero to be statistically significant.
We identified the individuals within those locations that were either first- or second-generation immigrants, measured as individual ancestry. This parameter estimated the probability that an individual originated from a different location (first-generation immigrant) or was an F1 descendant of the immigrant and a local animal (second-generation immigrant). To estimate the probabilities, we ran 100,000,000 Markov chain Monte Carlo iterations of a model that characterized changes in gene frequencies across populations due to migration. We used a 10,000,000-step burn-in period and a sampling interval of 500 steps. We tested multiple delta values for the mixing parameters of migration, allele frequencies and inbreeding values, where delta values were defined as the maximum amount a parameter could be changed between each iteration. Delta values set to 1 resulted in optimal acceptance rates for changes to each mixing parameter (between 20 and 60%). We conducted multiple runs initialized with dispersed starting values and compared the posterior mean parameter estimates for convergence. Migration rates and individual probability of being an immigrant were estimated using BAYESASS version 3.0 (Wilson and Rannala 2003).
Land Cover Categorization
Land cover data were categorized into six cover types: (1) closed canopy hardwood forest; (2) open canopy pine, prairie, and scrub (hereafter referred to as “open canopy”); (3) freshwater wetland; (4) lake and river; (5) agriculture; and (6) anthropogenic cover (Table 1). We created these broad-scale groups using the Cooperative Land Cover v3.2 Raster layer (10 m2 resolution derived from aerial photography, ground-truthing, and local knowledge; FWC and FNAI 2016) pursuant to the classification schemes described by Anderson et al. (1976), Knight et al. (2010) and Kawula (2014). We estimated the available land cover at each sampling site within a uniform spatial buffer with a radius of 5.75 km (area = 103.9 km2). This area encompassed the average home range size of wild pigs in the Gulf Coast forest habitat of the southeastern United States (mean = 4.8 km2, Garza et al. 2018) and thus represented the heterogeneity of the biophysical environments wild pigs might encounter within its home range. As such, this area represented a conservative proxy of the landscape scale where pathogens may interact with hosts across the studied ecological system (Meentemeyer et al. 2012). For sites where the exact location of wild pigs at time of death was unknown (i.e., where sampling occurred at hunter check stations), we placed the buffer around the geographic center of the managed area per the boundaries provided by the Florida Conservation Lands (FLMA) shapefile (FNAI 2016). For sites with exact geographic data for samples (i.e., where sampling occurred at the location of euthanasia), we overlaid the buffer around the geographic center of the site’s cluster of sampling points. The buffered area was then assessed for the proportion of the six land cover types, and the same measures of proportional land cover were assigned to all individuals within a given site. All spatial data collection was performed in ArcMap 10.4.1 (ESRI 2016).
Predictors of PrV and Brucella spp. Exposure in Wild Pigs
Within an Akaike information criterion (AIC) framework for model comparison (Burnham and Anderson 2002), we used logistic mixed effect regression models to assess the effect of the probability of recent (first or second-generation) individual migration, the proportion of each land cover type, age class (juvenile, sub-adult, or adult), and sex (male or female) on the odds of PrV and Brucella spp. exposure (seropositive = 1, seronegative = 0). We also included two-way interactions of agriculture with the other five land cover types to assess the influence of agricultural expansion on the relationship between PrV and Brucella spp. exposure and non-agricultural land cover. Prior to their inclusion in the models, predictor variables were tested for collinearity using Pearson correlation coefficients, and we found no terms exceeding the 0.7 threshold (Booth et al. 1994). To account for heterogeneity of PrV and Brucella spp. exposure across sampling sites, we included a random site-specific intercept in all models. Exploratory analyses indicated that including the random site effect significantly (p < 0.001) improved the overall model fit over the fixed-effects model (which included variables of migration, land cover, age class, sex and two-way interactions between land cover types). However, because the land cover types were spatially varying along with the site-specific random effect, the estimates for the regression coefficients of the fixed effects were confounded with the random intercepts. To alleviate the confounding of land cover with the random effects, we projected the random effects into the null space of the land cover variables so that the site-specific intercepts only accounted for variation not already explained by land cover (Reich et al. 2006). We fit all models and calculated regression coefficients and 95% confidence intervals (CIs) using the R package mgcv v1.8-16 (Wood 2006) and performed a likelihood ratio test as a measure of goodness of fit using the R package lmtest v0.9-36 (Zeileis and Hothorn 2002). Odds ratios for all variables were calculated by exponentiating the logistic regression coefficients, and statistical significance was determined as a 95% CI that did not include one.
Results
Population Genetic and Dispersal Analyses
The overall FST = 0.09 was statistically significant across all genotypes and loci (G-statistic = 26,334.4, p < 0.05). All pairwise FST values estimated between sampling locations were significantly different from zero (p < 0.05), which indicated genetic differentiation among sampling locations. FST values ranged from 0.020 (between locations 9 and 17) to 0.165 (between locations 1 and 2). Ten of 24 sampling sites showed moderate levels of genetic differentiation (all FST values > 0.05) compared to the rest of sampling sites (see Online Resource 1).
Analysis of dispersal patterns via estimation of migration rates revealed low and statistically insignificant migration among most sampling locations. However, we found significant mean posterior proportion of individuals that migrated between one site (location 17) and 15 other adjacent sampling sites throughout the Kissimmee Valley (ranging from 4 to 14% migrants between sites) (see Online Resource 2). For locations that had significant migration rates between them and location 17, we identified 130 of 156 wild pigs that exhibited a probability > 0.9 to be either first or second-generation immigrant from a source location different than the sampling location.
Predictors of PrV and Brucella spp. Exposure
Total sample sizes of wild pigs after omissions were 297 (25 juveniles, 24 sub-adults, 248 adults) for PrV exposure, and 291 (24 juveniles, 22 sub-adults, 245 adults) for Brucella spp. We observed roughly equivalent sex ratios within both sample populations (PrV: 148 males, 149 females; Brucella spp.: 146 males, 145 females). PrV-seropositive animals were detected at 21 of 23 sites (91.3%), and Brucella spp.-seropositive individuals were found at 14 of 23 sites (60.9%). Within each sampled population, 166 (55.9%; CI 50.0–61.6%) exhibited PrV-specific antibodies in their serum, and 35 (12.0%; CI 8.5–16.3%) exhibited Brucella spp.-specific antibodies.
We summarized the PrV and Brucella spp. seroprevalences, and the probability of recent migration and proportion of each land cover type as predictors of pathogen exposure across sampling locations (see Online Resource 3). The best-ranked AIC model predicting PrV exposure included the probability of recent migration, age class, open canopy, agriculture, and the interaction between agriculture and open canopy (Table 2). Odds of PrV exposure were over three times higher (odds ratio [OR] = 3.25) for recent migrant than for non-migrant wild pigs, and almost four times higher (OR = 3.56) for adults than for juveniles (Table 3). Odds of PrV exposure were also over 100 times higher for wild pigs on lands dominated by open canopy (OR = 214.86) and agriculture (OR = 170.72) than wild pigs in areas without these land cover types. Though both open canopy and agriculture had positive main effects on PrV exposure, the effect of open canopy on PrV exposure became increasingly negative as agricultural cover increased (Fig. 2). Both null and fixed-effects-only models exhibited ΔAIC > 2, and likelihood ratio tests confirmed that the best-ranked AIC model fit the PrV data significantly better than the null model (χ2 = 89.50, df = 18.10, p < 0.001) and the fixed-effects-only model (χ2 = 28.05, df = 3.10, p < 0.001). None of the remaining variables (sex, hardwood forest, freshwater wetland, lake and river, or anthropogenic land covers) were significantly related to changes in PrV exposure across all the candidate logistic regression models.
Main effects of (a) open canopy cover (pine, prairie, and scrub) and (b) agricultural cover (crop and pasture) proportions, and (c) two-way interaction between open canopy with agricultural as predictors of the probability of pseudorabies virus (PrV) exposure in wild pigs. In Figure 2c, X-axis and top legend depict the range of open canopy and agricultural cover proportions, respectively, estimated across 23 collection sites of the Kissimmee Valley of Florida.
The best-ranked AIC model predicting Brucella spp. exposure only included the probability that an individual was a first- or second-generation immigrant (Table 2). The odds of Brucella spp. exposure (odds ratio [OR] = 2.23) did not increase significantly for wild pigs that were recent immigrants (Table 3), but the 95% CIOR marginally included one. None of the remaining variables (any land cover class, age class, or sex) was a significant predictor of Brucella spp. exposure.
Discussion
Our findings suggest that wild pig dispersal and landscape composition influence pathogen exposure for PrV among wild pigs throughout the Kissimmee Valley of Florida. First- and second-generation immigrants contributed to increased PrV seroprevalence in wild pig populations, likely due to increased contact among infectious and susceptible individuals. Similarly, agriculture-dominated areas, which attract high densities of wild pigs due to the availability of abundant artificial food resources, had higher PrV seroprevalence than other land cover types. Contrary to expectations, the odds of PrV exposure were also higher at sites dominated by the resource-limited open canopy habitat. The mechanism driving these two apparently contradictory relationships may be the patchiness of resources within both land cover types. Indeed, the effect of open canopy on PrV exposure reversed when agricultural cover was available, suggesting that local distribution of resources may play a role in pathogen transmission. These results underscore the necessity for large-scale sampling both within and among populations to elucidate landscape-level disease dynamics and interactions among driving factors.
The role of movement in PrV exposure observed here corroborates previous observations that dispersal (both natural and anthropogenic) increases contact rates between pathogen-exposed and susceptible individuals, which contributes to disease spread at the population level (e.g., Zanardi et al. 2003; Hampton et al. 2006; Keuling et al. 2008; Pearson et al. 2014; Franckowiak and Poché 2018). In Florida, wild pig dispersal has been strongly influenced by successive events of human-assisted movement that have contributed to the geographical expansion of wild pigs throughout the Kissimmee Valley and adjacent regions (Hernández et al. 2018). Escapes from holding facilities, legal and illegal transport and release, and hunting pressures have contributed to the movement of wild pigs into areas that have less human disturbance or are unoccupied by wild pig social groups (Zanardi et al. 2003; Keuling et al. 2008). In the present study, much of the pattern of movement was driven by movement of animals into and out of location 17, which was within close proximity to a private hunting club known to transport animals into and out of the property. This anthropogenically induced movement likely resulted in the high levels of admixture and production of F1/F2 individuals from the mating between animals from location 17 and other source populations (see Hernández et al. 2018 for details), potentially affecting the opportunity for contact between naïve and infectious individuals, as suggested by previous studies (e.g., Zanardi et al. 2003; Cowled and Garner 2008). Because the movement of individual wild pigs transcended property boundaries and was facilitated by human-assisted movement, land managers wishing to control the spread of PrV among wild pigs, and from wild pigs to livestock and native wildlife, may benefit from cooperative efforts among public agencies and private landowners as well as from enforcement of animal movement laws in the state.
In addition to an animal’s dispersal history, the increase in PrV exposure was also associated with an increase in the proportion of both agricultural and open canopy cover within an animal’s home range. This finding suggests that small-scale spatial and temporal distribution of resources may be more important to the spread of directly transmitted diseases than overall resource abundance. Agricultural lands offer reliable access to diverse crop types (Genov 1981; Herrero et al. 2006), artificial water sources (Carrasco-Garcia et al. 2015; Payne et al. 2015) and supplemental feeding areas (Cross et al. 2007; Campbell et al. 2013). The availability of these resources not only drives overall wild pig densities up to four times higher in cropland and pasture than in surrounding habitat types (Caley 1993; Kay et al. 2017), but also attracts higher animal concentrations around the individual sources of food and water, creating local conditions for heightened exposure to directly transmitted pathogens. In contrast, water, thermal refugia, and highly preferred food resources are limited in open canopy (i.e., pine, prairie, and scrub) habitat, relative to hardwood forest and freshwater wetland habitats (Mayer and Brisbin Jr 2009; Saito et al. 2012; Keiter and Beasley 2017). However, the temporal patchiness of resources, either as food (Kurz and Marchinton 1972; Hughes 1985) or refugia from hunting (Gaston et al. 2008; Franckowiak and Poché 2018), within open canopy habitat may mimic the supplemental resources provided by agricultural areas in elevating local concentrations of wild pigs around discrete resources. This apparent role of small-scale distribution of resources in modulating disease transmission is bolstered by our finding that increasing agricultural cover reversed the effect of open canopy on PrV exposure. The local presence of artificial food and water sources may have allowed wild pigs to commute to agricultural fields from less attractive and sparse nutritional conditions in open canopy habitat, as demonstrated by previous studies (Gerard et al. 1991; Schley and Roper 2003; Herrero et al. 2006; Keuling et al. 2009). Consequently, contact and therefore pathogen exposure may be reduced among animals within the adjacent open canopy. Combined with the effects of wild pig dispersal, these results suggest that the processes driving pathogen exposure operate at a variety of spatial and temporal scales, reiterating the need for collaborative management efforts in controlling the spread of this non-native pathogen to species of conservation concern.
We detected no influence of land cover composition on Brucella spp.; while we found that wild pig dispersal weakly related to Brucella spp. exposure, we may be tempted to interpret recent migration as contributing to the persistence of this bacterial agent among wild pig populations. However, the relatively low number of wild pigs exposed to Brucella spp. (12%; 35/291) likely impeded our power to detect any significant predictor variables from the models. There are several known limitations of existing serological diagnostic tests for Brucella spp. (e.g., limited sensitivity and specificity and cross-reactivity with other pathogens) that tend to underestimate the proportion of wild pigs exposed to the bacteria (Pedersen et al. 2014, 2017). Larger sample sizes using improved serological tests would facilitate our understanding of the roles of animal movement and land cover for this pathogen.
While we suggest that host dispersal and resource-driven contacts predict the likelihood of PrV exposure among wild pigs, our study has methodological caveats that warrant a more cautious interpretation of results. First, considering that population density modulates the infection dynamics of several pathogens due to its impact on contact rates (Penrith et al. 2011; Pearson et al. 2016), variability in host population density may act as a potential confounding variable that influences PrV transmission between infected and susceptible individuals (e.g., Cowled et al. 2012). Unfortunately, lack of wild pig density data prevented us to include an independent measure of host density as potential predictor of pathogen exposure in our statistical models (i.e., there are no systematic records of hunting bag numbers across hunter check stations or public/private properties in the state of Florida). Second, the extreme odds of PrV exposure related to landscape composition were likely caused by the imbalanced number of animals opportunistically sampled across sites (range: 3–45 wild pigs per site after omissions), which may limit our inferences about the contribution of land cover on the risk of PrV spread among wild pigs.
Because of the risk of pathogen spill-over to other species, the findings presented here have direct implications to carnivore conservation. Movement of wild pigs infected with directly transmitted or water-borne pathogens has the potential to increase the risk of infection for other sympatric species (Hampton et al. 2006; Franckowiak and Poché 2018), and PrV is highly lethal to mammalian carnivores (Stallknecht and Howerth 2008). The results of this study suggest that patterns of wild pig dispersal and land cover composition may be used to predict habitats that present a high risk for cross-species transmission of PrV to endangered carnivores such as the Florida panther. Panthers are highly susceptible to this pig-borne pathogen (Glass et al. 1994), yet pigs represent the largest component of the Florida panther’s diet (Maehr et al. 1990). More globally, our approach could be extrapolated to understand which habitats within sympatric distributions of wild pigs and susceptible carnivores have a high risk of transmission and used to guide habitat management for endangered carnivores, such as the Iberian lynx (Lynx lynx,Masot et al. 2016) and European wolf (Canis lupus, Verpoest et al. 2014). Future studies may also test alternative hypotheses, such as the role of stress and its immunosuppressive effects as potential stimuli for increased disease transmission among wild pigs (Allwin et al.2015, 2016) and from wild pigs to endangered carnivore species. Finally, future landscape epidemiology studies should embrace multiscale data collection and analytical methods to better understand how host and landscape ecology influence the spread and persistence of pathogens across heterogeneous landscapes (Meentemeyer et al. 2012).
References
Alexander LJ, Troyerm DL, Rohrer GA, Smith TPL, Schook LB, Beattie CW (1996) Physical assignments of 68 porcine cosmid and lambda clones containing polymorphic microsatellites. Mammalian Genome 7:368-372
Allwin B, Jayathangaraj MG, Palanivelrajan M, Raman M (2015) Determination of endogenous faecal glucocorticoid metabolites to evaluate stress response in wild pigs interfering with agriculture adjoining forest regions in correlation with conflict and meteorological factors - a non invasive approach. Poultry Fish and Wildlife Science 3:127; https://doi.org/10.4172/2375-446x.1000127.
Allwin B, Gokarn NS, Vedamanickam S, Gopal S, Pandian SSA (2016) Occurrences of human wild pig conflict in Tamil Nadu - India. Journal of Ecosystem and Ecography 6:200; https://doi.org/10.4172/2157-7625.1000200
Altizer S, Bartel R, Han BA (2011) Animal migration and infectious disease risk. Science 331:296-302; https://doi.org/10.1126/science.1194694
Anderson JR, Hardy EE, Roach JT, Witmer RE (1976) A land use and land cover classification system for use with remote sensor data. Geological Survey Professional Paper 964. Washington, DC: United States Government Printing Office.
Barrios-Garcia MN, Ballari SA (2012) Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biological Invasions 14:2283-2300; https://doi.org/10.1007/s10530-012-0229-6
Barton HD, Gregory AJ, Davis R, Hanlon CA, and Wisely SM (2010) Contrasting landscape epidemiology of two sympatric rabies virus strains. Molecular Ecology 19:2725-2738; https://doi.org/10.1111/j.1365-294x.2010.04668.x.
Bevins SN, Pedersen K, Lutman MW (2014) Consequences associated with the recent range expansion of nonnative wild pig. Bioscience 64:291-299; https://doi.org/10.1093/biosci/biu015
Biek R, Real LA (2010) The landscape genetics of infectious disease emergence and spread. Molecular Ecology 19:3515-3531; https://doi.org/10.1111/j.1365-294x.2010.04679.x
Blanchong JA, Samuel MD, Scribner KT, Weckworth BV, Langenberg JA, Filcek KB (2008) Landscape genetics and the spatial distribution of chronic wasting disease. Biological Letters 4:130-133; https://doi.org/10.1098/rsbl.2007.0523
Booth GD, Niccolucci MJ, Schuster EG (1994) Identifying proxy sets in multiple linear regression: an aid to better coefficient interpretation. Research paper INT-470. Ogden, Utah: United States Department of Agriculture, Forest Service, pp 9
Burnham KP and Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer-Verlag, pp 485
Caley P (1993) Population-dynamics of feral pigs (Sus scrofa) in a tropical riverine habitat complex. Wildlife Research 20:625-636
Campbell TA, Long DB, and Shriner SA (2013) Wildlife contact rates at artificial feeding sites in Texas. Environmental Management 51:1187-1193; https://doi.org/10.1007/s00267-013-0046-4
Carrasco-Garcia R, Barasona JA, Gortazar C, Montoro V, Sanchez-Vizcaino JM, Vicente J (2015) Wildlife and livestock use of extensive farm resources in South Central Spain: implications for disease transmission. European Journal of Wildlife Research 62:65-78; https://doi.org/10.1007/s10344-015-0974-9
Choquenot D, Ruscoe WA (2003) Landscape complementation and food limitation of large herbivores: habitat-related constraints on the foraging efficiency of wild pigs. Journal of Animal Ecology 72:14-26; https://doi.org/10.1046/j.1365-2656.2003.00676.x
Collinge SK, Johnson WC, Ray C, Matchett R, Grensten J, Cully Jr JF, Gage KL, Kosoy MY, Loye JE, Martin AP (2005) Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the western USA. Landscape Ecology 20:941-955 https://doi.org/10.1007/s10980-005-4617-5
Cowled B, Garner G (2008) A review of geospatial and ecological factors affecting disease spread in wild pigs: considerations for models of foot-and-mouth disease spread. Journal of Preventive Veterinary Medicine 87:197-212; https://doi.org/10.1016/j.prevetmed.2008.03.012
Cowled BD, Ward MP, Laffan SW, Galea F, Garner MG, MacDonald AJ, Marsh I, Muellner P, Negus K, Quasim S, Woolnough AP, Sarre SD (2012) Integrating survey and molecular approaches to better understand wildlife disease ecology. PLoS ONE 7:e46310; https://doi.org/10.1371/journal.pone.0046310
Cross PC, Edwards WH, Scurlock BM, Maichak EJ, Rogerson JD (2007) Effects of management and climate on elk brucellosis in the Greater Yellowstone Ecosystem. Ecological Applications 17:957-964; https://doi.org/10.1890/06-1603
Cullingham CI, Pond BA, Kyle CJ, Rees EE, Rosatte RC, White BN (2008) Combining direct and indirect genetic methods to estimate dispersal for informing wildlife disease management decisions. Molecular Ecology 17:4874-4886; https://doi.org/10.1111/j.1365-294x.2008.03956.x
Cullingham CI, Merrill EH, Pybus MJ, Bollinger TK, Wilson GA, Coltman DW (2011) Broad and fine-scale genetic analysis of white-tailed deer populations: estimating the relative risk of chronic wasting disease spread. Evolutionary Applications 4:116-131; https://doi.org/10.1111/j.1752-4571.2010.00142.x
Ellegren H, Johansson M, Chowdhary BP, Marklund S, Ruyter D, Marklund L, Bräuner-Nielsen P, Edfors-Lilja I, Gustavsson I, Juneja RK, Andersson L (1993) Assignment of 20 microsatellite markers to the porcine linkage map. Genomics 16:431-439; https://doi.org/10.1006/geno.1993.1207
ESRI (Environmental Systems Resource Institute) (2016) ArcMap 10.4.1. ESRI, Redlands, California.
Florida Department of Health (2017) 2017 Florida Morbidity Statistics Report. Florida Department of Health, Tallahassee, Florida. pp 215
Florida Natural Areas Inventory (2016) Florida Conservation Lands. https://www.fnai.org/gisdata.cfm. Accessed 12 October 2020.
Franckowiak GA, Poché RM (2018) Short-term home range and habitat selection by feral hogs in northern Texas. The American Midland Naturalist Journal 179:28-37; https://doi.org/10.1674/0003-0031-179.1.28
Franco MP, Mulder M, Gilman RH, Smits HL (2007) Human brucellosis. The Lancet Infectious Diseases 7:775-786; https://doi.org/10.1016/s1473-3099(07)70286-4
Garza SJ, Tabak MA, Miller RS, Farnsworth ML, Burdett CL (2018) Abiotic and biotic influences on home-range size of wild pigs (Sus scrofa). Journal of Mammalogy, 99:97-107.
Gaston W, Armstrong JB, Arjo W, Stribling HL (2008) Home range and habitat use of feral hogs (Sus scrofa) on Lowndes County WMA, Alabama. National Conference on Feral Hogs. St Louis, Missouri: Internet Center for Wildlife Damage Management. pp 6
Geisser H, Reyer HU (2005) The influence of food and temperature on population density of wild boar Sus scrofa in the Thurgau (Switzerland). Journal of Zoology 267:89-96; https://doi.org/10.1017/s095283690500734x
Genov P (1981) Food composition of wild boar in northeastern and western Poland. Acta Theriologica 26:185-205
Gerard JF, Cargnelutti B, Spitz F, Valet G, Sardin T (1991) Habitat use of wild boar in a French agroecosystem from late winter to early summer. Acta Theriologica 36:119-129
Glass CM, McLean RG, Katz JB, Maehr DS, Cropp CB, Kirk LJ, McKeiman AJ, Evermann JF (1994) Isolation of pseudorabies (Aujeszky’s disease) virus from a Florida panther. Journal of Wildlife Diseases 30:180-184
Goudet J (2005) Hierfstat, a package for R to compute and test variance components and F-statistics. Molecular Ecology Notes 5:184-186
Hampton J, Spencer PBS, Elliot AD, Thompson RCA (2006) Prevalence of zoonotic pathogens from feral pigs in major public drinking water catchments in Western Australia. EcoHealth 3:103-108; https://doi.org/10.1007/s10393-006-0018-8
Hernández FA, Parker BM, Pylant CL, Smyser TJ, Piaggio AJ, Lance SL, Milleson MP, Austin JD, Wisely SM (2018) Invasion ecology of wild pigs (Sus scrofa) in Florida, USA: the role of humans in the expansion and colonization of an invasive wild ungulate. Biological Invasions 20:1865-1880; https://doi.org/10.1007/s10530-018-1667-6
Herrero J, Garcia-Serrano A, Couto S, Ortuno VM, Garcia-Gonzalez R (2006) Diet of wild boar Sus scrofa L. and crop damage in an intensive agroecosystem. European Journal of Wildlife Research 52:245-250; https://doi.org/10.1007/s10344-006-0045-3
Hosseini PR, Dhondt AA, Dobson AP (2006) Spatial spread of an emerging infectious disease: conjunctivitis in house finches. Ecology 87:3037-3046; https://doi.org/10.1890/0012-9658(2006)87%5b3037:ssoaei%5d2.0.co;2
Hughes TW (1985) Home range, habitat utilization, and pig survival of feral swine on the Savannah River Plant. M.S. Thesis, Clemson University, Clemson, South Carolina
Kawula R (2014) Florida land cover classification system. Florida Fish and Wildlife Conservation Commission, Florida Natural Areas Inventory. pp 65
Kay SL, Fisher JW, Monaghan AJ, Beasley JC, Boughton R, Campbell TA, Cooper SM, Ditchkoff SS, Hartley SB, Kilgo JC, Wisely SM, Wyckoff AC, VerCauteren KC, Pepin KM (2017) Quantifying drivers of wild pig movements across multiple spatial and temporal scales. Movement Ecology 5:14; https://doi.org/10.1186/s40462-017-0105-1
Keiter DA, Beasley JC (2017) Hog heaven? Challenges of managing introduced wild pigs in natural areas. Natural Areas Journal 37:6-16; https://doi.org/10.3375/043.037.0117
Kelly AC, Mateus-Pinilla NE, Douglas M, Douglas W, Brown W, Ruiz M, and Novakofski J (2010) Utilizing disease surveillance to examine gene flow and dispersal in white-tailed deer. Journal of Applied Ecology 47:1189-1198; https://doi.org/10.1111/j.1365-2664.2010.01868.x
Keuling O, Stier N, Roth M (2008) How does hunting influence and spatial usage in wild boar Sus scrofa L. European Journal of Wildlife Research 54:729-737; https://doi.org/10.1007/s10344-008-0204-9
Keuling O, Stier N, Roth M (2009) Commuting, shifting or remaining? Different spatial utilization patterns of wild boar Sus scrofa L. in forest and field crops during summer. Mammalian Biology-Z Für Säugetierkunde 74:145-152; https://doi.org/10.1016/j.mambio.2008.05.007
Knight GR, Knight A, Hipes D, NeSmith K, Gulledge K, Jenkins A, Elam C, Diamond P, Oetting J, Newberry A (2010) Development of a cooperative land cover map: final report. Florida’s Wildlife Legacy Initiative Project 08009. Florida Natural Areas Inventory pp 102
Kurz JC, Marchinton RC (1972) Radiotelemetry studies of feral pigs in South Carolina. Journal of Wildlife Management 36:1240-1248
Langlois JP, Fahrig L, Merriam G, Artsob H (2001) Landscape structure influences continental distribution of Hantavirus in deer mice. Landscape Ecology 16:255-266; https://doi.org/10.1023/a:1011148316537
Leiser OP, Corn JL, Schmit BS, Keim PS, Foster JT (2013) Feral swine brucellosis in the United States and prospective genomic techniques for disease epidemiology. Veterinary Microbiology 166:1-10; https://doi.org/10.1016/j.vetmic.2013.02.025
Macdonald DW, Laurenson MK (2006) Infectious disease: inextricable linkages between human and ecosystem health. Biological Conservation 131:143-150; https://doi.org/10.1016/j.biocon.2006.05.007
Maehr DS, Belden RC, Land ED, Wilkins L (1990) Food habits of panthers in southwest Florida. The Journal of Wildlife Management 54:420-423.
Masot AJ, Gil M, Risco D, Jiménez OM, Núñez JI, Redondo E (2016) Pseudorabies virus infection (Aujeszky’s disease) in an Iberian lynx (Lynx pardinus) in Spain: a case report. BMC Veterinary Research 13:6; https://doi.org/10.1186/s12917-016-0938-7
Matschke GH (1967) Aging European wild hogs by dentition. Journal of Wildlife Management 31:109-113
Mayer JJ, Brisbin Jr IL (2009) Wild pigs: biology, damage, control techniques and management. SRNL-RP-2009-00869. Savannah River National Laboratory: Aiken, South Carolina. pp 400
McAlpine C, Brearley G, Rhodes J, Bradley A, Baxter G, Seabrook L, Lunney D, Liu Y, Cottin M, Smith AG, and Timms P (2017) Time-delayed influence of urban landscape change on the susceptibility of koalas to chlamydiosis. Landscape Ecology 32:663-679; https://doi.org/10.1007/s10980-016-0479-2
McClure ML, Burdett CL, Farnsworth ML, Lutman MW, Theobald DM, Riggs PD, Grear DA, Miller RS (2015) Modeling and mapping the probability of occurrence of invasive wild pigs across the contiguous United States. PLoS ONE 10:e0133771; https://doi.org/10.1371/journal.pone.0133771
Meentemeyer RK, Haas SE, Vaclavik T (2012) Landscape epidemiology of emerging infectious diseases in natural and human altered ecosystems. Annual Review of Phytopathology 50:379-402; https://doi.org/10.1146/annurev-phyto-081211-172938
Meng XJ, Lindsay DS, Sriranganathan N (2009) Wild boars as sources for infectious diseases in livestock and humans. Philosophical Transactions of the Royal Society of London B R Soc Lond B Biological Sciences 364:2697-2707; https://doi.org/10.1098/rstb.2009.0086
Müller T, Hahn EC, Tottewitz F, Kramer M, Klupp BG, Mettenleiter TC, Freuling C (2011) Pseudorabies virus in wild swine: a global perspective. Archives of Virology 156:1691-1705; https://doi.org/10.1007/s00705-011-1080-2
Nielsen K, Gall D, Smith P, Vigliocco A, Perez B, Samartino L, Nicoletti P, Dajer A, Elzer P, Enright F (1999) Validation of the fluorescence polarization assay as a serological test for the presumptive diagnosis of porcine brucellosis. Veterinary Microbiology 68:245-253; https://doi.org/10.1016/s0378-1135(99)00077-2
Ostfeld RS, LoGiudice K (2003) Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology 84:1421-1427; https://doi.org/10.1890/02-3125
Pannwitz G, Freuling C, Denzin N, Schaarschmidt U, Nieper H, Hlinak A, Burkhardt S, Klopries M, Dedek J, Hoffmann L, Kramer M, Selhorst T, Conraths FJ, Mettenleiter T, Müller T (2012) A long-term serological survey on Aujeszky’s disease virus infections in wild boar in East Germany. Epidemiology and Infection 140:348-358; https://doi.org/10.1017/s0950268811000033
Payne A, Chappa S, Hars J, Dufour B, Gilot-Fromont E (2015) Wildlife visits to farm facilities assessed by camera traps in a bovine tuberculosis-infected area in France. European Journal of Wildlife Research 62:33-42; https://doi.org/10.1007/s10344-015-0970-0
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research – an update. Bioinformatics 28:2537-2539
Pearson HE, Toribio JLML, Hernandez-Jover M, Marshall D, Lapidge SJ (2014) Pathogen presence in feral pigs and their movement around two commercial piggeries in Queensland, Australia. Veterinary Record 174:325; https://doi.org/10.1136/vr.102019
Pearson HE, Toribio JLML, Lapidge SJ, Hernández-Jover M (2016) Evaluating the risk of pathogen transmission from wild animals to domestic pigs in Australia. Preventive Veterinary Medicine 123:39-51; https://doi.org/10.1016/j.prevetmed.2015.11.017
Pedersen K, Bevins SN, Schmit BS, Lutman MW, Milleson MP (2012) Apparent prevalence of swine brucellosis in feral swine in the United States. Human Wildlife Interactions 6:38-47
Pedersen K, Bevins SN, Baroch JA, Cumbee Jr JC, Chandler SC, Woodruff BS, Bigelow TT, DeLiberto TJ (2013) Pseudorabies in feral swine in the United States, 2009-2012. Journal of Wildlife Diseases 49:709-713; https://doi.org/10.7589/2012-12-314
Pedersen K, Quance CR, Robbe-Austerman S, Piaggio AJ, Bevins SN, Goldstein SM, Gaston WD, DeLiberto TJ (2014) Identification of Brucella suis from feral swine in selected states in the USA. Journal of Wildlife Diseases 50:171-179.
Pedersen K, Bauer NE, Olsen S, Arenas-Gamboa AM, Henry AC, Sibley TD, Gidlewski T (2017) Identification of Brucella spp. in feral swine (Sus scrofa) at abattoirs in Texas, USA. Zoonoses and Public Health 64:647-654.
Penrith ML Vosloo W, Mather C (2011) Classical swine fever (hog cholera): review of aspects relevant to control. Transboundary and Emerging Diseases 58:187-196; https://doi.org/10.1111/j.1865-1682.2011.01205.x
Rees EE, Pond BA, Cullingham CI, Tinline R, Ball D, Kyle CJ, White BN (2008) Assessing a landscape barrier using genetic simulation modeling: implications for raccoon rabies management. Preventive Veterinary Medicine 86:107-123; https://doi.org/10.1016/j.prevetmed.2008.03.007
Reich BJ, Hodges JS, Zadnik V (2006) Effects of residual smoothing on the posterior of the fixed effects in disease-mapping models. Biometrics 62:1197-1206; https://doi.org/10.1111/j.1541-0420.2006.00617.x
Riley S (2007). Large-scale spatial-transmission models of infectious disease. Science 316:1298-1301; https://doi.org/10.1126/science.1134695
Robic A, Dalens M, Woloszyn N, Milan D, Riquet J, Gellin J (1994) Isolation of 28 new porcine microsatellites revealing polymorphism. Mammalian Genome 5:580-583. https://doi.org/10.1007/bf00354935
Rohrer GA, Alexander LJ, Hu Z, Smith TPL, Keele JW, Beattie CW (1996) A comprehensive map of the porcine genome. Genome Research 6:371-391;
Root JJ, Puskas RB, Fischer JW, Swope CB, Neubaum MA, Reeder SA, Piaggio AJ (2009) Landscape genetics of raccoons (Procyon lotor) associated with ridges and valleys of Pennsylvania: implications for oral rabies vaccination programs. Vector-Borne and Zoonotic Diseases 9:583-588; https://doi.org/10.1089/vbz.2008.0110
Russell CA, Smith DL, Waller LA, Childs JE, Real LA (2004) A priori prediction of disease invasion dynamics in a novel environment. Proceedings of the Royal Society B Biological Sciences 271:21-25; https://doi.org/10.1098/rspb.2003.2559
Saito M, Koike F, Momose H, Mihira T, Uematsu S, Ohtani T, Sekiyama K (2012) Forecasting the range expansion of a recolonising wild boar Sus scrofa population. Wildlife Biology 18:383-392; https://doi.org/10.2981/11-110
Schley L, Roper TJ (2003) Diet of wild boar Sus scrofa in Western Europe, with particular reference to consumption of agricultural crops. Mammal Review 33:43-56; https://doi.org/10.1046/j.1365-2907.2003.00010.x
Seward NW, VerCauteren KC, Witmer GW, Engeman RM (2004) Feral swine impacts on agriculture and the environment. Sheep and Goat Research Journal 19:34-40
Snow NP, Jarzyna MA, VerCauteren KC (2017) Interpreting and predicting the spread of invasive wild pigs. Journal of Applied Ecology 54:2022-2232; https://doi.org/10.1111/1365-2664.12866
Stallknecht DE, Howerth EW (2008) Pseudorabies (Aujeszky’s Disease) In: Infectious Diseases of Wild Mammals, Williams ES, Barker IK (editors), Ames, Iowa: Iowa State University Press, pp 164-170
Storm DJ, Samuel MD, Rolley RE, Shelton P, Keuler NS, Richards BJ, Van Deelen TR (2013) Deer density and disease prevalence influence transmission of chronic wasting disease in white-tailed deer. Ecosphere 4:1-14; https://doi.org/10.1890/es12-00141.1
Tardy O, Massé A, Pelletier F, Fortin D (2018) Interplay between contact risk conspecific density, and landscape connectivity: An individual-based modeling framework. Ecological Modelling 373:25-38; https://doi.org/10.1016/j.ecolmodel.2018.02.003
Vander Wal E, Edye I, Paquet PC, Coltman DW, Bayne E, Brook RK, Andres JA (2013) Juxtaposition between host population structures: implications for disease transmission in a sympatric cervid community. Evolutionary Applications 6:1001-1011; https://doi.org/10.1111/eva.12065
Verpoest S, Cay AB, De Regge N (2014) Molecular characterization of Belgian pseudorabies virus isolates from domestic swine and wild boar. Veterinary Microbiology 172:72-77; https://doi.org/10.1016/j.vetmic.2014.05.001
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358-1370
Wilson GA, Rannala B (2003) Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163:1177-1191
Wood SN (2006) Generalized additive models: an introduction with R. Chapman and Hall/CRC pp 410
Zanardi G, Macchi C, Sacchi C, Rutili D (2003) Classical swine fever in wild boar in the Lombardy region of Italy from 1997 to 2002. Veterinary Record 152:461-465
Zeileis A, Hothorn T (2002) Diagnostic checking in regression relationships. R News 2:7-10
Acknowledgements
We thank USDA/APHIS/WS field personnel and several hunting check station operators for collecting samples on our behalf; J.C. Griffin, R. Boughton and M. Legare for assisting us with our sampling efforts in the field; M. Lopez, M. Anderson, P. Royston, B. Pace-Aldana, D. Watkins, and B. Camposano for helping us acquire sampling permits; and M. Tabak, H. Ernest and R. Beasley for providing technical support for the molecular work. FAH was supported by a doctoral fellowship from the Comisión Nacional de Ciencia y Tecnología de Chile (CONICYT-Chile) and by a postdoctoral fellowship from ANID/CONICYT (Fondecyt Postdoctorado Nº 3180111). This study was partially funded by USDA National Institute of Food and Agriculture, McIntire-Stennis Project No. 1015620 and a cooperative agreement with USDA APHIS WS National Wildlife Research Center, Agreement No. 12-7412-0896-CA to S.M. Wisely.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hernández, F.A., Carr, A.N., Milleson, M.P. et al. Dispersal and Land Cover Contribute to Pseudorabies Virus Exposure in Invasive Wild Pigs. EcoHealth 17, 498–511 (2020). https://doi.org/10.1007/s10393-020-01508-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-020-01508-6