Abstract
Purpose
Exposure to environmental heat stress increases carbohydrate oxidation and extracellular heat shock protein 70 (HSP70) concentrations during endurance exercise at matched absolute, external work rates. However, a reduction in absolute work rate typically occurs when unacclimated endurance athletes train and/or compete in hot environments. We sought to determine the effect of environmental heat stress on carbohydrate oxidation rates and plasma HSP70 expression during exercise at matched heart rates (HR).
Methods
Ten endurance-trained, male cyclists performed two experimental trials in an acute, randomised, counterbalanced cross-over design. Each trial involved a 90-min bout of cycling exercise at 95% of the HR associated with the first ventilatory threshold in either 18 (TEMP) or 33 °C (HEAT), with ~ 60% relative humidity.
Results
Mean power output (17 ± 11%, P < 0.001) and whole-body energy expenditure (14 ± 8%, P < 0.001) were significantly lower in HEAT. Whole-body carbohydrate oxidation rates were significantly lower in HEAT (19 ± 11%, P = 0.002), while fat oxidation rates were not different between-trials. The heat stress-induced reduction in carbohydrate oxidation was associated with the observed reduction in power output (r = 0.64, 95% CI, 0.01, 0.91, P = 0.05) and augmented sweat rates (r = 0.85, 95% CI, 0.49, 0.96, P = 0.002). Plasma HSP70 and adrenaline concentrations were not increased with exercise in either environment.
Conclusion
These data contribute to our understanding of how moderate environmental heat stress is likely to influence substrate oxidation and plasma HSP70 expression in an ecologically-valid model of endurance exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During endurance exercise, carbohydrates and fatty acids are the primary substrates oxidised to support the adenosine triphosphate (ATP) turnover required for repeated skeletal muscle contraction (Hawley and Leckey 2015; O’Brien et al. 1993; Watt et al. 2002). The absolute and relative contributions made by these substrates to total energy expenditure is largely determined by exercise intensity and duration (Jeukendrup et al. 1998; Romijn et al. 1993; Watt et al. 2002). Additionally, environmental heat stress increases carbohydrate and reduces fat oxidation during exercise at given work rates (Febbraio et al. 1994a, b; Jentjens et al. 2002; Hargreaves et al. 1996a). This shift in substrate utilisation may be explained by heat-stress-induced increases in core and muscle temperatures (Febbraio et al. 1994a, b; Fernández-Elías et al. 2015), dehydration (Wilson et al. 1977; Febbraio et al. 1996, 1998), and increases in circulating adrenaline concentrations (González-Alonso et al. 1999; Hargreaves et al. 1996b; Powers et al. 1985). The metabolic response to exercise has implications for fatigue (Bergström and Hultman 1967), the adaptive response to training (Philp et al. 2012), and refuelling requirements (Impey et al. 2018).
Exposure to environmental heat stress during endurance exercise also increases intracellular and extracellular heat shock protein 72 (HSP72) concentrations (Gibson et al. 2014; Magalhães et al. 2010; Marshall et al. 2006; Morton et al. 2006; Périard et al. 2012; Whitham et al. 2007). Intracellular HSP72 acts as a molecular chaperone that accompanies misfolded and denatured proteins to maintain cellular homeostasis (Whitham and Fortes 2008), and subsequently contributes to the development of thermotolerance (Li et al. 1995; Liu et al. 1992). In skeletal muscle, this chaperoning function of HSP72 may contribute to mitochondrial adaptations to endurance exercise training (Henstridge et al. 2014; Skidmore et al. 1995; Young et al. 2003). Mechanistically, HSP72 is released into the circulation through the α1-adrenoceptor pathway, and is therefore stimulated by circulating catecholamines (Johnson et al. 2005; Whitham et al. 2006), which are increased by exercise (Febbraio et al. 2002a; Walsh et al. 2001). During stress, the elevation of extracellular HSP72 expression may act a ‘danger signal’ to prime or enhance immunologic responses (Fleshner and Johnson 2005; Fleshner et al. 2003). It is possible that HSP72 responses are influenced by exercise in a duration-and-intensity dependent manner (Fehrenbach et al. 2005; Liu et al. 1999).
Previous research has studied these stimulatory effects of environmental heat stress on substrate metabolism (Maunder et al. 2020; Febbraio et al. 1994a; Jentjens et al. 2002; Young et al. 1985; Marino et al. 2001) and HSP72 expression (Gibson et al. 2014; Yamada et al. 2007; Walsh et al. 2001; Whitham et al. 2006) during endurance exercise at matched external work rates between-environments. In the real-world, however, endurance athletes are likely to experience a reduction in absolute work rates when training in hot vs. temperate conditions (Boynton et al. 2019; Lorenzo et al. 2010; Maunder et al. 2021a, b). Given that research supports the importance of exercise intensity in both substrate utilisation (Lorenzo et al. 2010; Boynton et al. 2019; Maunder et al. 2021a, b) and HSP72 expression (Gibson et al. 2014; Morton et al. 2009), it is possible that these acute effects of environmental heat stress may be at least partially blunted by the likely reduction in absolute work rates. Therefore, a relevant comparison for practitioners considering the likely metabolic effects of performing a training session under environmental heat stress may be using matched relative physiological stress, or heart rates.
Accordingly, the primary aim of the present investigation was to assess the effects of moderate environmental heat stress on substrate oxidation rates during heart rate-matched moderate-intensity cycling. Additionally, we investigated the response of plasma HSP70 to the exercise protocols. We hypothesised that lower rates of whole-body energy expenditure and carbohydrate oxidation would be observed under moderate environmental heat stress secondary to lower achieved power outputs, but that plasma HSP70 expression would still be elevated due to greater increases in core temperature and plasma adrenaline concentrations.
Methods
Ethical approval
This study was performed in accordance with the standards of the Declaration of Helsinki, 2013. The Auckland University of Technology Ethics Committee approved all procedures (21/121), and all participants provided written informed consent prior to participation. This study was not registered in a database. Data associated with this study are available from the corresponding author upon reasonable request.
Participants
Ten endurance-trained male cyclists and triathletes participated in this study (age, 31 ± 8 years; height, 181 ± 3 cm; body mass, 75.0 ± 5.7 kg; peak oxygen uptake [\({\dot{\text{V}}\text{O}}_{{{\text{2peak}}}}\)], 58.1 ± 6.8 mL·kg−1·min−1; first ventilatory threshold [VT1], 204 ± 46 W; overall training volume, 9 ± 3 h·wk−1; cycling volume, 6 ± 2 h·wk−1). Participants were free from viral infection (> 1 month), lower-limb injury (> 3 months), and had not suffered with any cardiovascular disease, or previously experienced exertional heat stress illness. Participants were unacclimated to exercise-heat stress, which was defined as not recently having undertaken specific heat acclimatisation training (≥ 6 months). Using 50% of the between-group effect size for post-exercise extracellular HSP72 concentration (Whitham et al. 2007), it was a priori calculated that a total sample size of 10 participants would be required to observe a between-group difference (P < 0.05) in post-exercise extracellular HSP72 concentration with 80% statistical power. This smaller effect size was utilised as smaller between-environment differences in post-exercise thermoregulatory variables were expected in this study (~ 1 °C difference in Tre based on Maunder et al. (2020), compared to the ~ 2 °C difference in Whitham et al. (2007)).
Study design
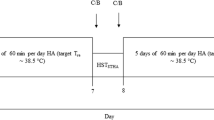
An acute, randomised, counterbalanced cross-over design was used in the present investigation. Participants visited the laboratory on three occasions, ~ 7 d apart, for: (i) a characterisation trial involving an incremental cycling test, and (ii) two experimental trials, which each involved a 90-min bout of cycling exercise at 95% of the HR associated with VT1 in either 18 (TEMP) or 33 °C (HEAT), with ~ 60% relative humidity (rH) (Fig. 1). The order in which participants completed the two experimental trials was randomised with a counterbalanced design. All laboratory trials were intended to be completed during the non-summer months in Auckland, New Zealand. However, data collection was interrupted by a nationwide lockdown for COVID-19. Consequently, four participants completed their trials during the winter months, and six of the ten participants were tested during the warmer months. Therefore, subgroup analyses were performed to assess seasonal effects on the primary outcome measures of this study.
Characterisation trial
Participants arrived the laboratory at ~ 7:00 am for the characterisation trial following an overnight fast, having refrained from caffeine, alcohol, and intense exercise for 24 h. On arrival, participants provided written informed consent and completed a health screening questionnaire. Height and body mass were first measured. Participants then mounted a laboratory ergometer (Excalibur Sport, Lode, Groningen, NET), and an incremental cycling test commenced at 95 W in a laboratory environment (18 °C , 60–80% rH). The power output was increased by 35 W every 3 min until the respiratory exchange ratio reached 1.0, after this point the duration of each stage was shortened to 1 min until task failure. Expired gases were collected throughout using a metabolic cart (TrueOne2400, ParvoMedics, Sandy, UT, US). \({\dot{\text{V}}\text{O}}_{{{\text{2peak}}}}\) was determined to ensure eligibility for study participation of \({\dot{\text{V}}\text{O}}_{{{\text{2peak}}}}\) ≥ 50 mL·kg−1·min−1, and VT1 was also determined to individualise the exercise intensity in the experimental trials. HR was also measured continuously using a chest-strap HR monitor (TICKR, Wahoo, Taiwan). The \({\dot{\text{V}}\text{O}}_{{{\text{2peak}}}}\) was identified as the highest 30-s average value for \({\dot{\text{V}}\text{O}}_{{2}}\) during the incremental cycling test. The VT1 was identified as the first increase in the ventilatory equivalent for oxygen (\({\dot{\text{V}}\text{E}}\cdot{\text{VO}}_{{2}}^{{ - {1}}}\)) without changes in ventilatory equivalent for carbon dioxide (\({\dot{\text{V}}\text{E}}\cdot{\text{VCO}}_{{2}}^{{ - {1}}}\)) (Lucía et al. 2000), and expressed as both a power output and HR value. Convective air flow was provided using a pedestal fan (GCPF340, Goldair, China).
Experimental trials
Participants arrived at the laboratory ~ 7 d following the characterisation trial at ~ 7:00 am, having adhered to the same pre-trial instructions described above, and made written records of their diet for 48 h and training for 7 d, such that these could be repeated in advance of the second experimental trial. On arrival, a 6-mL pre-exercise blood sample was drawn from an antecubital vein using the venepuncture technique. Pre-exercise body mass was measured in cycling clothes and then participants self-inserted a rectal thermometer ∼10 cm beyond the anal sphincter in privacy for determination of rectal temperature (Tre). A skin temperature thermistor was subsequently taped over the mid-belly of the vastus lateralis ∼15 cm above the patella, and covered with two pieces of 2-mm neoprene in order to insulate the skin underneath for continuous observation of insulated skin temperature (Tins), which was used for estimation of muscle temperature (Tmus) in line with recent work (Flouris et al. 2015). Participants then sat comfortably for 5 min for measurement of resting estimation Tmus and Tre in the laboratory environment before entering the environmental chamber, which was set at either 18 or 33 °C , with 60% rH. Participants then mounted their own road bicycle, which was connected to a calibrated, direct-drive indoor trainer (Kickr, Wahoo Fitness, Atlanta, USA), and were fitted with a chest-strap HR monitor (TICKR, Wahoo, Taiwan) for continuous observation of HR during exercise.
A 90-min bout of cycling then commenced. During the first 5-min of cycling, participants were asked to progressively increase their HR to a specified target equivalent to 95% (± 2 b·min−1) of the VT1 HR determined in the incremental cycling test, and then maintain their target HR until exercise cessation. Participants were reminded of this if their HR drifted outside the target range. Participants had ad libitum access to plain water throughout the cycling trial. Convective air flow (~ 3.2 m·s−1) was provided by an industrial fan (FS-75, FWL, Auckland, NZ). Expired gases were obtained for 4 min every 15 min using a metabolic cart (TrueOne2400, ParvoMedics, Sandy, UT, US). Rating of perceived exertion (RPE) on a scale of 6 to 20 (Borg 1982) was recorded every 15 min. Perceived thermal comfort on a 1-to-10 scale, and thermal sensation on a 1-to-14 scale, which were adapted from an previous work (Gagge et al. 1967), were also assessed every 15 min. The Tre was monitored throughout the cycling trial to ensure that it did not exceed 39.5 °C (Silva et al. 2019), and no participant reached this temperature.
Within 3-min after exercise cessation, a 6-mL post-exercise antecubital venous blood sample was obtained using venepuncture technique, and total water consumption was recorded through weighing of drink bottles. Participants subsequently removed the HR monitor, skin temperature thermistor, and rectal thermometer in privacy. Participants dried their skin using a towel, and post-exercise body mass was measured in cycling clothes for calculation of percentage dehydration (Eq. 1), and estimation of the magnitude of sweat loss during the trial, accounting for fluid consumption. Participants returned to the laboratory ~ 7 d later to perform the remaining experimental trial, having adhered to the same pre-trial instructions described above, and repeated their 48-h diet and 7-day training records in advance.
Equation 1 Calculation of percentage dehydration (%).
Expired gas analyses
In the incremental cycling test, V̇O2 and V̇CO2 from the last 1 min of each stage were used to estimate substrate oxidation rates using standard stoichiometric equations (Eq. 2) (Jeukendrup and Wallis 2005). Peak fat oxidation rate (g·min−1) was then identified as the highest fat oxidation rate during the test. In the experimental trials, expired gases from the final three minutes of each 4-min sampling time point were averaged and used for calculation of whole-body rates of energy expenditure, carbohydrate oxidation, and fat oxidation using standard equations (Eq. 2). The first minute of each sampling timepoint was discarded to minimise any potential hyperventilatory effect associated with reorganising the headgear, and to ensure that participants had resettled into their comfortable cycling position. The rate of metabolic heat production (Hprod) was calculated by subtracting external mechanical power output (W) from metabolic energy expenditure (W) (Ravanelli et al. 2020). The Hprod is expressed relative to body surface area according to the Dubois and Dubois formula (Du Bois and Du Bois 1916).
Equation 2 Estimates of whole-body rates of energy expenditure, carbohydrate oxidation, and fat oxidation, where both carbon dioxide production (\({\dot{\text{V}}\text{CO}}_{{2}}\)), and oxygen uptake (\({\dot{\text{V}}\text{O}}_{{2}}\)) are in L·min−1.
Thermoregulatory analyses
Mean values obtained from the rectal thermometer and skin temperature thermistor during the 30-s prior to each measurement timepoint were defined as Tre and Tins. The Tins was used for estimation of the vastus lateralis temperature (Tmus) in line with previous work (Eq. 3) (Flouris et al. 2015).
Equation 3 Estimates of insulated skin temperature over the vastus lateralis (Tins) at rest and during exercise, where TinsLag2 = Tins – Tins two min beforehand, etc.
Plasma analyses
Venous blood samples were stored on ice in pre-chilled ethylenediaminetetraacetic acid tubes until trial completion. In order to allow the correction of plasma concentrations for changes in plasma volume across the trial (van Beaumont et al. 1972), a small sample of whole blood was pipetted into duplicate capillary tubes before spinning for 3-min using a micro-haematocrit centrifuge (Haematospin 1400, Hawksley& Sons, Ltd., England), and then the proportion of red blood cells was manually measured (the coefficient of variation [CV], 1.55 ± 1.85%). Plasma was then extracted from the remaining whole blood by centrifugation in 4 °C for 10 min using a Heraeus Megafuge 16 Centrifuge (D-37520 Osterode, Thermo Fisher Scientific, Inc., Germany), and stored at − 80 °C for further analyses. Plasma HSP70 concentration was determined via an enzyme-linked immunosorbent assay (ELISA) (BMS2087, Thermo Fisher Scientific, Inc., US). Plasma adrenaline and noradrenaline concentrations were determined via ELISA kits (ab287788 and ab287789, Abcam®, UK). These assays were performed using commercially available kits in duplicate according to the manufacturer’s instructions. Achieved intra-assay CVs were 13.0% for plasma HSP70 concentration, 4.3% for plasma adrenaline concentration, and 16.6% for plasma noradrenaline concentration.
Statistical analyses
Statistical analysis was performed with GraphPad Prism Version 9.3.1 (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± standard deviation (SD) unless otherwise stated. The normality of data distributions were assessed using the Shapiro–Wilk test, which is considered as an appropriate test for small sample sizes (N < 50) (Rahman and Govindarajulu 1997). Within- and between-trial differences in physiological variables were assessed using two-way, repeated measures analyses of variance, with temperature and time as factors. Simple comparisons were made using paired t-tests (or the Wilcoxon signed-rank test for non-normal distributions). Where main effects of time were observed, exercise-induced changes (15 vs. 90 min) in variables were compared. Where main effects of environmental temperature were observed, end-exercise values were compared. Where interactions between trial and time were observed, the magnitude of exercise-induced changes (15 vs. 90 min) in variables were compared. Bivariate linear correlations (Pearson’s product-moment correlation coefficients or Spearman’s rank-order correlation coefficients, depending on the distribution of the data) were used to assess relationships between primary outcome measures (heat stress-induced changes in mean carbohydrate oxidation rate and post-exercise plasma HSP70 concentrations) and heat stress-induced changes in dehydration percentage, sweat rate, mean power output, mean Tre, and mean estimated Tmus. The level of statistical significance was set at P ≤ 0.05.
Results
Mean HR was not significantly different between TEMP and HEAT (P = 0.63, Fig. 2a); in all cases, the individual difference in mean HR between-trials was ≤ 4 b·min−1. Although a main effect of time for HR was observed (P = 0.01), there was no main effect of environmental temperature (P = 0.23), or interaction between time and environmental temperature (P = 0.53, Fig. 2b). Subgroup analyses indicated no significant effect of season for mean whole-body EE (P = 0.54), CHO oxidation rate (P = 0.58), fat oxidation rate (P = 0.78), or exercise-induced change in post-exercise plasma HSP70 concentration (P = 0.26).
Heart rate (HR, beats·min−1) and power output (W) during 90 min of moderate-intensity exercise at a target HR of 95% of the first ventilatory threshold in 18 °C (TEMP) and 33 °C (HEAT). a Mean HR during the 90-min exercise trials (beats·min−1), b HR at each timepoint throughout the 90-min exercise trials (beats·min.−1), c mean power output (W) during the 90-min exercise trials, and d power output (W) at each timepoint throughout the 90-min exercise trials. Bars indicate group mean values, and dots identify individual mean values. ***Indicates P ≤ 0.001
Mean power output was significantly lower in HEAT (17 ± 11%, P < 0.001, Fig. 2c); in all participants, mean power output was numerically lower in HEAT (5–64 W). A main effect of time was observed for power output, whereby power output significantly decreased as exercise progressed (P < 0.001, Fig. 2d). A significant interaction between time and environmental temperature was observed (P = 0.01, Fig. 2d). The reduction in power output over time (15 vs. 90 min) was greater in HEAT (22 ± 11 vs. 11 ± 7%, P = 0.005). Accordingly, the rate of metabolic heat production was lower in HEAT vs. TEMP (308 ± 77 vs. 354 ± 84 W·m−2, P < 0.001).
Both Tre and estimated Tmus significantly increased as exercise progressed (Tre, 1.1 ± 0.4 and 1.2 ± 0.6 °C ; estimated Tmus, 3.8 ± 0.7 and 4.5 ± 0.9, in TEMP and HEAT, respectively, from rest to 90 min, both P < 0.001, Fig. 3a, b). No main effect of environmental temperature (P = 0.49), or interaction between time and environmental temperature was observed for Tre (P = 0.58, Fig. 3a). A main effect of environmental temperature was observed for estimated Tmus, whereby the end-exercise estimated Tmus was greater in HEAT (1.0 ± 0.4 °C , P < 0.001, Fig. 3b). A significant interaction between time and environmental temperature was observed (P = 0.017). The increase in estimated Tmus from rest to 90 min was greater in HEAT (4.5 ± 0.9 vs. 3.8 ± 0.7 °C , P = 0.003). Sweat rate (1.1 ± 0.4 vs. 0.6 ± 0.3 L·h−1, P < 0.001) and dehydration (1.4 ± 0.6 vs. 0.9 ± 0.7% of pre-exercise body mass, P = 0.003) were significantly greater in HEAT.
Thermoregulatory and perceptual variables. a Rectal temperature (°C ), b estimated muscle temperature (°C ), c rating of perceived exertion (6–20, AU), d thermal comfort (1–10, AU), and e thermal sensation (1–14, AU) at each timepoint during 90 min of moderate-intensity exercise with a target HR of 95% of the first ventilatory threshold in 18 °C (TEMP) and 33 °C (HEAT). A solid line with clear markers indicates data obtained from TEMP, and a dashed line with solid markers indicate data obtained from HEAT
A main effect of time for RPE was observed, whereby it significantly increased as exercise progressed (P < 0.001, Fig. 3c). There was no significant main effect of environmental temperature (P = 0.09), or interaction between time and environmental temperature (P = 0.76) for RPE. No main effect of time was observed for thermal comfort (P = 0.12), but a significant effect of environmental temperature was observed, whereby thermal comfort was significantly higher in HEAT (P = 0.001, Fig. 3d). A significant interaction between time and environmental temperature was observed (P = 0.03), whereby participants felt less comfortable as exercise duration progressed in HEAT. No main effect of time was observed for thermal comfort (P = 0.10), but a significant effect of environmental temperature was observed, whereby thermal sensation was significantly higher in HEAT (P < 0.001, Fig. 3e). No interaction between time and environmental temperature interaction was observed for thermal sensation (P = 0.89).
Mean whole-body EE was significantly lower in HEAT (14 ± 8%, P < 0.001, Fig. 4a). A main effect of time was observed for whole-body EE (P = 0.002), whereby it significantly decreased as exercise progressed (12.0 ± 2.9 vs. 11.4 ± 2.9 kcal.min−1, at 15 vs. 90 min, respectively, P = 0.03), but there was no interaction between time and environmental temperature (P = 0.13, Fig. 4b). Mean CHO oxidation rate was significantly lower in HEAT (19 ± 11%, P = 0.002, Fig. 4c). A main effect of time was observed for CHO oxidation rate, whereby it significantly decreased as exercise progressed (P = 0.003, Fig. 4d). A significant interaction between time and environmental temperature was observed (P = 0.02). The exercise-induced reduction in CHO oxidation rate over time (15 vs. 90 min) was significantly greater in HEAT (27.1 ± 10.1 v. 9.6 ± 21.3%, P = 0.003, Fig. 4d). Mean fat oxidation rate was not significantly different between-trials (P = 0.54, Fig. 4e). A main effect of time was observed for fat oxidation (P < 0.001), whereby it significantly increased as exercise progressed (0.43 ± 0.18 and 0.52 ± 0.17 g·min−1, at 15 and 90 min, P = 0.001, Fig. 4f). No main effect of temperature (P = 0.54), or interaction between time and environmental temperature (P = 0.73), was observed for fat oxidation rate.
Whole-body substrate oxidation rates during 90 min of moderate-intensity exercise with a target HR of 95% of the first ventilatory threshold in 18 °C (TEMP) and 33 °C (HEAT). a Mean energy expenditure (kcal·min−1), b energy expenditure (kcal·min−1) at each 15-min timepoint, c mean carbohydrate oxidation rate (g·min−1), d carbohydrate oxidation rate (g·min−1) at each 15-min timepoint, e mean fat oxidation rate (g·min−1), f fat oxidation rate (g·min.−1) at each 15-min timepoint. Bars indicate group mean values, and dots identify individual mean values. ***Indicates P ≤ 0.001. **Indicates P ≤ 0.01
No main effect of time (P = 0.80), environmental temperature (P = 0.51), or interaction between time and environmental temperature (P = 0.30), was observed for plasma HSP70 concentration (Fig. 5a). No main effect of time (P = 0.65), environmental temperature (P = 0.31), or interaction between time and environmental temperature (P = 0.86), was observed for plasma adrenaline concentration (Fig. 5b). No main effect of time (P = 0.23), environmental temperature (P = 0.98), or interaction between time and environmental temperature (P = 0.91), was observed for plasma noradrenaline concentration (Fig. 5c).
Mean pre- and post-exercise plasma. a Heat shock protein 70 (ng·mL−1) (TEMP, N = 8; HEAT, N = 9), b adrenaline (pg·mL−1) (N = 9), and c noradrenaline (pg·mL−1) (N = 10) concentrations in 18 °C (TEMP) and 33 °C (HEAT). A solid line with clear markers indicates data obtained from TEMP, and a dashed line with solid markers indicate data obtained from HEAT
Heat stress-induced in changes in mean CHO oxidation rate were significantly correlated with heat stress-induced changes in power output (r = 0.64, P = 0.05), and sweat rate (r = 0.85, P = 0.002). No significant correlations were observed between heat stress-induced changes in post-exercise plasma HSP70 concentration and heat stress-induced changes in mean power output, dehydration, sweat rate, mean Tre, or mean estimated Tmus (Table 1).
Discussion
The purpose of the present investigation was to assess the effect of moderate environmental heat stress on substrate oxidation rates and plasma HSP70 expression in response to heart rate-matched prolonged moderate-intensity cycling. The primary findings were that (i) whole-body CHO oxidation rates were lower, but whole-body fat oxidation rates were similar, during moderate-intensity cycling in HEAT vs. TEMP, with these effects likely due to the reduction in power output and associated decrease in EE in HEAT, and (ii) moderate-intensity cycling did not increase plasma HSP70 expression in either HEAT or TEMP.
In this present study, exercise intensity was regulated in relation to HR thresholds (95% of the HR associated with VT1), and therefore, the reduced power output in HEAT vs. TEMP was not unexpected. Indeed, a decrease in mean power output was observed in all participants in HEAT (− 17 ± 11% from mean power output in TEMP, P = 0.001, Fig. 2c). Lower power output achieved during endurance exercise—at a given HR – in HEAT may be explained by increased peripheral blood flow to regulate temperature via sweating (Febbraio 2001). Greater peripheral blood flow in HEAT is supported by the observed elevated sweat rate in HEAT (1.1 ± 0.4 vs. 0.6 ± 0.3 L·h−1, P < 0.001). Greater demand for peripheral blood flow necessitates greater cardiac output and reduced stroke volume via the Frank-Starling mechanism, and therefore results in an increase in HR during exercise at given absolute work rates (Trinity et al. 2010). Thus, participants had to reduce power output to maintain the HR target in HEAT.
In alignment with our hypothesis, whole-body CHO oxidation rates were lower in HEAT vs. TEMP (1.48 ± 0.46 vs. 1.86 ± 0.61 g·min−1, P = 0.002, Fig. 4c). These findings contrast previous research demonstrating increased carbohydrate metabolism during endurance exercise performed under environmental heat stress (≥ 30 °C ) compared to equivalent exercise in temperate conditions at matched external work rates, where core temperatures were greater in the environmental heat stress trials and higher than in the present study (Febbraio et al. 1994a, b; Jentjens et al. 2002; Hargreaves et al. 1996a; Maunder et al. 2020). We took this approach in order to describe physiological responses to an ecologically valid scenario. The lack of difference in core temperature can be explained by the lower Hprod in HEAT vs. TEMP (308 ± 77 vs. 354 ± 84 W·m−2, P < 0.001), secondary to the lower EE and power output.
The bivariate correlational analyses indicate the observed heat stress-induced decrease in whole-body CHO oxidation rates may be explained by the associated reduction in power output in HEAT (Table 1). The lower absolute power output in HEAT reduced whole-body EE (− 14 ± 8% from mean EE in TEMP, P < 0.001, Fig. 4a), and therefore the demand for ATP production via carbohydrate metabolism. Interestingly, whole-body fat oxidation rates were unaffected by environmental temperature (P = 0.54, Fig. 4e). Therefore, the observed heat stress-induced reduction in whole-body EE associated with the lower power output during moderate-intensity cycling performed in HEAT is explained by reduced CHO oxidation and maintained fat oxidation rates. However, the addition of an experimental trial in HEAT with the exercise intensity at absolute power output matched to TEMP would be necessary to confirm this.
Furthermore, the observed heat stress-induced increase in sweat rate was related to the heat stress-induced decrease in whole-body CHO oxidation rate (Table 1). Plausibly, participants who had a larger increase in sweat rate in HEAT could dissipate heat more efficiently in HEAT, and therefore produce more metabolic heat for the same Tre response, resulting in a more subtle reduction in power output in HEAT vs. TEMP. Therefore, athletes with greater between-environment difference in sweat rate had smaller reductions in power output, and therefore smaller reductions in CHO oxidation rates.
However, this heat stress-induced decrease in whole-body CHO oxidation rate when exercising at matched HR might not be observed in endurance athletes acclimated to hot environments. Following acclimation, athletes produce more power at a given HR under heat stress (Yamada et al. 2007; Corbett et al. 2022), likely necessitating a greater demand for ATP synthesis, and therefore CHO oxidation, than when unacclimated. Accordingly, the between-trial differences we observed here might be reduced following a period of heat acclimation training.
Secondly, moderate-intensity endurance exercise did not increase plasma HSP70 expression in either TEMP or HEAT (Fig. 5a). These findings contrast previous studies reporting a significant increase in extracellular HSP72 concentration in unacclimated individuals following endurance exercise performed in temperate (18–24 °C ) (Whitham et al. 2007; Fehrenbach et al. 2005) and hot environments (35–40 °C ) (Magalhães et al. 2010; Marshall et al. 2006; Gibson et al. 2014; Périard et al. 2012; Whitham et al. 2007). The ELISA kit used in the present investigation quantified the concentration of the whole HSP70 family (including HSP72 and HSP73 isoforms). This could explain the greater plasma HSP70 concentrations (~ 6–9 ng·mL−1) observed in this present study, compared with the other studies measuring extracellular HSP72 (~ 1–6 ng·mL−1) (Magalhães et al. 2010; Marshall et al. 2006; Whitham et al. 2007; Périard et al. 2012; Fehrenbach et al. 2005). As HSP73 is unlikely to be stimulated by environmental heat stress (Kregel 2002; Locke 1997; Welch and Suhan 1986), it is plausible that the capture of the whole HSP70 may have obscured an effect of endurance exercise on plasma HSP72 expression. However, the lack of observed plasma HSP70 accumulation might also be explained by the absence of exercise-induced increase in plasma adrenaline concentration (Fig. 5b), given catecholamines stimulate the release of HSP72 into the circulation (Chin et al. 1996; Heneka et al. 2003).
The extracellular HSP72 response to endurance exercise is related to exercise intensity and duration (Fehrenbach et al. 2005), with the addition of internal thermal stress promoting further increases extracellular HSP72 responses (Marshall et al. 2006). In this present investigation, expired gases collected during TEMP indicated the exercise intensity corresponded to 60.7 ± 5.5%\({\dot{\text{V}}\text{O}}_{{{\text{2peak}}}}\). This is relatively similar to studies reporting increased concentrations of plasma HSP72 after endurance exercise intensity prescribed at ~ 60% \({\dot{\text{V}}\text{O}}_{{{\text{2peak}}}}\) (Whitham et al. 2007; Fehrenbach et al. 2005), but may have been insufficient in our cohort to induce increased extracellular HSP70 concentration. Similarly, insufficient exercise duration may explain the lack of stimulation of extracellular HSP70, as the aforementioned studies included longer exercise protocols (90 vs. 120 min) (Whitham et al. 2007; Fehrenbach et al. 2005). A longer exercise duration may further increase heat stress-induced physiological strain relevant to HSP72 accumulation, which may include elevated core and/or local temperatures (Morton et al. 2006; Cuthbert et al. 2019), or decreased carbohydrate availability (Dalgaard et al. 2022; Febbraio and Koukoulas 2000; Febbraio et al. 2002b). Plausibly, an exercise-induced increase in plasma HSP70 expression may have been observed in this present study if exercise duration was ≥ 120 min. Given HEAT did not increase Tre or plasma adrenaline concentrations vs. TEMP, it is unsurprising that a stimulatory effect of heat stress on plasma HSP70 expression was not observed.
From a practical standpoint, our descriptive data might be used to help inform nutrition practices in endurance training. Our data suggests that when endurance athletes are exposed to a hot environment during training, either incidentally (e.g. during summer months) or deliberately (e.g. heat acclimatisation training), the carbohydrate and overall energy cost of exercise might be reduced compared to training performed at a similar HR in temperate conditions.
In conclusion, heart rate-matched moderate-intensity cycling in a hot environment (33 °C ) reduced whole-body CHO oxidation rates, but did not alter fat oxidation rates, compared to the equivalent exercise performed in a temperate environment (18 °C ). This metabolic change was associated with the lower external power output in HEAT. Exercise-induced increases in plasma HSP70 expression did not occur in either condition. These data should be considered by practitioners working with endurance athletes who are exposed to moderate environmental heat stress, either incidentally or purposefully, during training and/or competition.
Availability of data and material
Data is available from the corresponding author upon reasonable request.
Code availability
Code is available upon reasonable request.
Abbreviations
- ATP:
-
Adenosine triphosphate
- CHO:
-
Carbohydrate
- EE:
-
Energy expenditure
- ELISA:
-
Enzyme-linked immunosorbent assay
- HR:
-
Heart rate
- HSP:
-
Heat shock protein
- rH:
-
Relative humidity
- RPE:
-
Rating of perceived exertion
- Tins :
-
Insulated skin temperature
- Tmus :
-
Muscle temperature
- Tre :
-
Rectal temperature
- \({\dot{\text{V}}\text{E}}\cdot{\text{VCO}}_{{2}}^{{ - {1}}}\) :
-
Ventilatory equivalent for carbon dioxide
- \({\dot{\text{V}}\text{E}}\cdot{\text{VO}}_{{2}}^{{ - {1}}}\) :
-
Ventilatory equivalent for oxygen
- \({\dot{\text{V}}\text{CO}}_{{2}}\) :
-
Volume of carbon dioxide production
- \({\dot{\text{V}}\text{O}}_{{2}}\) :
-
Volume of oxygen uptake
- \({\dot{\text{V}}\text{O}}_{{{\text{2peak}}}}\) :
-
Peak oxygen uptake
- VT1 :
-
First ventilatory threshold
References
Bergström J, Hultman E (1967) A study of the glycogen metabolism during exercise in man. Scand J Clin Lab Invest 19:218–228
Borg GAV (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381
Boynton JR, Danner F, Menaspa P, Peiffer JJ, Abbiss CR (2019) Effect of environmental temperature on high-intensity intervals in well-trained cyclists. Int J Sports Physiol Perform 14(10):1401–1407
Chin JH, Okazaki M, Hu ZW, Miller JW, Hoffman BB (1996) Activation of heat shock protein (hsp)70 and proto-oncogene expression by α1 adrenergic agonists in rat aorta with age. J Clin Investig 97(10):2316–2323. https://doi.org/10.1172/JCI118674
Corbett J, Massey HC, Costello JT, Tipton MJ, Neal RA (2022) The effect of medium-term heat acclimation on endurance performance in a temperate environment. Eur J Sport Sci. https://doi.org/10.1080/17461391.2020.1856935
Cuthbert RL, Shute RJ, Slivka DR (2019) Skeletal muscle cold shock and heat shock protein mRNA response to aerobic exercise in different environmental temperatures. Temperature 6(1):77–84. https://doi.org/10.1080/23328940.2018.1555414
Dalgaard LB, Ørtenblad N, Hvid LG, Gejl KD (2022) The expression of HSP70 in skeletal muscle is not associated with glycogen availability during recovery following prolonged exercise in elite endurance athletes. Eur J Appl Physiol. https://doi.org/10.1007/s00421-022-04955-x
Du Bois D, Du Bois EF (1916) A formula to estimate surface area if height and weight are known. Arch Intern Med 17:863–871
Febbraio MA (2001) Alterations in energy metabolism during exercise and heat stress. Sports Med 31(1):47–59
Febbraio MA, Koukoulas I (2000) HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. J Appl Physiol 89(3):1055–1060. https://doi.org/10.1152/jappl.2000.89.3.1055
Febbraio MA, Snow RJ, Hargreaves M, Stathis CG, Martin IK, Carey MF (1994a) Muscle metabolism during exercise and heat stress in trained men: effect of acclimation. J Appl Physiol 76(2):589–597. https://doi.org/10.1152/jappl.1994.76.2.589
Febbraio MA, Snow RJ, Stathis CG, Hargreaves M, Carey MF (1994b) Effect of heat stress on muscle energy metabolism during exercise. J Appl Physiol 77(6):2827–2831. https://doi.org/10.1152/jappl.1994.77.6.2827
Febbraio MA, Carey MF, Snow RJ, Stathis CG, Hargreaves M (1996) Influence of elevated muscle temperature on metabolism during intense, dynamic exercise. Am J Physiol 271(5):R1251–R1255. https://doi.org/10.1152/ajpregu.1996.271.5.R1251
Febbraio MA, Lambert DL, Starkie RL, Proietto J, Hargreaves M (1998) Effect of epinephrine on muscle glycogenolysis during exercise in trained men. J Appl Physiol 84(2):465–470. https://doi.org/10.1152/jappl.1998.84.2.465
Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK (2002a) Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol 544(3):957–962. https://doi.org/10.1113/jphysiol.2002.025148
Febbraio MA, Steensberg A, Walsh R, Koukoulas I, Van Hall G, Saltin BP, Klarlund B (2002b) Reduced glycogen availability is associated with an elevation in HSP72 in contracting human skeletal muscle. J Physiol 538(3):911–917. https://doi.org/10.1113/jphysiol.2001.013145
Fehrenbach E, Northoff H, Niess AM, Voelker K, Mooren FC (2005) Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med 26(7):552–557. https://doi.org/10.1055/s-2004-830334
Fernández-Elías VE, Hamouti N, Ortega JF, Mora-Rodríguez R (2015) Hyperthermia, but not muscle water deficit, increases glycogen use during intense exercise. Scand J Med Sci Sports 25(Suppl 1):126–134. https://doi.org/10.1111/sms.12368
Fleshner M, Johnson JD (2005) Endogenous extra-cellular heat shock protein 72: releasing signal(s) and function. Int J Hyperth 21(5):457–471. https://doi.org/10.1080/02656730500088211
Fleshner M, Campisi J, Johnson JD (2003) Can exercise stress facilitate innate immunity? A functional role for stress-induced extracellular Hsp72. Exerc Immunol Rev 9:6–24
Flouris AD, Webb P, Kenny GP (2015) Noninvasive assessment of muscle temperature during rest, exercise, and postexercise recovery in different environments. J Appl Physiol 118(10):1310–1320. https://doi.org/10.1152/japplphysiol.00932.2014
Gagge AP, Stolwijk JA, Hardy JD (1967) Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res 1(1):1–20. https://doi.org/10.1016/0013-9351(67)90002-3
Gibson OR, Dennis A, Parfitt T, Watt PW, Maxwell NS, Taylor L (2014) Extracellular Hsp72 concentration relates to a minimum endogenous criteria during acute exercise-heat exposure. Cell Stress Chaperones 19(3):389–400. https://doi.org/10.1007/s12192-013-0468-1
González-Alonso J, Calbet JA, Nielsen B (1999) Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans. J Physiol. https://doi.org/10.1111/j.1469-7793.1999.00577.x
Hargreaves M, Angus D, Howlett K, Conus NM, Febbraio M (1996a) Effect of heat stress on glucose kinetics during exercise. J Appl Physiol 81(4):1594–1597. https://doi.org/10.1152/jappl.1996.81.4.1594
Hargreaves M, Dillo P, Angus D, Febbraio M (1996b) Effect of fluid ingestion on muscle metabolism during prolonged exercise. J Appl Physiol 80(1):363–366. https://doi.org/10.1152/jappl.1996.80.1.363
Hawley JA, Leckey JJ (2015) Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med 45(Suppl 1):5–12. https://doi.org/10.1007/s40279-015-0400-1
Heneka MT, Gavrilyuk V, Landreth GE, O’Banion MK, Weinberg G, Feinstein DL (2003) Noradrenergic depletion increases inflammatory responses in brain: effects on IκB and HSP70 expression. J Neurochem 85(2):387–398. https://doi.org/10.1046/j.1471-4159.2003.01694.x
Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, Chung J, Watson N, Gardner T, Lee-Young RS, Connor T, Watt MJ, Carpenter K, Hargreaves M, McGee SL, Hevener AL, Febbraio MA (2014) Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 63(6):1881–1894. https://doi.org/10.2337/db13-0967
Impey SG, Hearris MA, Hammond KM, Louis J, Close GL, Morton JP, Bartlett JD (2018) Fuel for the work required: a theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med 48(5):1031–1048. https://doi.org/10.1007/s40279-018-0867-7
Jentjens RLPG, Wagenmakers AJM, Jeukendrup AE (2002) Heat stress increases muscle glycogen use but reduces the oxidation of ingested carbohydrates during exercise. J Appl Physiol 92(4):1562–1572. https://doi.org/10.1152/japplphysiol.00482.2001
Jeukendrup AE, Wallis GA (2005) Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 26:S28–S37
Jeukendrup AE, Saris WH, Wagenmakers AJ (1998) Fat metabolism during exercise: a review. Part I: fatty acid mobilization and muscle metabolism. Int J Sports Med 19(4):231–244. https://doi.org/10.1055/s-2007-971911
Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Fleshner M (2005) Adrenergic receptors mediate stress-induced elevations in extracellular Hsp72. J Appl Physiol 99(5):1789–1795. https://doi.org/10.1152/japplphysiol.00390.2005
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol (1985) 92(5):2177–2186. https://doi.org/10.1152/japplphysiol.01267.2001
Li GC, Weitzel G, Mivechi NF (1995) Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperth 11(4):459–488. https://doi.org/10.3109/02656739509022483
Liu RY, Li X, Li L, Li GC (1992) Expression of human hsp70 in rat fibroblasts enhances cell survival and facilitates recovery from translational and transcriptional inhibition following heat shocks. Can Res 52(13):3667–3673
Liu Y, Mayr S, Opitz-Gress A, Zeller C, Lormes W, Baur S, Lehmann M, Steinacker JM (1999) Human skeletal muscle HSP70 response to training in highly trained rowers. J Appl Physiol 86(1):101–104. https://doi.org/10.1152/jappl.1999.86.1.101
Locke M (1997) The cellular stress response to exercise: role of stress proteins. Exerc Sport Sci Rev 25:105–136
Lorenzo S, Halliwill JR, Sawka MN, Minson CT (2010) Heat acclimation improves exercise performance. J Appl Physiol 109(4):1140–1147
Lucía A, Hoyos J, Pérez M, Chicharro JL (2000) Heart rate and performance parameters in elite cyclists: a longitudinal study. Med Sci Sports Exerc 32(10):1777–1782
Magalhães FDC, Oliveira EM, Passos RLF, Fonseca MA, Oliveira KPM, Lima MRM, Guimarães JB, Ferreira-Júnior JB, Martini ARP, Lima NRV, Soares DD, Rodrigues LOC, Amorim FT (2010) Heat and exercise acclimation increases intracellular levels of Hsp72 and inhibits exercise-induced increase in intracellular and plasma Hsp72 in humans. Cell Stress Chaperones 15(6):885–895. https://doi.org/10.1007/s12192-010-0197-7
Marino FE, Mbambo Z, Kortekaas E, Wilson G, Lambert MI, Noakes TD, Dennis SC (2001) Influence of ambient temperature on plasma ammonia and lactate accumulation during prolonged submaximal and self-paced running. Eur J Appl Physiol 86(1):71–78. https://doi.org/10.1007/s004210100506
Marshall HC, Ferguson RA, Nimmo MA (2006) Human resting extracellular heat shock protein 72 concentration decreases during the initial adaptation to exercise in a hot, humid environment. Cell Stress Chaperones 11(2):129–134. https://doi.org/10.1379/csc-158r.1
Maunder E, Plews DJ, Merien F, Kilding AE (2020) Exercise intensity regulates the effect of heat stress on substrate oxidation rates during exercise. Eur J Sport Sci 20(7):935–943. https://doi.org/10.1080/17461391.2019.1674928
Maunder E, Plews DJ, Merien F, Kilding AE (2021a) Stability of heart rate at physiological thresholds between temperate and heat stress environments in endurance-trained males. Int J Sports Physiol Perform 16(8):1204–1207. https://doi.org/10.1123/ijspp.2020-0351
Maunder E, Plews DJ, Wallis GA, Brick MJ, Leigh WB, Chang WL, Watkins CM, Kilding AE (2021b) Temperate performance and metabolic adaptations following endurance training performed under environmental heat stress. Physiol Rep 9(9):1–14. https://doi.org/10.14814/phy2.14849
Morton JP, MacLaren DP, Cable NT, Bongers T, Griffiths RD, Campbell IT, Evans L, Kayani A, McArdle A, Drust B (2006) Time-course and differential expression of the major heat shock protein families in human skeletal muscle following acute non-damaging treadmill exercise. J Appl Physiol 101:176–182. https://doi.org/10.1152/japplphysiol.00046.2006
Morton JP, Holloway K, Woods P, Cable NT, Burniston J, Evans L, Kayani AC, McArdle A (2009) Exercise training-induced gender-specific heat shock protein adaptations in human skeletal muscle. Muscle Nerve 39(2):230–233. https://doi.org/10.1002/mus.21182
O’Brien M, Viguie C, Mazzeo R, Brooks G (1993) Carbohydrate dependence during marathon running. Med Sci Sports Exerc 25:1009–1017. https://doi.org/10.1249/00005768-199309000-00007
Périard JD, Ruell P, Caillaud C, Thompson MW (2012) Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones 17(3):375–383
Philp A, Hargreaves M, Baar K (2012) More than a store: regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am J Physiol Endocrinol Metab 302(11):E1343–E1351. https://doi.org/10.1152/ajpendo.00004.2012
Powers SK, Howley ET, Cox R (1985) Blood lactate concentrations during submaximal work under differing environmental conditions. J Sports Med Phys Fitness 25(3):84–89
Rahman MM, Govindarajulu Z (1997) A modification of the test of Shapiro and Wilk for normality. J Appl Stat 24(2):219–236. https://doi.org/10.1080/02664769723828
Ravanelli N, Imbeault P, Jay O (2020) Steady-state sweating during exercise is determined by the evaporative requirement for heat balance independently of absolute core and skin temperatures. J Physiol 598(13):2607–2619. https://doi.org/10.1113/JP279447
Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR (1993) Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. https://doi.org/10.1152/ajpendo.1993.265.3.e380
Silva RPM, Espindola FS, Penha-Silva N, Barros CLM, Mendes TT, Garcia ES, de Abreu LC, Garner DM, Valenti VE (2019) The influence of a hot environment on physiological stress responses in exercise until exhaustion. PLoS One. https://doi.org/10.1371/journal.pone.0209510
Skidmore R, Gutierrez JA, Guerriero V, Kregel KC (1995) HSP70 induction during exercise and heat stress in rats: role of internal temperature. Am J Physiol. https://doi.org/10.1152/ajpregu.1995.268.1.r92
Trinity JD, Pahnke MD, Lee JF, Coyle EF (2010) Interaction of hyperthermia and heart rate on stroke volume during prolonged exercise. J Appl Physiol 109(3):745–751. https://doi.org/10.1152/japplphysiol.00377.2010
van Beaumont W, Greenleaf JE, Juhos L (1972) Disproportional changes in hematocrit, plasma volume, and proteins during exercise and bed rest. J Appl Physiol 33(1):55–61. https://doi.org/10.1152/jappl.1972.33.1.55
Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA (2001) Exercise increases serum Hsp72 in humans. Cell Stress Chaperones 6(4):386–393
Watt MJ, Heigenhauser GJF, Dyck DJ, Spriet LL (2002) Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J Physiol 541(3):969–978. https://doi.org/10.1113/jphysiol.2002.018820
Welch WJ, Suhan JP (1986) Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol 103(5):2035–2052
Whitham M, Fortes MB (2008) Heat shock protein 72: release and biological significance during exercise. Front Biosci 13(4):1328–1339
Whitham M, Walker GJ, Bishop NC (2006) Effect of caffeine supplementation on the extracellular heat shock protein 72 response to exercise. J Appl Physiol 101(4):1222–1227. https://doi.org/10.1152/japplphysiol.00409.2006
Whitham M, Walsh NP, Laing SJ, Jackson A, Maassen N (2007) Effect of exercise with and without a thermal clamp on the plasma heat shock protein 72 response. J Appl Physiol 103(4):1251–1256. https://doi.org/10.1152/japplphysiol.00484.2007
Wilson DF, Erecińska M, Drown C, Silver IA (1977) Effect of oxygen tension on cellular energetics. Am J Physiol 233(5):C135-140
Yamada PM, Amorim FT, Robergs R, Schneider SM, Moseley P (2007) Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol 103(4):1196–1204. https://doi.org/10.1152/japplphysiol.00242.2007
Young AJ, Sawka MN, Levine L, Cadarette BS, Pandolf KB (1985) Skeletal muscle metabolism during exercise is influenced by heat acclimation. J Appl Physiol 59(6):1929–1935. https://doi.org/10.1152/jappl.1985.59.6.1929
Young JC, Hoogenraad NJ, Hartl FU (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112(1):41–50. https://doi.org/10.1016/s0092-8674(02)01250-3
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No sources of funding were used in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
TC, EM, and AEK conceived and designed the research. TC and EM conducted experiments and collected the data. TC and EM analysed the data. TC and EM drafted the manuscript. All authors read, revised, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests associated with this manuscript.
Ethical approval
The Auckland University of Technology Ethics Committee approved all procedures (21/121).
Consent to participate
All participants provided written informed consent.
Additional information
Communicated by Narihiko Kondo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Charoensap, T., Kilding, A.E. & Maunder, E. Carbohydrate, but not fat, oxidation is reduced during moderate-intensity exercise performed in 33 vs. 18 °C at matched heart rates. Eur J Appl Physiol 123, 2073–2085 (2023). https://doi.org/10.1007/s00421-023-05225-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05225-0