Abstract
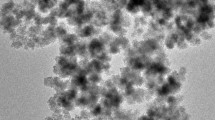
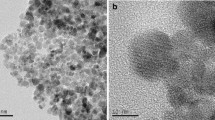
Carboxymethyl chitosan (CMCS)-conjugated magnetite (Fe3O4) nanoparticles (MNPs), which are denoted as CMCS-MNPs, were synthesized by covalently binding CMCS onto the surface of the MNPs via carbodiimide activation in a paraffin-acetic acid medium. The CMCS-MNPs exhibited a high level of CMCS binding (∼24.7 wt.%) and a spherical morphology with a mean diameter of 15 nm. In particular, they showed good water dispersity and a strong magnetic response. The sorption of Pb(II) on the CMCS-MNPs in aqueous solutions at different sorbent dosages (C s), pH, electrolyte (NaNO3) concentrations (C NaNO3), and temperatures (T) was investigated. The CMCS-MNPs showed high sorption capacity for Pb(II). The equilibrium amount increased with increasing pH but decreased with increasing C NaNO3 or T. In addition, a significant C s-effect was observed in the sorption equilibria. Two C s-dependent models, the Langmuir-SCA and Freundlich-SCA isotherms that were derived from a surface component activity (SCA) model, could describe the C s-effect observed. The changes in pH, C NaNO3, and T have no obvious influence on the C s-effect. In addition, the changes in the thermodynamic parameters, ∆G°, ∆H°, and ∆S°, for sorption were estimated, showing that the sorption process is spontaneous and exothermic.

Carboxymethyl chitosan (CMCS)-conjugated magnetite nanoparticles (MNPs), denoted as CMCS-MNPs, were synthesized. The CMCS-MNPs showed high sorption capacity for Pb(II). The sorbent effect (C s-effect) observed in the case of Pb (II) sorption on CMCS-MNPs could be described by the Langmuir-SCA and Freundlich-SCA isotherms, which were derived from a surface component activity (SCA) model.








Similar content being viewed by others
References
Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, Von Gunten U, Wehrli B (2006) The challenge of micropollutants in aquatic systems. Science 313:1072–1077
Ngah WSW, Teong LC, Hanafiah M (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83:1446–1456
Reddy DHK, Lee S-M (2013) Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv Colloid Interface Sci 201:68–93
Nguyen TAH, Ngo HH, Guo WS, Zhang J, Liang S, Yue QY, Li Q, Nguyen TV (2013) Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour Technol 148:574–585
Li Y-H, Wang S, Wei J, Zhang X, Xu C, Luan Z, Wu D, Wei B (2002) Lead adsorption on carbon nanotubes. Chem Phys Lett 357:263–266
Zhao G, Ren X, Gao X, Tan X, Li J, Chen C, Huang Y, Wang X (2011) Removal of Pb (II) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalton Trans 40:10945–10952
Madadrang CJ, Kim HY, Gao G, Wang N, Zhu J, Feng H, Gorring M, Kasner ML, Hou S (2012) Adsorption behavior of EDTA-graphene oxide for Pb (II) removal. ACS Appl Mater Interfaces 4:1186–1193
Sheindorf CH, Rebhun M, Sheintuch M (1981) A Freundlich-type multicomponent isotherm. J Colloid Interface Sci 79:136–142
Pan G, Liss PS (1998) Metastable-equilibrium adsorption theory: I. Theoretical. J Colloid Interface Sci 201:71–76
O’Connor DJ, Connolly JP (1980) The effect of concentration of adsorbing solids on the partition coefficient. Water Res 14:1517–1523
Voice TC, Weber WJ (1985) Sorbent concentration effects in liquid/solid partitioning. Environ Sci Technol 19:789–796
Di Toro DM, Mahony JD, Kirchgraber PR, O’Byrne AL, Pasquale LR, Piccirilli DC (1986) Effects of nonreversibility, particle concentration, and ionic strength on heavy-metal sorption. Environ Sci Technol 20:55–61
Helmy AK, Ferreiro EA, De Bussetti SG (2000) Effect of particle association on 2, 2′-bipyridyl adsorption onto kaolinite. J Colloid Interface Sci 225:398–402
Chang TW, Wang MK (2002) Assessment of sorbent/water ratio effect on adsorption using dimensional analysis and batch experiments. Chemosphere 48:419–426
Fehse K-U, Borg H, Sorkau E, Pilchowski K, Luckner L (2010) Correcting the effect of the sorbent to solution ratio on sorption isotherms from batch tests with soils and sediments. Water Air Soil Pollut 210:211–220
Guo Y, Hou W, Liang J, Liu J (2014) Sorbent concentration effect on adsorption of methyl orange on chitosan beads in aqueous solutions. Chem Res Chin Univ 30(5):837–843
Zhang F, Du N, Li H, Song S, Hou W (2015) Sorbent effect on the sorption of Cr (VI) on a Mg6AlFe-layered double hydroxide and its calcined product in aqueous solutions. Colloid Polym Sci 293:1961–1969
Zhang F, Du N, Song S, Hou W (2015) Mechano-hydrothermal synthesis of SDS intercalated LDH nanohybrids and their removal efficiency for 2,4-dichlorophenoxyacetic acid from aqueous solution. Mater Chem Phys 152:95–103
Zhang F, Song Y, Song S, Zhang R, Hou W (2015) Synthesis of magnetite–graphene oxide-layered double hydroxide composites and applications for the removal of Pb (II) and 2, 4-dichlorophenoxyacetic acid from aqueous solutions. ACS Appl Mater Interfaces 7:7251–7263
Zhao L-X, Hou W-G (2012) The effect of sorbent concentration on the partition coefficient of pollutants between aqueous and particulate phases. Colloids Surf A Physicochem Eng Asp 396:29–34
Zhao L-X, Song S-E, Du N, Hou W-G (2013) A sorbent concentration-dependent Freundlich isotherm. Colloid Polym Sci 291:541–550
Zhao L-X, Song S-E, Du N, Hou W-G (2012) A sorbent concentration-dependent Langmuir isotherm. Acta Phys-Chim Sin 28:2905–2910
Chang Y-C, Chen D-H (2005) Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu (II) ions. J Colloid Interface Sci 283:446–45
Chang YC, Chen DH (2005) Adsorption kinetics and thermodynamics of acid dyes on a carboxymethylated chitosan–conjugated magnetic nano–adsorbent. Macromol Biosci 5:254–261
Li G-y, Huang K-l, Jiang Y-r, Ding P, Yang D-l (2008) Preparation and characterization of carboxyl functionalization of chitosan derivative magnetic nanoparticles. Biochem Eng J 40:408–414
Kuang S-P, Wang Z-Z, Liu J, Wu Z-C (2013) Preparation of triethylene-tetramine grafted magnetic chitosan for adsorption of Pb (II) ion from aqueous solutions. J Hazard Mater 260:210–219
Mi F-L, Wu S-J, Chen Y-C (2015) Combination of carboxymethyl chitosan-coated magnetic nanoparticles and chitosan-citrate complex gel beads as a novel magnetic adsorbent. Carbohydr Polym 131:255–263
Nasirimoghaddam S, Zeinali S, Sabbaghi S (2015) Chitosan coated magnetic nanoparticles as nano-adsorbent for efficient removal of mercury contents from industrial aqueous and oily samples. J Ind Eng Chem 27:79–87
Muzzarelli RAA (2011) Potential of chitin/chitosan-bearing materials for uranium recovery: an interdisciplinary review. Carbohydr Polym 84:54–63
Hu X-j, Wang J-s, Liu Y-g, Li X, Zeng G-m, Bao Z-l, Zeng X-x, Chen A-w, Long F (2011) Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: isotherms, kinetics and thermodynamics. J Hazard Mater 185:306–314
Liao M-H, Chen D-H (2002) Preparation and characterization of a novel magnetic nano-adsorbent. J Mater Chem 12:3654–3659
Chen X-G, Park H-J (2003) Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr Polym 53:355–359
Yang J-H, Du Y-M, Qin C-Q (2003) Applications of infrared spectroscopy and nuclear magnetic resonance spectroscopy in the studies of the structure of chitin and chitosan. J Anal Sci 19:282–287
Guang G, Wu Z (2004) Structure and properties of carboxymethyl chitin. Polym Mater Sci Eng 20:107–110
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 21573133 and 21403128).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Research highlights:
1. Carboxymethyl chitosan-conjugated Fe3O4 nanoparticles, CMCS-MNPs, were synthesized.
2. CMCS-MNPs are an effective sorbent for the removal of Pb(II) from solution.
3. A sorbent effect (C s-effect) was observed for Pb(II) sorption on the CMCS-MNPs.
4. The C s-effect could be described using the Langmuir-SCA and Freundlich-SCA models.
5. The pH, electrolyte, and temperature had no obvious influence on the C s-effect.
6. Pb(II) sorption on the CMCS-MNPs is spontaneous and exothermic in nature.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 179 kb)
Rights and permissions
About this article
Cite this article
Lu, S., Li, H., Zhang, F. et al. Sorption of Pb(II) on carboxymethyl chitosan-conjugated magnetite nanoparticles: application of sorbent dosage-dependent isotherms. Colloid Polym Sci 294, 1369–1379 (2016). https://doi.org/10.1007/s00396-016-3893-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3893-8