Abstract
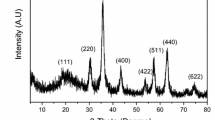
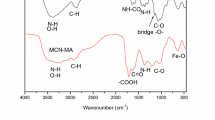
The polyethylenimine-functionalized magnetic chitosan nanoparticles (MCN-PEI) are synthesized and characterized by elemental analysis, TEM, FT-IR, XRD, etc. The MCN-PEI exhibits a core–shell structure, and had a saturation magnetization of 18.7 emu/g (which is high enough for fast magnetic separation). The maximum U(VI) sorption capacity for MCN-PEI reaches 134.6 mg/g at pH 5.0 and 298 K. The good-fitting of both sorption kinetics by pseudo-second-order model and sorption isotherms by Langmuir model indicates chemisorption mechanism. The thermodynamic parameters indicate the spontaneous and exothermic nature for U(VI) sorption. Finally, the magnetic nano-sorbents can be efficiently desorbed by acidified EDTA solution for regeneration.







Similar content being viewed by others
References
Zhang W, Jin C, Xiao D (2017) Treatment of low level radioactive waste liquid by air gap membrane distillation. J Nucl Radiochem 39:183–186
Parker BF, Zhang Z, Rao L, Arnold J (2018) An overview and recent progress in the chemistry of uranium extraction from seawater. Dalton Trans 47:639–644
Permogorov N (2016) Membrane technologies for water treatment: removal of toxic trace elements with emphasis on arsenic, fluoride and uranium. Johnson Matthey Technol Rev 60(2016):323–347
Quinn JE, Wilkins D, Soldenhoff KH (2013) Solvent extraction of uranium from saline leach liquors using DEHPA/alamine 336 mixed reagent. Hydrometallurgy 134:74–79
Davey PT, Scott T (1956) Adsorption of uranium on clay minerals. Nature 178:1195
Yang P, Post JE, Wang Q, Xu W, Geiss R, McCurdy PR, Zhu M (2019) Metal adsorption controls stability of layered manganese oxides. Environ Sci Technol 53:7453–7462
Kabay N, Demircioglu M, Yayh S, Günay E, Streat M (1998) Recovery of uranium from phosphoric acid solutions using chelating ion-exchange resins. Ind Eng Chem Res 37:1983–1990
Yuan D, Chen L, Xiong X, Yuan L, Liao S, Wang Y (2016) Removal of uranium(VI) from aqueous solution by amidoxime functionalized superparamagnetic polymer microspheresprepared by a controlled radical polymerization in the presence of DPE. Chem Eng J 285:358–367
Carboni M, Abney CW, Taylor-Pashow KM, Vivero-Escoto JL, Lin W (2013) Uranium sorption with functionalized mesoporous carbon materials. Ind Eng Chem Res 52:15187–15197
Zheng H, Zhou L, Liu Z, Le Z, Ouyang J, Huang G, Hamza S (2019) Functionalization of mesoporous Fe3O4@SiO2 nanospheres for highly efficient U(VI) adsorption. Micropor Mesopor Mater 279:316–322
Zhao Y, Wang X, Li J, Wang X (2015) Amidoxime functionalization of mesoporous silica and its high removal of U(VI). Polym Chem 6:5376–5384
Yan J, Wu T, Ding Z, Li X (2016) Preparation and characterization of carbon nanotubes/chitosan composite foam with enhanced elastic property. Carbohydr Polym 136:1288–1296
Huang Y, Lee X, Macazo FC, Grattieri M, Cai R, Minteer SD (2018) Fast and efficient removal of chromium(VI) anionic species by a reusable chitosan-modified multi-walled carbon nanotube composite. Chem Eng J 339:259–267
Zhou L, Shang C, Liu Z, Huang G, Adesina AA (2012) Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J Colloid Interface Sci 366:165–172
Hamza MF, Gamal A, Hussein G, Nagar MS, Abdel-Rahman AA, Wei Y, Guibal E (2019) Uranium(VI) and zirconium(IV) sorption on magnetic chitosan derivatives-effect of different functional groups on separation properties. J Chem Technol Biotechnol 94:3866–3882
Oshita K, Takayanagi T, Oshima M, Motomizu S (2007) Adsorption behavior of cationic and anionic species on chitosan resins possessing amino acid moieties. Anal Sci 23:1431–1434
Roosen J, Binnemans K (2014) Adsorption and chromatographic separation of rare earths with EDTA- and DTPA-functionalized chitosan biopolymers. J Mater Chem A 2:1530–1540
Ngah WSW, Teong LC, Hanafiah MA (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83:1446–1456
Wang JS, Peng RT, Yang JH, Liu YC, Hu XJ (2011) Preparation of ethylenediamine-modified magnetic chitosan complex for adsorption of uranyl ions. Carbohydr Polym 84:1169–1175
Liu H, Zhou Y, Yang Y, Zou K, Wu R, Xia K, Xie S (2019) Synthesis of polyethylenimine/graphene oxide for the adsorption of U(VI) from aqueous solution. Appl Surf Sci 471:88–95
Galhoum AA, Atia AA, Mahfouz MG, Abdel-Rehem ST, Gomaa NA, Vincent T (2015) Dy(III) recovery from dilute solutions using magnetic-chitosan nano-based particles grafted with amino acids. J Mater Sci 50:2832–2848
Das D, Sureshkumar MK, Koley S, Mithal N, Pillai CG (2010) Sorption of uranium on magnetite nanoparticles. J Radioanal Nucl Chem 285:447–454
Zhang X, Jiao C, Wang J, Liu Q, Li R, Yang P, Zhang M (2012) Removal of uranium(VI) from aqueous solutions by magnetic Schiff base: kinetic and thermodynamic investigation. Chem Eng J 198:412–419
Rahmati A, Ghaemi A, Samadfam M (2012) Kinetic and thermodynamic studies of uranium(VI) adsorption using Amberlite IRA-910 resin. Ann Nucl Energy 39:42–48
Wang H, Ma L, Cao K, Geng J, Liu J, Song Q, Yang X, Li S (2012) Selective solid phase extraction of uranium by salicylideneimine-functionalized hydrothermal carbon. J Hazard Mater 229:321–330
Hosoba M, Oshita K, Katarina RK, Takayanagi T, Oshima M, Motomizu S (2009) Synthesis of novel chitosan resin possessing histidine moiety and its application to the determination of trace silver by ICP-AES coupled with triplet automated-pretreatment system. Anal Chim Acta 639:51–56
Stopa LCB, Yamaura M (2010) Uranium removal by chitosan impregnated with magnetite nanoparticles: adsorption and desorption. Int J Nucl Energy Sci Technol 5:283–289
Zhou LM, Ouyang J, Hamza S, Le ZG, Li ZR, Adesoji AA (2018) Adsorption of U(VI) onto the carboxymethylated chitosan/Na-bentonite membranes: kinetic, isothermic and thermodynamic studies. J Radioanal Nucl Chem 317:1377–1385
Zhou LM, Ouyang J, Liu ZR, Huang G, Wang Y, Li ZG, Adesoji AA (2019) Highly efficient sorption of U(VI) from aqueous solution using amino/amine-functionalized magnetic mesoporous silica nanospheres. J Radioanal Nucl Chem 319:987–995
Zhao D, Wang X, Yang S, Guo Z, Sheng G (2012) Impact of water quality parameters on the sorption of U(VI) onto hematite. J Environ Radioact 103:20–29
Dimiropoulos V, Katsoyiannis I, Zouboulis A, Noli F, Simeonidis K, Mitrakas M (2015) Enhanced U(VI) removal from drinking water by nanostructured binary Fe/Mn oxy-hydroxides. J Water Proc Eng 7:227–236
Yusan S, Akyil S (2008) Sorption of uranium (VI) from aqueous solutions by akaganeite. J Hazard Mater 160:388–395
Van Horn JD, Huang H (2006) Uranium(VI) bio-coordination chemistry from biochemical, solution and protein structural data. Coord Chem Rev 250:765–775
Acknowledgements
The financial supports from National Natural Science Foundation (21667001; 21866002; 21866005; 21866006) and the Key Research and Development Program of Jiangxi Province (20192BBH80011) were acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, G., Zhou, L., Tang, X. et al. In situ formed magnetic chitosan nanoparticles functionalized with polyethylenimine for effective U(VI) sorption. J Radioanal Nucl Chem 325, 595–604 (2020). https://doi.org/10.1007/s10967-020-07230-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07230-5