Abstract
Background
In spite of the continuous progresses in pediatric neurosurgery, adamantinomatous craniopharyngioma (AC) remains a challenging tumor due to its proximity to optic pathways, pituitary gland, hypothalamus, and Willis’ circle, which can result in significant endocrine, cognitive, and neurological morbidity after treatment with subsequent impact on the patient’s quality of life (QoL). The relevance that QoL has today explains the changes in the management of AC observed over the time. The goal of the present article is to provide a historical background, to show the milestones in the changes of the AC treatment, and to analyze the current main options to manage such a challenging tumor.
Material and methods
The pertinent literature has been reviewed. Moreover, a comparison between the past and recent personal series is reported.
Results
Three main eras have been identified. The first (named Cushing era) was characterized by the need to realize a harmless surgery and to define the best way to approach AC; the second (microscope era) was characterized by a tremendous technical and technological development, with remarkable results in term of safe tumor resection and control but relatively poor QoL outcomes; and the third one (current period) is characterized by an increasing integration between surgery and adjuvant treatments, with relatively minor tumor control but significant improvement of QoL (comparable overall survival). The authors’ experience reflects these changes. Two groups of children were compared: 52 cases (mean follow-up: 17.5 years) belong to the historical series (group 1, 1985–2003, aggressive surgical management) and 41 (mean follow-up: 8.5 years) to the current one (Group 2, 2004–2021, integrated management). No significant differences between the two groups were detected about recurrence rate, surgical mortality, and overall survival. However, Group 2 showed significant lower rates of postoperative panhypopituitarism, obesity, and visual deterioration.
Conclusions
Radical surgery allows for a good AC control with a low rate of recurrence but high risk of permanent morbidity. Despite the greater number of recurrences and surgeries, the more conservative policy, based on a combination of treatments, seems to provide the same tumor control with a better QoL. The advances in trans-nasal and trans-ventricular endoscopy, in proton therapy and in the management of the AC cyst are the main factors that allowed such an improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adamantinomatous craniopharyngioma (AC) is a rare brain tumor classified by the World Health Organization as a tumor with low-grade malignancy, arising from remnants of the craniopharyngeal duct epithelium [1]. It accounts for 1.53–2.92/100,000 cases/year in children under 15 years and for 5–10% of sellar tumors in the pediatric population, representing 1.2–4% of all childhood intracranial tumors [2]. In spite of the “benign” pathological appearance, AC is a challenging disease because of the clinical and surgical implications (involvement of the optic-hypothalamic region and the Willis’ circle), thus rising a great interest among neurosurgeons since the birth of neurosurgery. Surgery still is the main treatment tool, cystic aspiration, intracystic chemotherapy, and irradiation being alternative options in selected patients. The increasing understanding of the AC genetic basis hopefully will provide targets for medical therapy. Anyway, a long-term multidisciplinary management is required for the follow-up, aiming at managing the possible surgical complications, the hypothalamic morbidity, the visual deficits, and the cognitive and neuropsychological sequelae that can impair the patient’s quality of life.
In this paper, the evolution of the management of AC is analyzed with particular emphasis on the main changes occurred in the different eras and on the new therapeutic options.
Background
The reasons of the challenge
As known, Harvey Cushing was used to refer to craniopharyngiomas as “the most forbidding of the intracranial tumors” [3]. This statement is still shareable. Actually, due to its proximity to highly eloquent (optic pathways, pituitary gland, and stalk) or vital structures (hypothalamus, Willis’ circle), both the growth and the treatment of AC can result in severe neurological, visual, and/or endocrine sequelae that can impact on quality of life (QoL), especially in long-term survivors. Indeed, 35% of AC patients can show already at the diagnosis a combination of symptoms related to hypothalamic dysfunction (increased body weight in 12–19%, behavioral changes, altered circadian rhythms, sleep irregularities, and imbalanced regulation of the body temperature, thirst, heart rate, and blood pressure) [4]. Postoperatively, moreover, hypothalamic dysfunction (mainly, severe obesity) can occur in a high number of patients, thus representing the main comorbidity. Despite an adequate hormonal substitution therapy, weight gain can present as well and is frequently exacerbated by other factors that limit physical activity. Visual impairment (reduced visual acuity and visual field with bi-temporal hemianopsia) can be detected in up to 50% of cases at the diagnosis [4]. Neurological sequelae, on the other hand, are more frequently observed in the postoperative period, including hemiparesis, epilepsy, cranial nerve deficits, and manifestations of cerebrovascular disease. In spite of the mainly temporary occurrence of these sequelae, long-term neurological deficits are found in about 8% of patients, with up to 35% of cases in children harboring huge AC [5].
The matter is complicated by the fact that surgery is the main therapeutic option, but, especially if compared with the papillary variant of craniopharyngioma, the complete surgical excision of AC is very hard to be obtained because of the possible low age of the patient, the often huge tumor size, the multi-cystic components, the encasement or the dense adhesions with the neighboring structures, and the presence of calcific portions often stick to the optic chiasm and the carotid artery (Fig. 1). In addition, AC is particularly prone to recurrence, even after an apparently gross total resection (GTR) [6].
Giant AC in a 11-year-old boy. Note the large and enhancing cystic components in the T1 sequences after gadolinium administration (A, B, C), which extend into the anterior, middle, and posterior cranial fossa. Also a biventricular hydrocephalus is evident (A–E). The cyst fluid is hyperintense compared to CSF in FLAIR sequence (D). Note that the solid component is a minor part of the tumor (E, arrow) but involves the region of the Willis’ circle (F, asterisk)
Evolution of treatment options
The first operation for craniopharyngioma we are aware of was performed more than one Century ago by Halstead in Chicago on July 1909 [7]. The author was unaware that the tumor was not an “ordinary” pituitary adenoma, and during surgery, he detected a cyst containing cholesterol crystals. After the operation, the patient’s vision improved. Since that time, the treatment management of AC showed epochal changes, mainly resulting from the technical and technological development and, more recently, the analysis of the results. According to Castro-Dufourny, 4 main eras in AC management can be recognized: (1) prior to 1970: in this era, loupes/headlight were the tools used for the surgical operation, brain RT was based on the sixties rudimental techniques, and the outcome was measured by the analysis of the morbidity and mortality rates; (2) 1970–1990: in this next generation, the introduction of the microscope and microsurgical techniques gave a significant input to the surgical management of AC. 2D conformal RT opened the modern era of radiation treatment and the GOS (Glasgow Outcome Score) started a new type of outcome assessment; (3) 1990–2010: the modern era was enriched by important technical advances, such as the development of endoscopic skull base surgery, or new kind of treatments, like the intracystic therapies, while RT evolved into the 3D conformal treatments. The outcome was assessed by general outcome scales (e.g., Karnofsky scale); (4) present time: in the current time, finally, the maintenance of a good QoL has become a crucial goal of the treatment and a way to assess the outcome; therefore, it is evaluated by specific scales and by neuropsychological tests. The treatment is tailored according to the patients’ characteristics taking into account not only the effectiveness of the therapy but also its side effects (the protons therapy summarizes the possibility to maximize the efficacy of a treatment without increasing the complications) [8].
The evolution of the diagnostic tools (X-rays, ventriculography, and angiography in the early phases, CT scan and standard MRI afterwards, and refined MRI with functional studies other than augmented reality simulation programs in current times) went with the aforementioned changes in the management and are strictly related to them.
In the following paragraphs, the main shifts in the AC management are summarized according the main technical and technological advances.
The beginning and the Cushing’s experience
The first description of AC was probably provided by Erdheim in 1904, a pathologist from the General Hospital of Vienna, who performed an autopsy on a woman who died from cardiac insufficiency [9]. The author found a cyst on the anterior surface of the pituitary gland, whose wall was lined by squamous epithelium, different from the Rathke’s pouch. Therefore, the author concluded that the origin of this cyst was from vestiges of not involuted hypophyseal–pharyngeal duct and named it as “hypophyseal duct tumor.” In 1932, Cushing first used the term “craniopharyngioma”: ‘‘This admittedly somewhat cumbersome term has been employed, for want of something more brief to include the kaleidoscopic tumors, solid, and cystic, which take their origin from epithelial rests ascribable to an imperfect closure of the hypophysial or craniopharyngeal duct [10]. The Cushing’s series accounted for 124 tumors with the characteristics of craniopharyngioma. He performed 198 surgical operations in the patients of his series who underwent a neurosurgical treatment, with a 23% mortality rate calculated within the first 2 months from surgery. Also the other experiences in the same period were characterized by high mortality rates, ranging from 29 to 41% [11,12,13,14]. The Cushing’s career on craniopharyngioma surgery can be divided in 3 main periods which anticipate the need to progressively develop the management of such a challenging tumor (Table 1): early period, characterized by the use of the transsphenoidal approach and a limited drainage of the cystic component of the tumor; intermediate period, in which the subfrontal approach was adopted to attempt a maximal tumor removal; and late period, characterized by the use of air ventriculography for topographical diagnosis and limited tumor resection via transventricular approach. In spite of the highest rates of radical tumor resection and the good outcome related to the subfrontal approach period, Cushing was not completely satisfied about that. Actually, postoperative symptoms related to hypothalamic dysfunction occurred in 53% of cases. The removal of AC with third ventricle invasion was associated with the highest rates of hypothalamic injury. Among the complications, indeed, severe hypothalamic disturbances (malignant hyperthermia, impairment of consciousness, severe electrolyte imbalances) occurred in 18% of cases as consequence of traumatic or ischemic injury of the hypothalamus [15]. Cushing realized the importance of preserving the pituitary stalk and the hypothalamus. His legacy was received by the development of microsurgery or, more recently, by the extended transsphenoidal approaches [16, 17].
In summary, this era showed that AC is a challenging tumor whose main treatment is the surgical excision. In this period, the main efforts were devoted in favoring anatomical studies and refining the surgical techniques to find the best surgical way to approach such a complex tumor. The final goal was to reduce the excessively high rates of surgical mortality and morbidity.
The changing management of AC
The “modern” eras are characterized by the advent of new concepts on neurosurgical techniques and new diagnostic and surgical technological tools that significantly contributed to improve the management of AC. Such a pathway has been covered together with the parallel development of medical and radiation therapies.
It is worth noting that the beginning of the microscope era coincided with the birth of the International Society for Pediatric Neurosurgery (ISPN). As a result, large part of evolution and changes in the AC management were due to ISPN members.
The microscope era: “The more the better”
With the introduction of the operating microscope, a new management strategy started to be adopted in many centers: the GTR of the tumor. Thanks to the operating microscope, the wall of AC was more safely separable from the surrounding vessels and the chiasm, thus reducing the risk of ischemic complications [18, 19]. Due to the contemporary improvements in the anesthesiology and intensive care management, GTR in childhood became more and more possible without significant surgical mortality and with a limited neurological morbidity. At the same time, the development of reliable substitutive drugs provided a further impulse in enlarging the surgical limits thanks to the possibility to support adequately the patients with postoperative hormone imbalance. The histologically benign features of AC, finally, theoretically made it an ideal target for curative radical surgical resection, namely with the goal of preserving the visual function and field [17, 19,20,21,22]. In 1969, Matson et al. reported on series of 57 patients where GTR was obtained in 77% of cases: the authors emphasized that the ideal treatment of AC in children should consist of making every efforts to achieve GTR at the first operation, followed by conscientious regular endocrine and neurological evaluation [15]. Hoffman et al. “took to extremes” this surgical strategy by achieving a 90% GTR in their 50 patients and a SR in the remaining 10% [20]. The authors had one surgical death (2%). Most of postoperative morbidity occurred after the second or third operation (among them, weight gain, hyperosmolality, panhyipopituitarism, severe and prolonged convulsion, water intoxication occurred). These reoperations were necessary because, in spite of the high rate of GTR, 34% of AC recurred after a 32.6-month mean period. Among 46 patients with complete follow-up, 60% had a normal or nearly normal life.
The magnification due to the operating microscope, the resectability of AC and the fear for a possible recurrence led to consider GTR so mandatory that even the voluntary excision of the pituitary stalk was justified to avoid AC remnants [23]. It is worth noting that such an “aggressive” policy did not result only by the need to obtain GTR but also from the evidence of a very high rate of children with endocrine dysfunction already at the diagnosis [24]. The progresses in the neuroimaging techniques other than in microsurgery increased the recognition of the appropriate surgical approaches, thus enhancing the surgeon’s ability to attain GTR in AC and explaining the peak of enthusiasm that craniopharyngioma surgery reached at the beginning of 1990s. Such an enthusiasm was justified by the significant drop of the tumor-related mortality (up to 50% at 10 years before the aggressive strategy) and the tumor recurrence (up to 50% in some series) [17, 20, 21, 25,26,27,28,29,30,31,32,33,34,35,36,37].
Unexpectedly, even the endoscopic techniques started to evolve in this rich historical period. AC was actually considered ideal to be reached through the transsphenoidal approach since the beginning. However, the first surgical approaches to the pituitary region were extensive and disfiguring; other than burdened by a significant morbidity [38]. The introduction of transcranial microsurgery gave a significant input also to the evolution of transsphenoidal surgery, favoring more precise, narrow, and effective approaches. Starting from the 1960s, several technical and technological tools were introduced to enhance the transsphenoidal route: Dott and Guiot started using lighted nasal speculums and intraoperative image intensification and fluoroscopy and Hardy introduced the binocular microscope [39]. Finally, Apuzzo and coworkers, in 1977, described the use of a side-viewing endoscope to provide access to angles hidden from the microsurgical approach [40]. These advances forewarn the endoscopic renaissance (both ventricular and endonasal) that will characterize the next era.
In the meanwhile, similar progresses were being obtained in radiotherapy techniques. From 1970s, the linear accelerator started replacing the 60Co radiation therapy (RT), and due to the diffusion of CT scan, the calculation of the radiation field became more precise. Both these advances allowed the clinicians to evaluate the tumor response to RT more precisely. Therefore, it was evident that external-beam photon RT was effective in attenuating tumor growth and preventing recurrences but, at the same time, also the damages resulting from the wide irradiated brain area were evident, especially in children. Already in 1961, Kramer et al. published a report on the role of limited surgery followed by RT in 6 children with AC treated at the Royal Marsden Hospital between 1952 and 1954 (combined surgery and external beam radiation therapy delivering 5500 Roentgen over 6 weeks) [41]. RT was delivered along the sella turcica and at level of the calcifications beyond the sella, based on X-rays. All patients were alive and free from recurrence after 6 years. During the following years, the series undergoing conservative surgery and adjuvant RT at the same institution progressively enlarged [36, 42, 43]. More recent series (between 1980 and 2000) confirmed the good control of the disease, with a progression free survival ranging from 79 to 84% [29, 32, 44,45,46]. It is not surprising, therefore, to find data supporting the reliability of partial resection of AC plus adjuvant RT compared with GTR in the era of the maximal surgical resection strategy. Nevertheless, since RT was not so developed to result curative at that time, to make any efforts to remove AC at the first operation was the main suggestion given by experienced neurosurgeons [47].
In summary, this era was characterized by a stimulating research on the way to maximize the surgical excision and the overall survival of patients with AC (Table 2) [17, 20, 27, 28, 34, 36, 48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88]. Actually, the tumor recurrence/regrowth appeared as the worst complication in the management of this tumor while, at the same time, the concept of caring the QoL was not so developed yet (e.g., note the high rate of not available data on obesity in Table 2). As a result, the technical and technological progresses (not only in neurosurgery but also in the general care of patients), which made surgery safer and more effective, allowed to push more and more ahead the limits of surgery, in spite of the risk of postoperative complications. However, always during this period, the improvement of the adjuvant therapies and the introduction of the evaluation of the functional outcomes laid the foundations for changes characterizing the next era.
How to bright the dark side of moon: the surgical pendulum era
The search for GTR showed the “dark side of the moon” of AC surgery with the recognition of too high rates of complications and relatively high rates of recurrence even after an apparently GTR [28, 35, 108]. Subsequently, this era, which started at the beginning of the current century and still lasts, is characterized by the experimentation of new management solutions and different combination of treatments, the main goals of these treatment being to preserve the patient’s QoL. Indeed, the developing age requires efforts to safeguard the physical, sexual, and psychomotor development of children affected by AC. Therefore, strategies favoring limited surgical excision of AC followed by RT or options encompassing temporary measures to postpone the surgical operation (e.g., use of intracystic drugs) have been more and more used during this time period. Right now, just at the end of this well-balanced era, there is certain trend to go back to a prevalently surgical management of AC (probably because of the improvement in endoscopic endonasal surgery). The most important and probably most recent acquisition of this time period is to consider and manage AC as a chronic disease, providing solutions not only to minimize the treatment complications but also for long-term clinical and neuropsychological interventions [109]. Actually, the survival analysis in large series, demonstrated that, unlikely most brain tumors, the extent of resection does not critically affect the overall survival in AC [110]. The Kraniopharygeom 2007 project can be mentioned as a summary of this period. Such a German project, indeed, had/has the goal to study AC from 2007 in all its main features to provide information and increase the knowledge on the outcome [111] and about very specific aspects, like cerebral infarction [112] or cardiac remodeling in AC [113] or pregnancy after childhood onset AC [114].
Among the several centers that emphasized the need to bright the dark side of the moon, the Catholic University’s group in Rome and the Necker’s group in Paris published focused articles on this subject. In his personal communications, done during the previous era and often criticized at that time, Di Rocco first started questioning the need to obtain GTR at any cost in AC. Actually, several subsequent studies, carried out with his group, clearly demonstrated that surgery for AC was feasible and GTR could be obtained without a significant risk of mortality and permanent neurolgoical morbidity, but the weight of the hypothalamic dysfunction was not more acceptable according to the modern standard of QoL [5, 53, 91]. These studies showed the changes in the management policy of the Rome group over the time, shifting from an aggressive surgical management to a less aggressive approach tailored on the patient’s characteristics: GTR in AC with poor hypothalamic involvement and old children/adolescents, subtotal or even partial resection followed by RT in case of hypothalamic involvement, and use of intracystic interferon-alpha to postpone surgery and RT as much as possible in young children. In the same period, Sainte-Rose and the Necker’s group, by reviewing their 20-year long experience, found that QoL of AC patients correlates with the degree of hypothalamic damage evident on the postoperative MRI [76, 115]. The authors proposed a multimodal treatment based on the possibility to predict the hypothalamic damage according to the degree of hypothalamic involvement on preoperative MRI. According to the Puget’s classification, such an involvement was classified in 3 types: type 0: no involvement of hypothalamus (GTR is suitable); type 1: compression but not invasion of the hypothalamus (GTR can be still attempted); and type 2: hypthalamus invasion (limited surgical excision followed by RT) [75]. Although questioned because of the poor correlation between MRI and intraoperative possibility to detect a hypothalamic invasion, this classification suggested the need to let the surgical pendulum swing from an aggressive to a conservative management. The most important argument against the conservative management came from the increase of tumor recurrences (often 100%) so that, since the beginning of the new era, the need of a personalized approach to AC was emphasized [61]. As showed by Boop in the Saint-Jude’s experience, a personalized approach, including conservative surgery (aimed at decompressing the optic pathways by leaving intact the arachnoid plane for a possible second look surgery) and conformal RT was the best solution to preserve both the tumor control and the QoL [116].
A great support to such a swinging of the pendulum was related to the advances in neuroimaging and conformal RT that led to a continuous refinement of the techniques to localize the tumor and limit the dose of radiation delivered, making more suitable the goal to reduce neurocognitive and endocrinological damages and to improve the patients’ QoL. The benefit in reducing all the RT complications (visual deterioration, optic neuropathies, vasculopathies, and Moyamoya disease, RT-related secondary tumors) was evident [117]. Moreover, the possibility to achieve the same long-term control of the disease than GTR resulted crucial to move several centers to prefer the partial/subtotal resection plus RT option [110, 118]. Yang and colleagues published a systematic review comparing the efficacy of different treatment options in more than 400 patients with craniopharyngiomas [110]. GTR was achieved in 58% of cases, subtotal resection (ST) in 23%, and ST and RT in 19%. The 5- and 10-year overall survival rates of GTR and STR+RT did not differ (98% versus 95%) as well as the progression free survival (67% versus 69%). As an evolution, more and more focused RT variants were explored to preserve cognition and improve patients’ QoL. Gamma knife radiosurgery (GKR) was realized to maximize the RT doses deliverable to the tumor by limiting those involving the surrounding tissue (namely, the optic pathways). GKR consists of the stereotactic administration of a single dose of 12–30 Gy or two to five fractionated doses, in case of near proximity with the optic pathways. Losa et al. proposed hypofractionated GKR to deliver high doses to the tumor and to reduce the risk of damaging the optic ways [119]. Ogino et al. found that the tumor control can be maximized, by reducing the risk of complication at the same time, when > 85% of AC receives at least 12 Gy [120]. More in details, the study included 53 patients undergoing a single-session stereotactic radiosurgery for recurrent or residual AC, when ≥ 85% of the tumor received ≥ 12 Gy, the tumor control rates at 3-, 5-, and 10-year were 100%, 93.3%, and 93.3%, respectively.
A further, relevant evolution of RT was represented by intensity-modulated radiation therapy (IMRT), once again designed to spare surrounding structures from high doses of radiation and toxicity (merchant 2022). In the management of AC, IMRT showed no significant difference in PFS (65.8% at 5 years) and OS (96% at 5 years) rates compared with 2D and non-IMRT 3D RT techniques, the solid component demonstrating a better response than the cystic part [121,122,123].
The most recent and promising evolution of RT is the proton-beam therapy (PBT), which allows the patient to receive a greater dose distribution with reduced risk of injury of the surrounding, normal tissues taking advantage of the different radiation physics of the particle beams and the different deposition of the radiation dose in the tissues. The final result is the reduction of brain irradiation, especially on the temporal lobes [104]. PBT is administered by means of charged particles with mass which concentrate the dose on the target with only a small amount of energy being delivered along the particle path. Merchant et al. provided a large analysis of long-term outcome in a series of 94 children with AC undergoing PBT [100] who were compared with their historical series of 101 pediatric patients treated by photon RT [99]. Taking into account the limitations of the heterogeneous protocols characterizing the historical series, the authors found similar results between their two series as far as survival outcomes and severe complications (endocrine deficits, vasculopathy, neurological, or visual deficits) were concerned. However, a significant decrease in neurocognitive dysfunction was detected compared to conventional radiotherapy. The still limited availability and the high costs are the main limits of PBT [104].
The concept of maximal safe resection plus RT and the concomitant advent of endoscopic transshpenoidal surgery (which seemed to be more promising than transcranial surgery about the safety of resection) raised a great debate on the use of the transcranial microsurgical approach (TMA) versus the endoscopic endonasal transsphenoidal approach (EETA) [89, 124]. Such a debate was really common during this era. Initially, it was in favor of TMA because EETA was limited to sellar or suprasellar, infradiaphragmatic AC, preferably in patients with a well-pneumatized sphenoid sinus and enlargement of the pituitary fossa [124]. Afterwards, EETA, which was more and more diffused and technically improved, gained a relevant consent because of some obvious advantages (direct access to the tumor, no brain manipulation) [125, 126]. At present, finally, it has been realized that these two approaches are both effective but should be performed in different population of children, alone (EETA in patients with mainly sellar/suprasellar midline AC while TMA in patients with large and laterally extending AC) or even in combination (huge AC) [6, 89].
As mentioned, EETA could have contributed to a certain renaissance of the surgical excision of AC in the current times. Actually, EETA was initially dedicated to the skull base lesions in adults and became very popular thanks to the absence of brain retraction, the faster recovery and the shorter hospital stay, the absence of a visible surgical scar, and the direct endoscopic visualization (close to the tumor and possible also on hidden angles) compared with TMA [127,128,129,130,131,132]. The major limitations of EETA, which are represented by tumors with lateral and superior extensions, limited possibility to repair directly microvascular damages and CSF leakage have been overcome by the introduction of extended approaches, the learning curve, and the specific reconstruction techniques, respectively [16, 133, 134]. A recent multicentric, Italian study analyzed the impact of EETA in adult and pediatric infradiaphragmatic craniopharyngiomas (84 patients treated between 2000 and 2021 in 6 different centers) [135]. A good rate of GTR (72%) was detected in case of tumor involving the region below the chiasmatic cistern together with a satisfactory visual outcome (improvement in 75%) confirming the safety and efficacy of this approach. GTR in tumors extending above the chiasmatic cistern was lower (54%) but visual function improved in the majority of cases even in this group (72%). The most common complication remained CSF leakage (14%). Just to remain on the recent literature, Cao Lei et al. reported about a very large series of 182 patients (both children and adults), treated at a single Institution, and compared EETA with TMA [136]. The EETA group showed higher GTR rate than TMA (97% vs 61% for intrasellar tumors and 93% vs 77% for suprasellar ones), lower incidence of new onset hypopituitarism (69% vs 32%), and better visual outcome. About the latter: in case of intrasellar AC, an improvement was detected in 60.3% of cases (36 patients with EETA and 5 with TMA) and a deterioration in 10.3% (3 patients with EETA and 4 with TMA); for suprasellar AC, an improvement occurred in 60.5% of cases (31 patients with EETA and 15 with TMA) while a deterioration in 14.5% (4 with EETA and 7 with TMA). No significant difference in the incidence of diabetes insipidus was found between the 2 groups (23–32%). The authors concluded that EETA should be considered as the first-line surgical modality for craniopharyngioma.
The theoretical limits of EETA in the pediatric population, manly related to the different anatomic conditions (e.g., small nostrils, poorly developed paranasal sinuses, growing bony structures) or to the lack of a dedicated surgical instruments have been overcome too thanks to the refinement of the technique and the technology so that EETA is more and more used to approach anterior skull base tumors in children or even those located in the third ventricle [97, 101, 137]. According to a personal experience comparing EETA with sub-labial microsurgery (31 children, 35% with AC), a significant difference between the two approaches was found about admission to ICU (35% vs 100%), blood transfusion (23% vs 71%), duration of hospital stay (4 vs 5.7 days), and pain perception scores (2.05±0.74 versus 2.92±0.91 in the early postoperative course, 0.82±0.95 versus 1.64±0.84 in the late one) [138]. No differences in term of early complications and functional or oncological outcome were found. Therefore, EETA resulted more advantageous than microsurgery as far as the trans-sphenoidal route is concerned. More recently, Mazzatenta et al. analyzed a series of 25 consecutive cases of pediatric AC treated by EETA, showing a very high rate of GTR (92%) and normalization/improvement of visual function (43%) [139]. On the other hand, complication as postoperative endocrine dysfunction and CSF leak remained as high as 92% and 24%, respectively. The recurrence rate was 19%. These figures demonstrated that EETA is a reliable approach in children too but also that, in spite of the effectiveness the technique, AC remains a challenging tumor as far as complication and risk of recurrence are concerned. Another implication of EETA concerns the reduced efficacy in case of tumor recurrence. Indeed, the possibility to achieve GTR is higher at the time of the first operation than at the time of the tumor recurrence [107] although such a difference may be not statistically significant [130].
A further contribute to the recent surgical “revival” comes from the evidence of long-term effects of RT on the neurocognitive development. Actually, recent studies did not show differences between GTR and partial resection (PR) plus RT as far as intellectual outcome and QoL scale scores were concerned, GRT being even superior in term of improvement in adaptive behavior and conceptual skills [140]. It is worth noting, however, that proton RT maintains the same neuro-psychological outcomes than GTR but with a minor rate of hypothalamic dysfunction [99, 141].
Overview of the current options for the management of the cyst
The AC-related cyst deserves a special mention for several reasons. First, AC is mainly characterized for the presence of a predominant cystic component (about 90% of cases) [142]. Second, the surgical excision of the whole cyst is often impossible because of the adherence of the cystic wall to vessels, optic ways, and third ventricle. Therefore, the risk of cyst recurrence remains a challenge. Third, RT is effective on the solid part of AC while it shows an unpredictable but usually minor effect on the cystic component. Fourth, the often rapid growth of the cystic part may cause problems during RT due to the need to modify accordingly the irradiation field [96]. Fifth, fortunately, the cystic component may benefit from dedicated treatments. Most of these treatments were designed during the “surgery” era for unresectable or recurrent AC but are more extensively adopted during the current era to reduce the impact of surgery and RT [143].
Cyst aspiration
The intracystic positioning of an Ommaya reservoir is one of the most common techniques, used for a cyst aspiration and/or to deliver drugs into the cyst itself. The placement of a catheter inside the cyst can be performed under direct vision, or under stereotactic or ultrasounds or ventriculoscopic or fluoroscopic guidance, as well as with stereotactic endoscopic technique [144, 145]. Currently, the navigated endoscopic transventricular approach is the most commonly used technique [146]. This approach, indeed, is mini-invasive (neuroendoscopy) and reliable (neuronavigation); moreover, it allows the surgeon to visualize directly the position of the catheter across the cyst and to perform additional surgical maneuvers (tumor biopsy, cyst aspiration, third ventriculostomy, septostomy), if required.
The fluid aspiration is the most “basic” approach to the cyst, being realized simply through an intracystic catheter connected to a subcutaneous reservoir (Rickham or Ommaya). It is used to obtain a quick tumor decompression and to re-aspirate fluid in case of cyst recurrence. As expected, this technique is a transient measure to be adopted to gain time for a more radical treatment in quickly growing AC or in case of poorly clinical condition. However, the cyst aspiration alone has been proved to be effective even in the long-term period. Actually, Moussa et al. reported that up to 70% of their patients did not show a cyst recurrence and did not need further treatments in a 7-year follow-up [147]. Only 19% of patients required a repeated procedure every 6 months and 8% of patients received further treatments.
In case of partially solid or giant AC, the cyst aspiration may be used as part of multimodal treatment involving TMA or EETA. The cyst drainage, indeed, can facilitate the surgical tumor removal by reducing the tumor volume and, therefore, can reduce the risk of complication [148,149,150]. Similarly, the fluid subtraction favors RT by removing a not radiosensitive component of the tumor and by reducing the irradiation field. Finally, such multimodal management may involve neuroendoscopy both for fluid aspiration and for tumor removal in case of AC mainly involving the third ventricle [151].
Beta-emitting radionuclides
Thanks to their penetration width of 3–4 mm, the β-radiations are effective in damaging the cyst wall so that different β-emitting sources (Yttrium90, Rhenium186, Aurum198, or Phosphorous32) have been proposed to manage cystic AC. Phosphorus32 (P-32) is the most used one because if offers longer half-life, lower required dose and lower half-value tissue penetrance than other radionuclides [98]. The treatment with P-32 alone can induce a cyst reduction in up to 70–80% of cases in the short-term period, but it is burdened by a high rate of recurrence in the long-term one [152,153,154]. On the other hand, if used in combination with cyst aspiration, P-32 is able to stop the AC growth in to 75% of cases and to maintain the result for some years (mean follow-up reported: 48.6 months) [155]. In a recent series of 32 patients treated by P-32 for recurrent craniopharyngioma, Hu et al. identified 4 types of tumors according to the thickness of the cyst wall and the expression of vascular endothelial growth factor (VEGF) and VEGF receptor-2 (VEGFR-2) [156]. Thin cyst (type I and II) and expression of VEGFR-2 were predictive of a good response to P-32, whilst types III and IV (and no VEGFR-2 expression) were not.
The current use of radionuclides is limited by several factors, as their possible side effects, the complex protocols for their transport, handle and disposal, and their poor availability [157]. The most feared risk remains the spillover of the radionuclide into the cerebrospinal fluid, although its incidence and impact are hard to be assessed. According to one of the largest series (53 patients) published so far by Kickingereder et al., the risk of permanent complications of this treatment does not overcome that of other therapies, the permanent neurological and hormone deficits being observed in 4% and 2% of cases, respectively [94].
Bleomycin
First discovered by Umezawa in 1966 and launched in Japan by Kayaku in 1969, such a “natural” antibiotic (bleomycin is a glicopeptide secreted by Streptomyces Verticillus) was proved to be effective against several epithelial tumors [158]. Therefore, the squamous epithelium of AC cyst wall was considered an ideal target for bleomycin since the beginning: Kubo obtained good result against cultured craniopharyngioma cells already in 1971 [106] and Takahashi reported on the first clinical series (7 patients) in 1985 where bleomycin was observed to be effective on the cystic component of AC but not on the solid portion [159]. Since there, it was more and more used with different protocols varying according to the different centers.
Several studies demonstrate the effectiveness of bleomycin in the transient control of the AC-related cyst. Hukin et al. analyzing their large experience on pediatric cases from 1982 to 2003 found that intracystic bleomycin definitely allows to postpone surgery as much as possible and should be preferred to other more invasive treatment in selected patients [160]. Mottolese and coworkers compared a group of 36 patients treated by direct surgical approach alone with a group of 24 patients managed by intracystic bleomycin administration (with or without surgery) and observed a significantly lower rate of morbidity and mortality in the bleomycin group [72]. In spite of these good results and the large cumulative experience in the literature, there is not enough evidence yet to recommend bleomycin to manage AC because of the heterogeneity of the series, the lack of randomized trials and the risk of adverse events [102]. Although carried out in a small sample of patient (7 cases), the randomized controlled trial by Jiang et al. on the comparison of bleomycin and P-32 alone and in combination demonstrated the superiority of the combination treatment in shrinking the cyst [161]. However, the authors acknowledged an important limitation of this approach in term of endocrine imbalance and other severe adverse events (bilateral thalamic infarction in 22% of cases). The main side effects of bleomycin are fever (up to 70% of cases), headache, and nausea, followed by delayed toxic effects due to damage to neural and vascular structures (transient neurological deficits, ischemic vasculopathy, permanent optic pathway, and/or hypothalamic injury [162,163,164].
Interferon alpha
Interferon-alpha (IA) is a cytokine produced by macrophages and lymphocytes as response to viral infection which can have also an antitumoral activity because it promotes the differentiation of T cells into T-helper lymphocytes, it stimulates the proliferation on natural killer cells and macrophages, it provides the production of IL-1 and IA itself, and finally, it increases the expression of the MHC complex class 1 and surface antigens [165, 166]. As for bleomycin, the experience with IA started with skin epithelial tumors (squamous and basal cell carcinomas) that share the same origin with AC [167]. Its use on AC was favored not only by the epithelial origin of AC but also by the evidence of the predominant role of inflammation in the growth of AC-related cyst [168,169,170,171]. After the first, positive experience with the systemic (subcutaneous) administration of IA in reducing the AC growth, Cavalheiro et al. realized a trial on the intracystic use of IA, reporting on a complete response in about two thirds of children in a 1.7-year mean follow-up [145]. Other than the good initial results, the most important factor which contributed to the diffusion of IA was the occurrence of only mild adverse events (self-limiting headaches, fever, fatigue).
Apart from some minimal variants, the protocol for IA administration (given through an Ommaya reservoir and an intracystic catheter) is based on 3 million International Units (IU) of interferon-α-2a every other day for a total of 12 administrations per cycle (36 million IU), the cycle being repeated 1 month after the end of the previous one for 3 or more times [103]. The first multicenter study, provided by Cavalheiro et al. on 2010, recruited 60 pediatric patients from 2000 to 2009 and showed a tumor control in 47 patients (78.3%), with no mortality and very low morbidity rates [172]. More in details, 30% of children had minor side effects (like headache, fever, palpebral edema, fatigue, arthritis) that were easily controlled and disappeared after treatment. The follow-up ranged from 4 to 84 months: 13.3% of patients developed new endocrinological deficits, and the tumor control was obtained in 78.3% patients (13 children needed new surgery) with no differences in the disease course based on whether the patient had undergone previous treatments or not. The international multicenter cooperative study by Kilday et al., published in 2017 on 56 children (median age: 6.3 years), only 23% of whom received IA as a first treatment, showed the following results after 5.1-year median follow-up: (1) 75% of patients had a clinical or radiological progression during the follow-up even though 33% of them did not require any treatment for this progression, and (2) 41% of patients did not present adverse events [173]. The remaining children experienced influenza-like malaise (29%), headaches (18%), fatigue (13%), transient hyponatremia (2%), appetite loss (2%), and weight loss (2%). None of these symptoms was severe: (3) two cases of brain toxicity were registered (optic pathway edema and brain atrophy with hydrocephalus) due to the suspected spillover of IA in the subarachnoid spaces. Accordingly, the use of IA was encouraged to delay AC progression, thus postponing surgery or RT radiotherapy (which is crucial for children during the development age). Sometimes, IA is able to efface completely the cyst while, sometimes, just to shrink it (Fig. 2), in any case allowing to postpone surgery and to gain time for the child development. A possible limitation of IA is the thickening of the tumor wall after the treatment that can be often observed during surgery, which can make its removal more difficult (Fig. 3).
Pre-treatment T1 sagittal (A), axial (B), and coronal MRI (C) of AC in a 5-year-old girl. Note the relatively small, solid, sella portion, and the large suprasellar/third ventricle cystic component. The same sequences performed 2 years later (D, E, F), at the end of 3 cycles of IA, show a quite good control of the disease, with shrinkage of the cyst. The cyst wall remains thick and present a “rigid” appearance
Intraoperative view of the same case in Fig. 2 (the child was operated when she was 8 years old, 3 years after the IA treatment, because of initial tumor-regrowth). In spite of the subtraction of the cystic fluid (A, B, C), the tumor capsule remains somewhat fibrotic, thicker, and more difficult do detach from the surrounding structures, and it does not deflate after the cyst fluid removal (D, E, F). This makes the tumor excision more complicated (G, H, I)
The main limitation of the use of intracystic IA is the current unavailability of the drug. Actually, due to the update of international regulation on the use of drugs, such a precious tool has been recently withdrawn from the market. Therefore, the interest could be addressed to the administration of subcutaneous pegylated IA (PIA). Yeung et al. proposed a protocol based on 1–3 μg/kg/week of PIA for 2 years [174]. Goldman and coworkers recently reported on 23 children and young adults with unresectable craniopharyngioma who were treated by the aforementioned protocol for 108 weeks [175]. The patients were divided in two groups: the first one included patients previously treated with surgery while the second one was composed by patients who had received radiation therapy. 28.8% of patients of the first group showed a partial response to the treatment but none of the second group presented a radiographically significant response. The progression free-survival was 17.7 and 19.5 months, respectively. As expected, the systemic toxicity of PIA was higher than intracystic IA, although only grade 2 and 3 hematologic toxicities occurred (no grade 4 or 5).
Basic research
Under a biologically and molecular point of view, AC is a surprisingly rich tumor. The dysregulation of WNT/B-catenin pathway, necessary for the organ formation during the embryogenesis, for maintaining the stem cells in adulthood and controlling gene transcription and cell adhesion and migration, is typical of AC, being observed in 57–96% of cases [176]. Both cystic and solid components express various cytokines, chemokines, and inflammatory mediators which can be involved in cancer cells activities and play a promising role not only to explain the tumor development and progression but also to find possible therapeutic targets. For example, the preoperative high levels of α-defensins found in the fluid of AC could indicate a role of inflammation in stimulating the secretion of cyst fluid by the epithelial cells of the cyst wall, whilst the decrease of defensins in the postoperative period would account for the reduced fluid production and the cyst shrinkage [177]. Moreover, the treatment with IA has been found to reduce the defensins levels through an antitumoral effect on the squamous epithelial cells, an immuno-modulatory action on the recruitment of inflammatory cells, and an anti-angiogenic activity, further supporting the previous, inflammatory hypothesis [177]. Furthermore, AC was found also to present high immunoreactivity response to retinoic acid receptors (RARs), which have a role in cell maturation and differentiation and could be responsible, at least in part, of the recurrence of AC according to the expression of specific cathepsins (which are important for the cell turnover) [93, 105, 178]. More in details, according to Lubansu et al., recurrent AC show low levels of RAR-beta and high levels of RAR-gamma: AC with low RAR-beta express low levels of cathepsin D while AC with high RAR-gamma express high levels of cathepsin K [92]. Considering other factors and pathways, PD-1/PD-L1 expression, SHH signaling upregulation, BMP and MMP pathways, and BRAF mutations can represent potential goals for target treatment in AC [90, 179, 180], thus explaining the increasing number of trials on AC [181].
Actually, in the current era, which is more and more searching for target therapies, the results of the base research on AC are advocated to possibly develop new therapeutic agents (both intracystic and systemic) which could interfere with AC molecular pathways. As mentioned, the inflammatory mediators seem to be promising in AC since they are involved in cancer cell activities (tumor development and progression) and could be targeted for possible therapies. IL-6 pathway is an example of a pathway showing target drugs that have been successfully used in AC [182, 183]. Tocilizumab (an anti-IL6 monoclonal antibody) has been systemically administered to two patients with recurrent AC after cyst aspiration and RT [95, 184, 185]. The preliminary results showed that one patient had a tumor volume reduction and stability without side effects after a 7-month cycle and the other one had to interrupt the first cycle after 8 months because of disease progression. It is worth noting that, in this second patient, the tumor control was obtained afterwards by a combination of tocilizumab and bevacizumab (an anti-angiogenetic, anti-VEGF monoclonal antibody). Neutropenia is the main side effect of this treatment.
Further, potential targets could come from the analysis of the clonal mutations that drive the oncogenesis of craniopharyngioma [186, 187]. AC is characterized by alterations in the Wnt/b-catenin pathway, mainly involving the central regulatory gene CTNNB1, while papillary craniopharyngioma is mostly driven by the V600E mutation in the BRAF gene, which activates the mitogen-activated protein kinase (MAPK) signaling pathway [186,187,188,189]. These molecular changes could disclose potential targets for new therapeutic approaches to improve long term tumor control volume and to decrease the associated morbidity [190, 191]. Calvanese et al. reported two cases of adult patients with papillary craniopharyngioma who underwent anti-BRAF/MEK combined therapy as adjuvant (case 1) or neoadjuvant (case 2) treatment [192]. Case 1 underwent a SR through EETA (the tumor harbored the BRAF V600E mutation) and a subsequent adjuvant target therapy with Dobrafenib and Trametinib with a 40% reduction in tumor volume after 2 months and a 90% reduction after 5 months. Case 2 underwent a transventricular endoscopic biopsy and, afterwards, started the same combined therapy with a 80% reduction in tumor volume after 2 months and a 90% reduction after 4 months. Hopefully, similar treatments will be available also for AC.
Personal experience: results of the paradigm shift
All children with AC consecutively treated at our institution between January 1985 and July 2021 have been considered for the present report to briefly summarize the personal model shift in the management of such a challenging tumor.
Patients and methods
Only children with comprehensive preoperative and postoperative work-up and with complete, 2-year minimum follow-up were considered for the present study. Ninety-three children were therefore enrolled among 114 patients composing the whole AC series: 52 patients belonged to the “old era” (group 1: 1985–2003), where the goal of treatment was to achieve GTR, while 41 patients to the “new era” (group 2: 2004–2021), where a more conservative, multimodal approach was used including SR/PR, RT and intra-cystic IA administration. The statistical analysis was done by using the chi-squared test and by setting the significance with a p value < 0.05.
Group 1 consists of 33 boys and 19 girls, with a 9-year mean age at surgery (ranging from 20 months to 16 years). The main clinical findings were increased intracranial pressure signs/symptoms (62% of cases), growth retardation or low stature (44%), deterioration of visual acuity (25%), hemianopia or other visual filed restrictions (17%), asthenia and hypotension (21%), diabetes insipidus (19%), hypo/anorexia and weight loss (17%), and hyperphagia and weight (6%). Preoperative panhypopituitarism was detected in 10 cases (19%) and selected hormone deficits in 14 cases (27%). Neuroradiological work-up included CT scan and/or MRI: AC was mainly cystic in 24 cases (46%), mainly solid in 8 (16%), and solid-cystic in 20 (38%); calcifications were detected in 32 cases (62%). The purely intrasellar location was evident only in 3 cases (6%), while the remaining children harbored a sellar/suprasellar prechiasmatic AC (24 cases, 46%) or a sellar/suprasellar retrochiasmatic AC (14 cases, 27%) or a giant AC (11 cases, 21%). Obstructive hydrocephalus at the time of diagnosis was present in 17 cases (33%).
Group 2 is composed by 24 boys and 17 females, with a mean age at surgery of 8.5 years (range: 12 months–17 years). The main clinical manifestations were related to raised intracranial pressure in 61% of cases, growth retardation or low stature in 17%, asthenia and/or hypotension in 12%, diabetes insipidus, delayed sexual development, weight loss and weight gain in 7% each, visual disturbances (including reduction of visual acuity, cranial nerves deficits and strabismus, visual field restriction and nystagmus) in 85%, hemiparesis and gait ataxia in 9.5% each, behavioral disturbances in 5%, and dysregulation of the sleep rhythm in 2.5%. Preoperative laboratory investigation revealed a panhypopituitarism in 2 cases (5%) and partial hormone deficits in 17 patients (41%). All patients received both CT scan and MRI. A mainly cystic AC was evident in 9 cases (22%), a mainly solid one in 10 cases (24%) and a solid-cystic one in 22 (54%); calcifications were detected in 15 cases (37%). Considering the tumor location, AC was purely intrasellar in 4 patients (10%), sellar/suprasellar with prechiasmatic growth in 24 cases (58%), sellar/suprasellar with retrochiasmatic growth in 10 cases (24%), and giant in 3 cases (8%). Hydrocephalus was present in 16 cases (39%).
Results
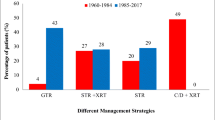
In group 1, surgery was realized through a single approach in 47 patients (90%) and a combined approach in the remaining 5 (10%). More in details, 41 patients underwent a TMA alone, 6 children were operated via transsphenoidal sublabial route, 4 children underwent a transsphenoidal approach followed by craniotomy and one child a craniotomy followed by a transsphenoidal approach. GTR was achieved in 40 cases (77%), SR in 17%, and PR in 6%. The associated hydrocephalus required a separate surgical operation in 7 out of 17 cases. Two surgery-related deaths (3.8%) occurred in this group, both following a transsphenoidal sublabial procedure: the first one as a consequence of a massive intra-operative bleeding in an attempt to remove a calcification on the lateral wall of the sella, the second one as a consequence of a gram-negative CSF infection complicated by cerebral venous sinus thrombosis. The most common mechanical complication was represented by postoperative subdural collection in 8 cases, requiring a drainage in 3 cases. Visual deficits improved in 40% of cases but worsened in 36%, and neurological deficits improved in 64% of cases (Table 3).
Nine children in group 1 presented a tumor recurrence/regrowth at variable time interval from the surgical treatment (17%). In 3 of them (6% of the whole group), a true recurrence after GTR occurred; in the remaining cases, it was about a re-growth of residual tumor left behind at operation. All the recurrences were treated surgically: GTR was achieved in five cases and SR/PR 4 cases. One postoperative death was recorded (surgical mortality for recurrence: 10%).
RT was administered postoperatively to 12 children of this group (23%); only one of them developed recurrence after the treatment. The tumor control was obtained in 92% of cases. One boy experienced the novel development of a malignant thalamic glioma 8 years after the completion of RT and required new surgery and chemotherapy.
The mean follow-up of this group was 17.5 years. After this period, 46 patients were alive (88%). Other than the 2 surgical mortalities, the causes of death were late post-RT malignancy (1 case), tumor progression (2 cases), and late systemic complications (1 case). The residual visual deficit did not prevent all but 3 patients from attending standard school. Two patients presented permanent hemiparesis, and 2 needed anti-epileptic drugs for postoperative epilepsy. Forty-three percent of patients required treatment for diabetes insipidus, 80% developed panhypopituitarism requiring hormone replacement, and 23% developed hypothalamic obesity (Table 4).
In group 2, 17 patients (41%) underwent surgical treatment by TMA and 24 (59%) by EETA. GTR was obtained in 17 patients (41%), SR in 15%, and partial in 12%. 32% of patients received the implantation of an intracystic catheter plus subcutaneous reservoir for cyst aspiration (in 3 cases) and to deliver IA (in the remaining 10 cases) (Fig. 4). Hydrocephalus had to be surgically addressed in 4 out of 16 affected children. No surgery-related deaths were recorded in this group. Nine patients developed a subdural hygroma; one of them required surgical drainage and another required a permanent subdural peritoneal shunt system. In the early period, visual deficits were improved in 54% of patients and worsened in 12%. Neurological deficits were improved in 85% of them (Table 3).
T1 MRI before (A) and after gadolinium administration (B) showing the presence of AC in a 7-year-old girl; C, D the same MRI sequences after navigation-guided placement of intracystic catheter and aspiration of motor-oil fluid, which allowed a significant reduction of the cyst with decompression of the surrounding structures; E, F CT scan performed at the last follow-up (4 years later and after 3 cycles of IA) showing the effacement of the cystic component with evidence of a small, calcified intrasellar, tumor solid portion
In group 2, 6 children showed a tumor recurrence after GTR (15%), while 20 children had a tumor re-growth (48%). Recurrences were treated surgically in a single procedure in 14 cases, in 2 steps in 6 cases, in 3 or more steps in 6 cases. No postoperative deaths occurred.
RT was administered postoperatively to 8 patients of this group (19.5%). Three of them developed recurrence after the treatment: therefore, the disease was controlled in 63% of them. Also, in this group, one patient developed a malignant thalamic glioma 10 years after the completion of RT and required radiation therapy and chemotherapy.
All but 3 patients (93%) are still alive after a 8.5-year mean follow-up. One patient died because of tumor progression, one patient because of complication of obesity surgery and one patient because of late RT-induced malignancy. Neurological and visual deficit improvement was demonstrated in up to 80% of patients compared with the diagnosis. Twenty-four percent of patients developed panhypopituitarism requiring hormone replacement, and 7% developed hypothalamic obesity (Table 4).
Summary
In synthesis, the personal experience confirms the advantages of the multimodal treatment in reducing the late sequelae of AC management. Indeed, the comparison between the two groups shows a statistically significant reduction of visual worsening and a relevant increase of neurological improvement during the modern era compared with the past one (Table 3). Moreover, both the occurrence of panhypopituitarism and obesity were significantly reduced in the postoperative period by the multimodal treatment (Table 4). These results were obtained without relevant differences between the two groups in terms of tumor recurrence and overall survival.
Conclusions
Both the historical excursus with the review of the literature and the personal experience suggest that radical surgery allows for a good AC control with a lower rate of recurrence, but it is burdened by relatively high risk of mortality and significant, permanent morbidity as far as visual, neurological and endocrine outcomes are concerned. For this reason, a more conservative policy has been recently adopted that, despite the greater number of recurrences and surgeries, seems to be safer both in terms of complications and mortality: the higher recurrence risk is balanced by better clinical conditions, preservation of endocrine functions, and minor development of hypothalamic obesity. In current times, where the QoL has gained more and more importance so that it is often considered by patients more relevant than the survival itself, the efforts made to enhance the multimodal management of AC seem to be the best choice to preserve QoL. Actually, the improvement in EETA and neuroendoscopy, in the proton RT and in the management of the cyst offers the pediatric neurosurgeon a great possibility to treat AC both with good oncological and functional outcomes: the key point is to use these therapeutic options together and not singularly. AC is still a challenging tumor and an ideal treatment is not yet available but, hopefully, the results coming from the basic research could enrich the multimodal treatment up to win such a challenge.
References
Prabhu VC, Brown HG (2005) The pathogenesis of craniopharyngiomas. Childs Nerv Syst 21(8–9):622–627
Wang K-C, Hong SH, Kim S-K, Cho B-K (2005) Origin of craniopharyngiomas: implication on the growth pattern. Childs Nerv Syst 21(8–9):628–634
Tritos NA (2015) Is there a role for targeted medical therapies in patients with craniopharyngiomas? Future Oncol 11(24):3221–3223
Castle-Kirszbaum M, Shi MDY, Goldschlager T (2022) Quality of life in craniopharyngioma: a systematic review. World Neurosurg 164:424-435.e2
Caldarelli M, Massimi L, Tamburrini G, Cappa M, Di Rocco C (2005) Long-term results of the surgical treatment of craniopharyngioma: the experience at the Policlinico Gemelli, Catholic University, Rome. Childs Nerv Syst 21(8–9):747–757
Bianchi F, Benato A, Massimi L (2022) Treatment of cystic craniopharyngiomas: an update. Adv Tech Stand Neurosurg 45:139–176
(2012) 1 History of Pituitary Surgery, 2012th ed. Endoscopic Pituitary Surgery. https://doi.org/10.1055/b-0034-82119
Castro-Dufourny I, Carrasco R, Prieto R, Barrios L, Pascual J-M (2017) The first sixty-five craniopharyngioma operations in France. Rev Neurol (Paris) 173(4):180–188
Lindholm J, Nielsen EH (2009) Craniopharyngioma: historical notes. Pituitary 12(4):352–359
Harvey Cushing: a journey through his life intracranial tumors, p. 46-47 Harvey Cushing: A Journey Through His Life Yale University Library Online Exhibitions. https://onlineexhibits.library.yale.edu/s/harvey-cushing/item/8017#?c=&m=&s=&cv=&xywh=-1447%2C0%2C5117%2C1654. Accessed 24 Jul 2023
Gordy PD, Peet MM, Kahn EA (1949) The surgery of the craniopharyngiomas. J Neurosurg 6(6):503–517
Grant FC (1948) Surgical experience with tumors of the pituitary gland. J Am Med Assoc 136(10):668–672
Love JG, Marshall TM (1950) Craniopharyngiomas. Surg Gynecol Obstet 90(5):591–601
Muller R, Wohlfart G (1950) Craniopharyngiomas. Acta Med Scand 138(2):121–138
Prieto R, Pascual JM, Barrios L (2018) Harvey Cushing’s craniopharyngioma treatment: Part 2. Surgical strategies and results of his pioneering series. J Neurosurg 131(3):964–978
Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM (2008) Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg 108(4):715–728
Yaşargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P (1990) Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg 73(1):3–11
Bergland R (1969) The arterial supply of the human optic chiasm. J Neurosurg 31(3):327–334
Hoffman HJ, Hendrick EB, Humphreys RP, Buncic JR, Armstrong DL, Jenkin RD (1977) Management of craniopharyngioma in children. J Neurosurg 47(2):218–227
Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SI (1992) Aggressive surgical management of craniopharyngiomas in children. J Neurosurg 76(1):47–52
Pierre-Kahn A, Brauner R, Renier D, Sainte-Rose C, Gangemi MA, Rappaport R, Hirsch JF (1988) Treatment of craniopharyngiomas in children. Retrospective analysis of 50 cases. Arch Fr Pediatr 45(3):163–167
Puget S (2012) Treatment strategies in childhood craniopharyngioma. Front Endocrinol (Lausanne) 3:64
Choux M, Lena G, Genitori L (1995) Craniopharyngioma in children: surgical considerations. In: Broggi G (ed) Craniopharyngioma. Springer Milan, Milano, pp 20–30
Lucas C, Benderitter T, Choux M (1982) Pre- and postoperative endocrine function in children with craniopharyngioma. Arch Fr Pediatr 39(5):303–307
Caldarelli M, di Rocco C, Papacci F, Colosimo C (1998) Management of recurrent craniopharyngioma. Acta Neurochir (Wien) 140(5):447–454
Carmel PW, Antunes JL, Chang CH (1982) Craniopharyngiomas in children. Neurosurgery 11(3):382–389
De Vile CJ, Grant DB, Kendall BE, Neville BG, Stanhope R, Watkins KE, Hayward RD (1996) Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted? J Neurosurg 85(1):73–81
Fischer EG, Welch K, Shillito J, Winston KR, Tarbell NJ (1990) Craniopharyngiomas in children. Long-term effects of conservative surgical procedures combined with radiation therapy. J Neurosurg 73(4):534–540
Hetelekidis S, Barnes PD, Tao ML, Fischer EG, Schneider L, Scott RM, Tarbell NJ (1993) 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys 27(2):189–195
McLone DG, Raimondi AJ, Naidich TP (1982) Craniopharyngiomas. Childs. Brain 9(3–4):188–200
Shapiro K, Till K, Grant DN (1979) Craniopharyngiomas in childhood. A rational approach to treatment. J Neurosurg 50(5):617–623
Sung DI (1982) Suprasellar tumors in children: a review of clinical manifestations and managements. Cancer 50(7):1420–1425
Till K (1982) Craniopharyngioma. Childs. Brain 9(3–4):179–187
Tomita T, McLone DG (1993) Radical resections of childhood craniopharyngiomas. Pediatr Neurosurg 19(1):6–14
Villani RM, Tomei G, Bello L, Sganzerla E, Ambrosi B, Re T, Giovanelli Barilari M (1997) Long-term results of treatment for craniopharyngioma in children. Childs Nerv Syst 13(7):397–405
Zuccaro G (2005) Radical resection of craniopharyngioma. Childs Nerv Syst 21(8–9):679–690
Zuccaro G, Jaimovich R, Mantese B, Monges J (1996) Complications in paediatric craniopharyngioma treatment. Childs Nerv Syst 12(7):385–390. discussion 390-391
Cavallo LM, Somma T, Solari D, Iannuzzo G, Frio F, Baiano C, Cappabianca P (2019) Endoscopic endonasal transsphenoidal surgery: history and evolution. World Neurosurg 127:686–694
Hardy J (2007) Transsphenoidal hypophysectomy. 1971. J Neurosurg 107(2):458–471
Apuzzo ML, Heifetz MD, Weiss MH, Kurze T (1977) Neurosurgical endoscopy using the side-viewing telescope. J Neurosurg 46(3):398–400
Kramer S, Mckissock W, Concannon JP (1961) Craniopharyngiomas. Treatment by combined surgery and radiation therapy. J Neurosurg 18:217–226
Bloom HJ, Glees J, Bell J, Ashley SE, Gorman C (1990) The treatment and long-term prognosis of children with intracranial tumors: a study of 610 cases, 1950–1981. Int J Radiat Oncol Biol Phys 18(4):723–745
Rajan B, Ashley S, Thomas DG, Marsh H, Britton J, Brada M (1997) Craniopharyngioma: improving outcome by early recognition and treatment of acute complications. Int J Radiat Oncol Biol Phys 37(3):517–521
Cabezudo JM, Vaquero J, Areitio E, Martinez R, de Sola RG, Bravo G (1981) Craniopharyngiomas: a critical approach to treatment. J Neurosurg 55(3):371–375
Rajan B, Ashley S, Gorman C, Jose CC, Horwich A, Bloom HJ, Marsh H, Brada M (1993) Craniopharyngioma–a long-term results following limited surgery and radiotherapy. Radiother Oncol 26(1):1–10
Thomsett MJ, Conte FA, Kaplan SL, Grumbach MM (1980) Endocrine and neurologic outcome in childhood craniopharyngioma: review of effect of treatment in 42 patients. J Pediatr 97(5):728–735
Tomita T (1988) Management of craniopharyngiomas in children. Pediatr Neurosci 14(4):204–211
Albright AL, Hadjipanayis CG, Lunsford LD, Kondziolka D, Pollack IF, Adelson PD (2005) Individualized treatment of pediatric craniopharyngiomas. Childs Nerv Syst 21(8–9):649–654
Bülow B, Attewell R, Hagmar L, Malmström P, Nordström CH, Erfurth EM (1998) Postoperative prognosis in craniopharyngioma with respect to cardiovascular mortality, survival, and tumor recurrence. J Clin Endocrinol Metab 83(11):3897–3904
Chen C, Okera S, Davies PE, Selva D, Crompton JL (2003) Craniopharyngioma: a review of long-term visual outcome. Clin Exp Ophthalmol 31(3):220–228
Colangelo M, Ambrosio A, Ambrosio C (1990) Neurological and behavioral sequelae following different approaches to craniopharyngioma. Long-term follow-up review and therapeutic guidelines. Childs Nerv Syst 6(7):379–382
Dhellemmes P, Vinchon M (2006) Radical resection for craniopharyngiomas in children: surgical technique and clinical results. J Pediatr Endocrinol Metab 19(Suppl 1):329–335
Di Rocco C, Caldarelli M, Tamburrini G, Massimi L (2006) Surgical management of craniopharyngiomas–experience with a pediatric series. J Pediatr Endocrinol Metab 19(Suppl 1):355–366
Elliott RE, Hsieh K, Hochm T, Belitskaya-Levy I, Wisoff J, Wisoff JH (2010) Efficacy and safety of radical resection of primary and recurrent craniopharyngiomas in 86 children. J Neurosurg Pediatr 5(1):30–48
Erşahin Y, Yurtseven T, Ozgiray E, Mutluer S (2005) Craniopharyngiomas in children: Turkey experience. Childs Nerv Syst 21(8–9):766–772
Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M (1999) Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg 90(2):237–250
Fisher PG, Jenab J, Gopldthwaite PT, Tihan T, Wharam MD, Foer DR, Burger PC (1998) Outcomes and failure patterns in childhood craniopharyngiomas. Childs Nerv Syst 14(10):558–563
Gonc EN, Yordam N, Ozon A, Alikasifoglu A, Kandemir N (2004) Endocrinological outcome of different treatment options in children with craniopharyngioma: a retrospective analysis of 66 cases. Pediatr Neurosurg 40(3):112–119
Gupta DK, Ojha BK, Sarkar C, Mahapatra AK, Mehta VS (2006) Recurrence in craniopharyngiomas: analysis of clinical and histological features. J Clin Neurosci 13(4):438–442
Hafez MA, ElMekkawy S, AbdelBadie H, Mohy M, Omar M (2006) Pediatric craniopharyngioma–rationale for multimodal management: the Egyptian experience. J Pediatr Endocrinol Metab 19(Suppl 1):371–380
Kalapurakal JA, Goldman S, Hsieh YC, Tomita T, Marymont MH (2003) Clinical outcome in children with craniopharyngioma treated with primary surgery and radiotherapy deferred until relapse. Med Pediatr Oncol 40(4):214–218
Karavitaki N, Brufani C, Warner JT, Adams CBT, Richards P, Ansorge O, Shine B, Turner HE, Wass JAH (2005) Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf) 62(4):397–409
Kawamata T, Amano K, Aihara Y, Kubo O, Hori T (2010) Optimal treatment strategy for craniopharyngiomas based on long-term functional outcomes of recent and past treatment modalities. Neurosurg Rev 33(1):71–81
Khafaga Y, Jenkin D, Kanaan I, Hassounah M, Al Shabanah M, Gray A (1998) Craniopharyngioma in children. Int J Radiat Oncol Biol Phys 42(3):601–606
Kim SK, Wang KC, Shin SH, Choe G, Chi JG, Cho BK (2001) Radical excision of pediatric craniopharyngioma: recurrence pattern and prognostic factors. Childs Nerv Syst 17(9):531–536. discussion 537
Lee Y-Y, Wong T-T, Fang Y-T, Chang K-P, Chen Y-W, Niu D-M (2008) Comparison of hypothalamopituitary axis dysfunction of intrasellar and third ventricular craniopharyngiomas in children. Brain Dev 30(3):189–194
Lena G, Paz Paredes A, Scavarda D, Giusiano B (2005) Craniopharyngioma in children: Marseille experience. Childs Nerv Syst 21(8–9):778–784
Maira G, Anile C, Rossi GF, Colosimo C (1995) Surgical treatment of craniopharyngiomas: an evaluation of the transsphenoidal and pterional approaches. Neurosurgery 36(4):715–724
Mark RJ, Lutge WR, Shimizu KT, Tran LM, Selch MT, Parker RG (1995) Craniopharyngioma: treatment in the CT and MR imaging era. Radiology 197(1):195–198
Merchant TE, Kiehna EN, Sanford RA, Mulhern RK, Thompson SJ, Wilson MW, Lustig RH, Kun LE (2002) Craniopharyngioma: the St. Jude Children’s Research Hospital experience 1984–2001. Int J Radiat Oncol Biol Phys 53(3):533–542
Minamida Y, Mikami T, Hashi K, Houkin K (2005) Surgical management of the recurrence and regrowth of craniopharyngiomas. J Neurosurg 103(2):224–232
Mottolese C, Szathmari A, Berlier P, Hermier M (2005) Craniopharyngiomas: our experience in Lyon. Childs Nerv Syst 21(8–9):790–798
Ohmori K, Collins J, Fukushima T (2007) Craniopharyngiomas in children. Pediatr Neurosurg 43(4):265–278
Pierre-Kahn A, Sainte-Rose C, Renier D (1994) Surgical approach to children with craniopharyngiomas and severely impaired vision: special considerations. Pediatr Neurosurg 21(Suppl 1):50–56
Puget S, Garnett M, Wray A et al (2007) Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg 106(1 Suppl):3–12
Sainte-Rose C, Puget S, Wray A, Zerah M, Grill J, Brauner R, Boddaert N, Pierre-Kahn A (2005) Craniopharyngioma: the pendulum of surgical management. Childs Nerv Syst 21(8–9):691–695
Samii M, Bini W (1991) Surgical treatment of craniopharyngiomas. Zentralbl Neurochir 52(1):17–23
Shi X-E, Wu B, Fan T, Zhou Z-Q, Zhang Y-L (2008) Craniopharyngioma: surgical experience of 309 cases in China. Clin Neurol Neurosurg 110(2):151–159
Shibuya M, Takayasu M, Suzuki Y, Saito K, Sugita K (1996) Bifrontal basal interhemispheric approach to craniopharyngioma resection with or without division of the anterior communicating artery. J Neurosurg 84(6):951–956
Shirane R, Hayashi T, Tominaga T (2005) Fronto-basal interhemispheric approach for craniopharyngiomas extending outside the suprasellar cistern. Childs Nerv Syst 21(8–9):669–678
Sosa IJ, Krieger MD, McComb JG (2005) Craniopharyngiomas of childhood: the CHLA experience. Childs Nerv Syst 21(8–9):785–789
Stripp DCH, Maity A, Janss AJ et al (2004) Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys 58(3):714–720
Symon L, Pell MF, Habib AH (1991) Radical excision of craniopharyngioma by the temporal route: a review of 50 patients. Br J Neurosurg 5(6):539–549
Thompson D, Phipps K, Hayward R (2005) Craniopharyngioma in childhood: our evidence-based approach to management. Childs Nerv Syst 21(8–9):660–668
Tomita T, Bowman RM (2005) Craniopharyngiomas in children: surgical experience at Children’s Memorial Hospital. Childs Nerv Syst 21(8–9):729–746
Van Effenterre R, Boch A-L (2002) Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg 97(1):3–11
Xu J, You C, Cai B, Jiang S, Sun H, Guo F, Yang Y, Wu B (2005) Microsurgical resection of craniopharyngioma of the third ventricle via an improved transventricular approach. Chin Med J (Engl) 118(10):806–811
Zhang YQ, Ma ZY, Wu ZB, Luo SQ, Wang ZC (2008) Radical resection of 202 pediatric craniopharyngiomas with special reference to the surgical approaches and hypothalamic protection. Pediatr Neurosurg 44(6):435–443
Elliott RE, Jane JA, Wisoff JH (2011) Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery 69(3):630–643. discussion 643
Wang C-H, Qi S-T, Fan J, Pan J, Peng J-X, Nie J, Bao Y, Liu Y-W, Zhang X, Liu Y (2019) Identification of tumor stem-like cells in admanatimomatous craniopharyngioma and determination of these cells’ pathological significance. J Neurosurg 1–11
Pancucci G, Massimi L, Caldarelli M, D’Angelo L, Sturiale C, Tamburrini G, Tufo T, Di Rocco C (2007) Pediatric craniopharyngioma: long-term results in 61 cases. Minerva Pediatr 59(3):219–231
Lubansu A, Ruchoux M-M, Brotchi J, Salmon I, Kiss R, Lefranc F (2003) Cathepsin B, D and K expression in adamantinomatous craniopharyngiomas relates to their levels of differentiation as determined by the patterns of retinoic acid receptor expression. Histopathology 43(6):563–572
Coury JR, Davis BN, Koumas CP, Manzano GS, Dehdashti AR (2020) Histopathological and molecular predictors of growth patterns and recurrence in craniopharyngiomas: a systematic review. Neurosurg Rev 43(1):41–48
Kickingereder P, Maarouf M, El Majdoub F et al (2012) Intracavitary brachytherapy using stereotactically applied phosphorus-32 colloid for treatment of cystic craniopharyngiomas in 53 patients. J Neurooncol 109(2):365–374
Jones G, Sebba A, Gu J et al (2010) Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 69(1):88–96
Freeman CR, Patrocinio H, Farmer J-P (2003) Highly conformal radiotherapy for craniopharyngioma: (potentially) throwing the baby out with the bathwater. Med Pediatr Oncol 40(5):340–341. author reply 341
de Divitiis E, Cappabianca P, Gangemi M, Cavallo LM (2000) The role of the endoscopic transsphenoidal approach in pediatric neurosurgery. Childs Nerv Syst 16(10–11):692–696
Julow JV (2013) Intracystic irradiation for craniopharyngiomas. Pituitary 16(1):34–45
Merchant TE, Edmonston DY, Wu S, Li Y, Boop FA, Lustig RH (2022) Endocrine outcomes after limited surgery and conformal photon radiation therapy for pediatric craniopharyngioma: Long-term results from the RT1 protocol. Neuro Oncol 24(12):2210–2220
Merchant TE, Hoehn ME, Khan RB et al (2023) Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): a single-arm, phase 2 study. Lancet Oncol 24(5):523–534
Tanoue Y, Morisako H, Sasaki T, Ikegami M, Goto T (2023) Endoscopic endonasal approach to remove pediatric intraventricular tumors of the third ventricle. Childs Nerv Syst. https://doi.org/10.1007/s00381-023-05989-y
Zhang S, Fang Y, Cai BW, Xu JG, You C (2016) Intracystic bleomycin for cystic craniopharyngiomas in children. Cochrane Database Syst Rev 7(7):CD008890
Dastoli PA, Nicácio JM, Silva NS, Capellano AM, Toledo SRC, Ierardi D, Cavalheiro S (2011) Cystic craniopharyngioma: intratumoral chemotherapy with alpha interferon. Arq Neuropsiquiatr 69(1):50–55
Grossman A, Kosmin M (2023) Craniopharyngiomas and proton beam therapy: worth the expense? Lancet Oncol 24(5):422–423
Gupta S, Bi WL, Giantini Larsen A, Al-Abdulmohsen S, Abedalthagafi M, Dunn IF (2018) Craniopharyngioma: a roadmap for scientific translation. Neurosurg Focus 44(6):E12
Kubo O, Takakura K, Miki Y, Okino T, Kitamura K (1974) Intracystic therapy of bleomycin for craniopharyngioma–effect of bleomycin for cultured craniopharyngioma cells and intracystic concentration of bleomycin (author’s transl). No Shinkei Geka 2(10):683–688
Yamada S, Fukuhara N, Yamaguchi-Okada M, Nishioka H, Takeshita A, Takeuchi Y, Inoshita N, Ito J (2018) Therapeutic outcomes of transsphenoidal surgery in pediatric patients with craniopharyngiomas: a single-center study. J Neurosurg Pediatr 21(6):549–562
Adamson TE, Wiestler OD, Kleihues P, Yaşargil MG (1990) Correlation of clinical and pathological features in surgically treated craniopharyngiomas. J Neurosurg 73(1):12–17
Müller HL (2020) The diagnosis and treatment of craniopharyngioma. Neuroendocrinology 110(9–10):753–766
Yang I, Sughrue ME, Rutkowski MJ, Kaur R, Ivan ME, Aranda D, Barani IJ, Parsa AT (2010) Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus 28(4):E5
Beckhaus J, Friedrich C, Boekhoff S, Calaminus G, Bison B, Eveslage M, Timmermann B, Flitsch J, Müller HL (2023) Outcome after pediatric craniopharyngioma: the role of age at diagnosis and hypothalamic damage. Eur J Endocrinol. https://doi.org/10.1093/ejendo/lvad027
Boekhoff S, Bison B, Genzel D, Eveslage M, Otte A, Friedrich C, Flitsch J, Müller HL (2021) Cerebral infarction in childhood-onset craniopharyngioma patients: results of KRANIOPHARYNGEOM 2007. Front Oncol 11:698150
Sowithayasakul P, Buschmann LK, Boekhoff S, Müller HL (2021) Cardiac remodeling in patients with childhood-onset craniopharyngioma-results of HIT-Endo and KRANIOPHARYNGEOM 2000/2007. Eur J Pediatr 180(5):1593–1602
Sowithayasakul P, Boekhoff S, Bison B, Müller HL (2021) Pregnancies after childhood craniopharyngioma: results of KRANIOPHARYNGEOM 2000/2007 and review of the literature. Neuroendocrinology 111(1–2):16–26
Puget S, Grill J, Habrand J-L, Sainte-Rose C (2006) Multimodal treatment of craniopharyngioma: defining a risk-adapted strategy. J Pediatr Endocrinol Metab 19(Suppl 1):367–370
Boop FA (2007) Craniopharyngioma. J Neurosurg 106(1 Suppl):1–2. discussion 2
Kiehna EN, Merchant TE (2010) Radiation therapy for pediatric craniopharyngioma. Neurosurg Focus 28(4):E10
Clark AJ, Cage TA, Aranda D, Parsa AT, Sun PP, Auguste KI, Gupta N (2013) A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Childs Nerv Syst 29(2):231–238
Losa M, Pieri V, Bailo M, Gagliardi F, Barzaghi LR, Gioia L, Del Vecchio A, Bolognesi A, Mortini P (2018) Single fraction and multisession Gamma Knife radiosurgery for craniopharyngioma. Pituitary 21(5):499–506
Ogino A, Niranjan A, Kano H, Flickinger JC, Lunsford LD (2021) Optimizing stereotactic radiosurgery in patients with recurrent or residual craniopharyngiomas. J Neurooncol 154(1):113–120
Bishop AJ, Greenfield B, Mahajan A et al (2014) Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys 90(2):354–361
Greenfield BJ, Okcu MF, Baxter PA, Chintagumpala M, Teh BS, Dauser RC, Su J, Desai SS, Paulino AC (2015) Long-term disease control and toxicity outcomes following surgery and intensity modulated radiation therapy (IMRT) in pediatric craniopharyngioma. Radiother Oncol 114(2):224–229
Klimo P, Venable GT, Boop FA, Merchant TE (2015) Recurrent craniopharyngioma after conformal radiation in children and the burden of treatment. J Neurosurg Pediatr 15(5):499–505
Reddy GD, Hansen D, Patel A, Lin Y, Jea A, Lam S (2016) Treatment options for pediatric craniopharyngioma. Surg Neurol Int 7(Suppl 6):S174-178
Chakrabarty A, Mitchell P, Bridges LR (1998) Craniopharyngioma invading the nasal and paranasal spaces, and presenting as nasal obstruction. Br J Neurosurg 12(4):361–363
de Divitiis E, Cappabianca P, Cavallo LM, Esposito F, de Divitiis O, Messina A (2007) Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery 61(5 Suppl 2):219–227. discussion 228
Cappabianca P, Alfieri A, de Divitiis E (1998) Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS). Minim Invasive Neurosurg 41(2):66–73
Cavallo LM, Frank G, Cappabianca P, Solari D, Mazzatenta D, Villa A, Zoli M, D’Enza AI, Esposito F, Pasquini E (2014) The endoscopic endonasal approach for the management of craniopharyngiomas: a series of 103 patients. J Neurosurg 121(1):100–113
Ciric I, Ragin A, Baumgartner C, Pierce D (1997) Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery 40(2):225–236. discussion 236-237
Dhandapani S, Singh H, Negm HM, Cohen S, Souweidane MM, Greenfield JP, Anand VK, Schwartz TH (2017) Endonasal endoscopic reoperation for residual or recurrent craniopharyngiomas. J Neurosurg 126(2):418–430
Jho HD, Carrau RL (1997) Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg 87(1):44–51
Tabaee A, Anand VK, Barrón Y, Hiltzik DH, Brown SM, Kacker A, Mazumdar M, Schwartz TH (2009) Endoscopic pituitary surgery: a systematic review and meta-analysis. J Neurosurg 111(3):545–554
Alalade AF, Ogando-Rivas E, Boatey J, Souweidane MM, Anand VK, Greenfield JP, Schwartz TH (2018) Suprasellar and recurrent pediatric craniopharyngiomas: expanding indications for the extended endoscopic transsphenoidal approach. J Neurosurg Pediatr 21(1):72–80
Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, Mintz A (2006) A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116(10):1882–1886
Solari D, d’Avella E, Agresta G et al (2023) Endoscopic endonasal approach for infradiaphragmatic craniopharyngiomas: a multicentric Italian study. J Neurosurg 138(2):522–532
Lei C, Chuzhong L, Chunhui L, Peng Z, Jiwei B, Xinsheng W, Yazhuo Z, Songbai G (2021) Approach selection and outcomes of craniopharyngioma resection: a single-institute study. Neurosurg Rev 44(3):1737–1746
Locatelli D, Massimi L, Rigante M, Custodi V, Paludetti G, Castelnuovo P, Di Rocco C (2010) Endoscopic endonasal transsphenoidal surgery for sellar tumors in children. Int J Pediatr Otorhinolaryngol 74(11):1298–1302
Massimi L, Rigante M, D’Angelo L, Paternoster G, Leonardi P, Paludetti G, Di Rocco C (2011) Quality of postoperative course in children: endoscopic endonasal surgery versus sublabial microsurgery. Acta Neurochir (Wien) 153(4):843–849
Mazzatenta D, Zoli M, Guaraldi F, Ambrosi F, Faustini Fustini M, Pasquini E, Asioli S, Zucchelli M (2020) Outcome of endoscopic endonasal surgery in pediatric craniopharyngiomas. World Neurosurg 134:e277–e288
Aldave G, Okcu MF, Chintagumpala M et al (2023) Comparison of neurocognitive and quality-of-life outcomes in pediatric craniopharyngioma patients treated with partial resection and radiotherapy versus gross-total resection only. J Neurosurg Pediatr 31(5):453–462
Kahalley LS, Douglas Ris M, Mahajan A et al (2019) Prospective, longitudinal comparison of neurocognitive change in pediatric brain tumor patients treated with proton radiotherapy versus surgery only. Neuro Oncol 21(6):809–818
Müller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera J-P, Puget S (2019) Craniopharyngioma. Nat Rev Dis Primers 5(1):75
Bartels U, Laperriere N, Bouffet E, Drake J (2012) Intracystic therapies for cystic craniopharyngioma in childhood. Front Endocrinol (Lausanne) 3:39
Alén JF, Boto GR, Lagares A, de la Lama A, Gómez PA, Lobato RD (2002) Intratumoural bleomycin as a treatment for recurrent cystic craniopharyngioma Case report and review of the literature. Neurocirugia (Astur) 13(6):479–485. discussion 485
Cavalheiro S, Dastoli PA, Silva NS, Toledo S, Lederman H, da Silva MC (2005) Use of interferon alpha in intratumoral chemotherapy for cystic craniopharyngioma. Childs Nerv Syst 21(8–9):719–724
Frio F, Solari D, Cavallo LM, Cappabianca P, Raverot G, Jouanneau E (2019) Ommaya reservoir system for the treatment of cystic craniopharyngiomas: surgical results in a series of 11 adult patients and review of the literature. World Neurosurg 132:e869–e877
Moussa AH, Kerasha AA, Mahmoud ME (2013) Surprising outcome of ommaya reservoir in treating cystic craniopharyngioma: a retrospective study. Br J Neurosurg 27(3):370–373
Jarebi M, Coutte A, Bartier F, Khormi Y, Peltier J, Lefranc M (2019) A novel, hybrid, stereotactic approach (radiosurgery and dual Ommaya reservoirs) for the treatment of mixed (polycystic and solid) craniopharyngioma. Stereotact Funct Neurosurg 97(4):266–271
Laville A, Coutte A, Capel C, Maroote J, Lefranc M (2020) Dosimetric and volumetric outcomes of combining cyst puncture through an Ommaya reservoir with index-optimized hypofractionated stereotactic radiotherapy in the treatment of craniopharyngioma. Clin Transl Radiat Oncol 23:66–71
Zhu W, Li X, He J, Sun T, Li C, Gong J (2017) A reformed surgical treatment modality for children with giant cystic craniopharyngioma. Childs Nerv Syst 33(9):1491–1500
Ndukuba K, Ogiwara T, Nakamura T, Abe D, Ichinose S, Horiuchi T, Ohaegbulam S, Hongo K (2020) Cyst fenestration and Ommaya reservoir placement in endoscopic transcortical transventricular approach for recurrent suprasellar cystic craniopharyngioma without ventriculomegaly. J Clin Neurosci 72:425–428
Ansari SF, Moore RJ, Boaz JC, Fulkerson DH (2016) Efficacy of phosphorus-32 brachytherapy without external-beam radiation for long-term tumor control in patients with craniopharyngioma. J Neurosurg Pediatr 17(4):439–445
Barriger RB, Chang A, Lo SS, Timmerman RD, DesRosiers C, Boaz JC, Fakiris AJ (2011) Phosphorus-32 therapy for cystic craniopharyngiomas. Radiother Oncol 98(2):207–212
Maarouf M, El Majdoub F, Fuetsch M, Hoevels M, Lehrke R, Berthold F, Voges J, Sturm V (2016) Stereotactic intracavitary brachytherapy with P-32 for cystic craniopharyngiomas in children. Strahlenther Onkol 192(3):157–165
Interstitial radiotherapy using phosphorus-32 for giant posterior fossa cystic craniopharyngiomas - PubMed. https://pubmed.ncbi.nlm.nih.gov/25679384/. Accessed 24 Jul 2023
Hu C, Chen J, Meng Y, Zhang J, Wang Y, Liu R, Yu X (2018) Phosphorus-32 interstitial radiotherapy for recurrent craniopharyngioma: expressions of vascular endothelial growth factor and its receptor-2 and imaging features of tumors are associated with tumor radiosensitivity. Medicine (Baltimore) 97(26):e11136
Chang H, Zhang J, Cao W, Wang Y, Zhao H, Liu R, Guo S (2018) Drug distribution and clinical safety in treating cystic craniopharyngiomas using intracavitary radiotherapy with phosphorus-32 colloid. Oncol Lett 15(4):4997–5003
Umezawa H, Maeda K, Takeuchi T, Okami Y (1966) New antibiotics, bleomycin A and B. J Antibiot (Tokyo) 19(5):200–209
Takahashi H, Nakazawa S, Shimura T (1985) Evaluation of postoperative intratumoral injection of bleomycin for craniopharyngioma in children. J Neurosurg 62(1):120–127
Hukin J, Visser J, Sargent M, Goddard K, Fryer C, Steinbok P (2005) Childhood craniopharyngioma: Vancouver experience. Childs Nerv Syst 21(8–9):758–765
Jiang R, Liu Z, Zhu C (2002) Preliminary exploration of the clinical effect of bleomycin on craniopharyngiomas. Stereotact Funct Neurosurg 78(2):84–94
Belen D, Er U, Yigitkanli K, Bolay H (2007) Delayed neurotoxic complication of intracavitary bleomycin therapy for craniopharyngioma in a child who had previously undergone radiosurgery. Case report. J Neurosurg 106(5 Suppl):391–393
Cho W-S, Kim S-K, Wang K-C, Phi JH, Cho B-K (2012) Vasculopathy after intracystic bleomycin administration for a recurrent cystic craniopharyngioma: case report. J Neurosurg Pediatr 9(4):394–399
Lafay-Cousin L, Bartels U, Raybaud C, Kulkarni AV, Guger S, Huang A, Bouffet E (2007) Neuroradiological findings of bleomycin leakage in cystic craniopharyngioma. Report of three cases. J Neurosurg 107(4 Suppl):318–323
Jonasch E, Haluska FG (2001) Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist 6(1):34–55
Taniguchi T, Ogasawara K, Takaoka A, Tanaka N (2001) IRF family of transcription factors as regulators of host defense. Annu Rev Immunol 19:623–655
Jakacki RI, Cohen BH, Jamison C et al (2000) Phase II evaluation of interferon-alpha-2a for progressive or recurrent craniopharyngiomas. J Neurosurg 92(2):255–260
Ierardi DF, Fernandes MJS, Silva IR, Thomazini-Gouveia J, Silva NS, Dastoli P, Toledo SRC, Cavalheiro S (2007) Apoptosis in alpha interferon (IFN-alpha) intratumoral chemotherapy for cystic craniopharyngiomas. Childs Nerv Syst 23(9):1041–1046
Jokonya L, Reid T, Kasambala M, Mduluza-Jokonya TL, Fieggen G, Mduluza T, Kalangu KKN, Naicker T (2020) Antimicrobial effects of craniopharyngioma cystic fluid. Childs Nerv Syst 36(11):2641–2646
Massimi L, Martelli C, Caldarelli M, Castagnola M, Desiderio C (2017) Proteomics in pediatric cystic craniopharyngioma. Brain Pathol 27(3):370–376
Zhou J, Zhang C, Pan J, Chen L, Qi S-T (2017) Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human adamantinomatous craniopharyngioma cells and promotes tumor cell migration. Mol Med Rep 15(6):4123–4131
Cavalheiro S, Di Rocco C, Valenzuela S et al (2010) Craniopharyngiomas: intratumoral chemotherapy with interferon-alpha: a multicenter preliminary study with 60 cases. Neurosurg Focus 28(4):E12
Kilday J-P, Caldarelli M, Massimi L et al (2017) Intracystic interferon-alpha in pediatric craniopharyngioma patients: an international multicenter assessment on behalf of SIOPE and ISPN. Neuro Oncol 19(10):1398–1407
Yeung JT, Pollack IF, Panigrahy A, Jakacki RI (2012) Pegylated interferon-α-2b for children with recurrent craniopharyngioma. J Neurosurg Pediatr 10(6):498–503
Goldman S, Pollack IF, Jakacki RI et al (2020) Phase II study of peginterferon alpha-2b for patients with unresectable or recurrent craniopharyngiomas: a Pediatric Brain Tumor Consortium report. Neuro Oncol 22(11):1696–1704
Hengartner AC, Prince E, Vijmasi T, Hankinson TC (2019) Adamantinomatous craniopharyngioma: moving toward targeted therapies. Neurosurg Focus 48(1):E7
Pettorini BL, Inzitari R, Massimi L, Tamburrini G, Caldarelli M, Fanali C, Cabras T, Messana I, Castagnola M, Di Rocco C (2010) The role of inflammation in the genesis of the cystic component of craniopharyngiomas. Childs Nerv Syst 26(12):1779–1784
Lefranc F, Chevalier C, Vinchon M et al (2003) Characterization of the levels of expression of retinoic acid receptors, galectin-3, macrophage migration inhibiting factor, and p53 in 51 adamantinomatous craniopharyngiomas. J Neurosurg 98(1):145–153
Coy S, Rashid R, Lin J-R et al (2018) Multiplexed immunofluorescence reveals potential PD-1/PD-L1 pathway vulnerabilities in craniopharyngioma. Neuro Oncol 20(8):1101–1112
Gump JM, Donson AM, Birks DK et al (2015) Identification of targets for rational pharmacological therapy in childhood craniopharyngioma. Acta Neuropathol Commun 3:30
Apps JR, Muller HL, Hankinson TC, Yock TI, Martinez-Barbera JP (2023) Contemporary biological insights and clinical management of craniopharyngioma. Endocr Rev 44(3):518–538
Grob S, Mirsky DM, Donson AM, Dahl N, Foreman NK, Hoffman LM, Hankinson TC, Mulcahy Levy JM (2019) Targeting IL-6 is a potential treatment for primary cystic craniopharyngioma. Front Oncol 9:791
Whelan R, Prince E, Gilani A, Hankinson T (2020) The inflammatory milieu of adamantinomatous craniopharyngioma and its implications for treatment. J Clin Med 9(2):519
Markham A, Patel T (2014) Siltuximab: first global approval. Drugs 74(10):1147–1152
Zhou Z, Zhang S, Hu F (2021) Endocrine disorder in patients with craniopharyngioma. Front Neurol 12:737743
Andoniadou CL, Gaston-Massuet C, Reddy R, Schneider RP, Blasco MA, Le Tissier P, Jacques TS, Pevny LH, Dattani MT, Martinez-Barbera JP (2012) Identification of novel pathways involved in the pathogenesis of human adamantinomatous craniopharyngioma. Acta Neuropathol 124(2):259–271
Jannelli G, Calvanese F, Paun L, Raverot G, Jouanneau E (2023) Current advances in papillary craniopharyngioma: state-of-the-art therapies and overview of the literature. Brain Sci 13(3):515
Cani CMG, Matushita H, Carvalho LRS, Soares IC, Brito LP, Almeida MQ, Mendonça BB (2011) PROP1 and CTNNB1 expression in adamantinomatous craniopharyngiomas with or without β-catenin mutations. Clinics (Sao Paulo) 66(11):1849–1854
Carreno G, Boult JKR, Apps J et al (2019) SHH pathway inhibition is protumourigenic in adamantinomatous craniopharyngioma. Endocr Relat Cancer 26(3):355–366
Laws ER Jr (2012) History of Pituitary Surgery, In: Schwartz, Theodore H. et al.: 2012 Endoscopic Pituitary Surgery. https://doi.org/10.1055/b-0034-82119
Harvey Cushing (1932) A journey through his life. Intracranial Tumors p. 46–47. Yale University Library Online Exhibitions. https://onlineexhibits.library.yale.edu/s/harvey-cushing/item/8017#?c=&m=&s=&cv=&xywh=-770%2C118%2C3513%2C1531. Accessed 24 Jul 2023
Yu X, Zhang J, Liu R, Wang Y, Wang H, Wang P, Chen J, Liu S (2015) Interstitial radiotherapy using phosphorus-32 for giant posterior fossa cystic craniopharyngiomas J Neurosurg Pediatr 15(5):510–518. https://doi.org/10.3171/2014.10.PEDS14302. Epub 2015 Feb 13
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
L.M. and C.D.R. designed the work and wrote the main manuscript text; D.P., A.M., F.B. and P.F. collected and analyzed the data; L.M. prepared the figures; L.M. and G.T. critically revised the manuscript; and all authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Massimi, L., Palombi, D., Musarra, A. et al. Adamantinomatous craniopharyngioma: evolution in the management. Childs Nerv Syst 39, 2613–2632 (2023). https://doi.org/10.1007/s00381-023-06143-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06143-4