Abstract
A humidity sensor plays a crucial role in determining the efficiency of materials and the precision of apparatuses. To measure and control humidity, a non-stoichiometric Li1.1Co0.3Fe2.1O4 mesopore sensor is synthesized by a modified citrate auto combustion technique. The XRD study confirms that prepared nanoparticles are cubic spinel structures having an Fd3m space group. The crystallite size is approximately 36 nm. Thermal analysis measurements show that samples become thermally stable at a temperature of 600 °C. Additionally, the kinetic studies of the prepared samples are calculated via a pseudo-first-order kinetic model. The temperature dependence of AC conductivity is found to increase with increasing temperature. These observations are explained in various models. The resistivity mechanism of humidity sensors is studied via complex impedance spectroscopy (CIS). Its impedance data are fitted to a corresponding circuit, to achieve a simulation of the sample under study. This fitting is detected by the Nyquist plot (Cole–Cole). The obtained data confirm that the studied samples are very sensitive to humidity and can be commercially used as a humidity sensing element.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Precision humidity measurement in the surrounding environment has acquired the curiosity of the scientific community because it has a direct impact on many industries and living organisms [1]. Impedance-based humidity sensors have been proposed using nanostructured metal oxides, ceramics, and polymers. Ceramics and polymers have been suggested as the best candidates for humidity sensors because no expensive noble metal catalyst or complicated synthesizing procedures or treatments are required to achieve moderate sensor performance [2]. The efficiency of the humidity sensors depends on several fascinating factors such as wide span of operating range, fast recovery, and response, promoted sensitivity, stability over long span periods, and durability [3, 4]. Humidity sensors are usually designed and constructed by depositing the sensing materials between two conductive electrodes, where the adsorbed water molecules cause a change in the physical properties of the sensing material [5]. Different configurations of humidity sensors have been proposed and constructed, such as quartz crystal microbalance (QCM), surface acoustic waves (SAW), capacitive, field-effect transistor (FET), resistive, and impedance. The resistive and impedance types are attractive as they are easily fabricated at low cost, have good linearity, and have high sensitivity [6, 7]. Spinel ferrite nanoparticles can be used as humidity sensors due to their high porosity, large surface/volume ratio [8].
Ferrite is an important material whose structure and physical properties are very favorable on a nanometric scale [9]. Among ferrite nanoparticles, lithium ferrite is a class of soft spinel ferrite materials having a square hysteresis loop, excellent dielectric properties, a high Curie temperature, and high saturation magnetization [10]. Besides the mentioned properties, lithium ferrite is a significant transition metal spinel oxide with the advantages of low price, environmental friendliness, and ease of manufacture [11].
It plays a crucial role as a functional material for different applications such as lithium-ion batteries, electrochromic displays [12] storage devices [13], and sensors [14] [15]. For the synthesis of ferrites, many methods have been proposed and investigated, including high-temperature solid-state reaction [16], co-precipitation, hydrothermal [17], sol–gel [18], high-energy ball mill, and auto combustion [19]. Among all the mentioned methods, auto combustion has the advantages of simplicity, low cost, fast, and additionally, no need for high temperatures [10, 20].
The Li ferrite nanoparticles are indexed according to the space group Fd3m and can be characterized as (A)[B2] O4. A and B represent the tetrahedral and octahedral sites, respectively [21].
The effect of cation substitutions on the physical properties of Li nano ferrites has been examined by many researchers [22, 23]. The reversible loss of lithium and oxygen during the sintering process is the main issue that restricts the synthesizing procedure of the lithium (Li) ferrites. Consequently, Li ferrite is often doped with other cations to adjust its physical properties [24].
The nano ferrite is categorized as a high resistivity material. The increase in surrounding humidity reduces the resistivity of the ferrite nanoparticles. The surrounding humidity can change the resistivity of the ferrite by nearly three orders of magnitude. Generally, the humidity is specified as relative humidity (RH) which signifies the amount of water vapors in the air at a specified temperature. According to a thorough review of the literature, the vast majority of sensors are based on ferrite nanoparticles [25].
The main challenge of this study is to advance ferrite nanoparticles with high quality, and low cost. Through this study, the structural, morphological, thermal, and electrical properties of the Li1.1Co0.3Fe2.1O4 ferrite nanoparticles were elucidated. The structural parameters lattice parameter, the X-ray density (DX), experimental density (deep), and porosity (P) were calculated. A detailed thermal study was conducted to calculate the half-life (t1/2), entropy (ΔSo), enthalpy (ΔHo), and Gibbs free energy (ΔGo). The electrical properties of the Li1.1Co0.3Fe2.1O4 nanoparticles were studied at different testing frequencies and different temperatures. The humidity sensing performance of the prepared ferrite was evaluated over a wide span of relative humidity at different testing frequencies. Finally, the humidity sensing mechanism was clarified using Nyquist complex impedance plots over a frequency ranging from 50 Hz up to 5 MHz and at various humidity levels from 40 up to 90%. The current study offers a good reference point for how to maximize the performance of the examined samples in the HS applications.
2 Experimental work
2.1 Materials and sample preparation:
Iron nitrate Fe (NO3)3·9H2O, cobalt nitrate Co (NO3)2·6H2O, and lithium nitrate La (NO3)3 were purchased from Sigma-Aldrich. The citric acid (C6H8O7) was acquired from ACROS Organics. As reported previously [26], LiCoFeO nanoparticles were prepared by mixing non-stoichiometric proportions of Fe, Co, and Li nitrates with a calculated amount of C6H8O7 by the citrate auto combustion technique. The ammonia solution was used as a fuel and was added drop by drop to the metal solution to adjust the pH value to 7. The sample was first heated at 80 ℃ for 60 min with stirring, and then heated at 200 ℃ till the final product became a fluffy powder. The sample was grinded using an agate mortar for 120 min. The obtained power was calcinated at 600 ℃ using a rate of 4 ℃/min and grinded again for 30 min. Figure 1 illustrates the flowchart for the preparation method of Li1.1Co0.3Fe2.1O4.
2.2 Sensor fabrication and evaluations
The transparent Fluorine doped Tin Oxide (FTO) coated glass substrate has been cleaned to be utilized for sensor fabrication. The electrical contacts were made by connecting two copper foils with conductive adhesive onto the FTO substrate. In brief, a proper amount of sensing material (Li1.1Co0.3Fe2.1O4) has been grinded carefully in an agate mortar for 10 min, then mixed with a suitable amount of distilled water and grinded again for an additional 10 min to form a paste. The slurry was poured on the surface of the pre-cleaned FTO substrate and dried at 60 °C for 12 h. It was conditioned at 90% RH and AC voltage (1 V, 1 kHz) for 24 h to enhance its stability. The behavior and performance of the sensor were evaluated in a homemade humidity-controlled testing chamber as shown in Fig. 2.
The humidity sensor performance was evaluated in a custom-made humidity chamber with a controlled RH value. A commercially available TSI-IAQ thermohygrometer and a 241 PG ultrasonic particle generator were used to measure the RH value and to generate the required level of humidity inside the testing chamber, respectively. The sensor response was measured using an LCR meter (HIOKI 3532-50) with an applied voltage of 1 V-AC and a measurement frequency from 100 to 100 kHz in the range of RH from 40 to 90%. The humidity controller is connected automatically with the ultrasonic humidifier, and it was adjusted to a specific value of relative humidity. The humidity will enter the closed chamber until it reaches the specific value. Simultaneously, the sensor of the humidity controller will sense it at that moment, the humidifier will be off and the measurements will be done [27].
2.3 Characterization of ferrite nanoparticles
The X-ray diffraction (XRD) pattern of ferrite powder nanoparticles was obtained by an X-ray diffractometer (Xpert PRO MPD) using CuKα radiation. The shape and size distribution of the nanoparticles were investigated using a field emission scanning electron microscope (FESEM, model Quanta 250). STD Q-600 (TGA/DTA) with a heating rate of 20 °C/min in the temperature range of 25–1000 °C under nitrogen atmosphere was used to analyze the phase decomposition and thermal stability of the samples.
In addition, the thermodynamic parameters were determined using the 1st order reaction rate equation as given in the following relation [28]:
where x is the fracture of a sample decomposed at time t, k is the rate constant of reaction, wi is the initial weight, wf is the final weight, and wt is the weight of the sample at a particular time t.
3 Results and discussion
3.1 The X-ray diffraction (XRD)
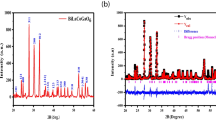
Figure 3. illustrates the XRD pattern of the Li1.1Co0.3Fe2.1O4 sample powder. The diffraction pattern exhibited diffraction peaks at 18.43°, 30.37°, 37.53°, 43.67°, 53.95°, 57.43°, and 63.1° correspondence to (111), (220), (311), (222), (400), (422), (511), and (440) diffraction plans respectively and coincidence with JCPDS card 04-022-8066. No additional peaks were detected in the pattern, signifying that Li1.1Co0.3Fe2.1O4 nanoparticles are completely formed with high purity. The formation of a single-phase cubic spinel structure with a space group (Fd-3 m) is ratified. The average crystallite size is calculated based on the Debye–Scherer’s relation as mentioned in the previous work [29,30,31].
The value of the lattice parameter (aexp) is estimated using the following relation [32]
where d is the d-spacing and (hkl) are the Miller indices of the planes associated with characteristic peaks. The X-ray density (DX), experimental density (dexp), and porosity (P), for Li1.1Co0.3Fe2.1O4 are demonstrated using Eqs. 4, 5 and 6 respectively [33]. The calculated data are illustrated in Table 1.
where Z is the number of molecules per unit cell (for spinel ferrites Z = 8), M is the molecular weight of the sample (g/mole), NA is the Avogadro's number (6.023 × 1023 atom/mole), and m, r, and t are the mass, radius, and thickness (t = 3.47 mm), respectively, of each pellet of Li1.1Co0.3Fe2.1O4 nano ferrite sample. All the structural related parameters are presented in Table 1.
3.2 Field emission scanning electron microscope (FESEM)
The FESEM images of the investigated sample are presented in Fig. 4a, b. The FE-SME images of Li1.1Co0.3Fe2.1O4 nanoparticles demonstrate a sponge and fluffy like structure. At low magnification (Fig. 4a) the Li1.1Co0.3Fe2.1O4 seems to be agglomerated without any obvious details regarding the grain size, structure, or orientation. At high magnification (Fig. 4b) the particles appear as sponges with noticeable agglomeration. The grains, with their porous nature and rocky-like shape clearly appeared. The appearance of pores can be attributed to the escape of gasses during the combustion process. The same behavior was attained for copper nano ferrites [34].
The FESEM images show that some of the particles combined with each other to form clusters and leave some spaces as pores. These pores serve as humidity or gas adsorption sites, as will be discussed later. The detected porosity from XRD agrees with the FESEM images. This morphology of Li1.1Co0.3Fe2.1O4 is highly recommended for humidity sensor applications.
3.3 Thermal analysis
TGA-DTA curves of the Li1.1Co0.3Fe2.1O4 ferrite in the temperature range 25–1000 °C under an N2 atmosphere with a heating rate of 20 °C min−1 are shown in Fig. 5a. The TGA thermogram of the sample exhibits three weight losses in the temperature ranges of (1) 30–170 °C, (2) 170–430 °C, and (3) 450–800 °C. The first stage of weight loss (WL) corresponds to 0.9% due to the evaporation of residual water during the preparation technique. Similarly, the DTA curve of the Li1.1Co0.3Fe2.1O4 represents an exothermic peak around 130 °C due to water evaporation.
In the second stage, WL ≈ (1.62%) corresponds to the volatilization of the organic solvents and represents an exothermic peak (DTG) around 400 °C. The third step of the TGA plot is after 600 °C where the WL is in the range of (0.58%). This WL may be due to improving the crystallization process of the materials.
The TGA thermogram shows no further WL above 650 °C, confirming the formation of the stable Li1.1Co0.3Fe2.1O4 nano ferrite sample. The exothermic peaks in the DTA curve are consistent with the change regions in the TGA pattern. The kinetic study of the prepared sample is calculated via a pseudo-first-order kinetic model. The rate constant (k) can be obtained from the slope of the linear plot of ln (1 − \(x\)) against time (t) using the next equation [35, 36]. The data are plotted in Fig. 5b.
The half-life (t1/2) is determined using Eq. (8), and the obtained results are illustrated in Table 2.
The Coats and Redfern model can be used to detect the kinetics parameters as designated in the following formula [28]:
where A, β, and R are the pre-exponential parameter, the heating rate (20 °C/min), and the universal gas constant (8.3143 Jmol−1 K−1) respectively. While Ea and T are the activation energy and the temperature (K) respectively. The Ea can be calculated by plotting ln [ln (1 − x)] versus 1000/T, as shown in Fig. 5c.
In the present case the chemically controlled reaction is predominant as ratified by Ea values (> 25 kJ mol−1). Other parameters, such as change of entropy (ΔSo), enthalpy (ΔHo), and Gibbs free energy (ΔGo), are calculated using basic thermodynamic equations [37]
where h and K are the Blank constant and Boltzmann constant, respectively. The results for each stage are tabulated in Table 2. The positive value of ΔHo designates the input heat energy which is required for the reactants. The degree of disturbance of the system can be identified by ΔS. The negative value of ΔSo specifies that the transition state orientation is higher compared to the reactants in the ground state. Additionally, it signifies that the Li2O + Co O + Fe O join together to form LiCoFeO which is more organized and stable compared to the ground state and subsequently decreases the randomness of the system. The ΔG gives an idea of either the non-spontaneity or spontaneity of the reaction depending on the + ve or − ve values of ΔGo. In the present study, + ve values of ΔG indicate the non-spontaneous nature of the process.
3.4 Electrical properties
The electrical properties of doped nano ferrites are the most important characteristics, since they have a significant impact on the enhancement of the humidity application. As shown in Fig. 6a, b, the dielectric constant (ε′) gradually increases with increasing temperature and decreases with increasing frequency. In the first region of the temperature, the thermal energy provided to the system is insufficient to liberate the localized dipoles until T ≈ 550 K.
In the second region, the thermal energy liberates more localized dipoles, and the field accompanied by the applied frequency aligns them in its direction up to peak T≈ 650 K. According to Koop’s’ model, ferrites are comprised of two layers, the first is low resistance grains, and the other is high-resistance grain boundaries.
When the electric field is applied to the investigated sample, electrons accumulate at grain boundaries and hinder electron conduction. Electron hopping occurs primarily between the elements at the B site as Fe2+–Fe3+ and Co2+–Co3+ ions in Li1.1Co0.3Fe2.1O4 [38].
The excess of metal ions in Li1.1Co0.3Fe2.1O4 (B cations > 2) will greatly increase the probability of electron hopping between Fe2+/Fe3+, and Co2+/Co3+ ions.
This hopping causes local displacement in the external field direction, producing a change in polarization as well as ε′. The decrease in ε′ after the peak is due to an increase in the lattice vibration, which causes scattering of charge carriers as well as disordering of the dipoles, with the result that ε′ decreases. As shown from the figures, the dielectric transition point shifts slightly to a lower value with increasing frequency which means that the ferrimagnetic region decreases and the paramagnetic region (disordered state) increases. This can be attributed to the disturbing effect of an oscillating electric field as its frequency increases.
The drop in `ε with increasing frequency is due to the fast alternation of the field accompanied by the applied frequency, where the alternation of the dipoles increases as well as the friction between them. The quantity of heat dissipated in the entire volume of the sample increases, and the aligned dipoles will be disturbed with the result of decreasing `ε.
The relation of ln σ (σ: conductivity) versus 1000/T at various frequencies is shown in Fig. 7. This plot is fitted for each region to the linear regression line with the appropriate parameter R2 ≈ 0.99.
Furthermore, it increases with increasing temperature, confirming the semiconducting-like behavior. The plot of Fig. 7 can be divided into three numerous temperature regions corresponding to three numerous conduction mechanisms.
The first is the hopping mechanism that appears below the transition temperature Tc, where the σ is frequency and temperature-dependent. The second mechanism above Tc is temperature-dependent and frequency-independent. It is related to the drift mobility of the thermally activated electrons and not to the thermal creation of the charge carriers.
In the third region, from 300 to 386 K, the σ slightly decreases with increasing temperature giving rise to a metallic like behavior. The Tc of the investigated sample occurs at ≅ 605 K.
The activation energies (AE) at different frequencies are estimated from the Arrhenius relation [39] and given in Table 3. The AE for electron hopping (Fe2+/Fe3+) is of the order of 0.1 eV as estimated by Ateia et al. [40, 41]. However, the double exchange (DE) electron hopping as Fe2+ + Co3+ ⇔ Fe3+ + Co2+ requires more energy for the conduction, and then the AE would naturally be considerably larger. Accordingly, in the present case, the obtained AE in the range of 0.2–0.3 eV suggests that the DE interaction process is more predominant. The obtained values of AE agree well with those reported for DE electron hopping (0.25 eV) [42].
Figure 7b illustrates the relation of ln σ (σ: conductivity) and ln ω (ω: angular frequency) for the Li1.1Co0.3Fe2.1O4 as a function of the absolute temperature. The σac obeyed the power law that was discussed previously [42].
where σac is the ac conductivity, ω = 2πf denotes the angular frequency, B denotes a temperature-dependent constant and S denotes the frequency-dependent exponent. The slope of lines represents the value of the exponent factor (S). The experimental value of the slope S attained for Li1.1Co0.3Fe2.1O4 is of the order of 0.57. The attained value agrees well with the values (0.6–1.0) found for the hopping mechanism in most transition metal oxide materials [43].
3.5 The molar magnetic susceptibility (χM)
The thermal variation of χM is illustrated in Fig. 8a. Ferrimagnetic behavior is the main trend over a wide temperature region. The data reveal the slow decrease in χM with increasing temperature. The thermal energy given to the sample is insufficient to overcome the magnetic field effect. At high temperatures in the para magnetic region (above Tc) the thermal energy due to heating increases the lattice vibration as well as the spin randomization. In the case study, the disturbance in the system increases leading to a drop in the magneto crystalline anisotropy and overcoming the field effect. The TC is calculated from dχm/dT as an accurate value. It is demonstrated in the inset of the figure.
The obtained data obey the well-known Curie–Weiss law where 1/χm varies linearly with temperature in the paramagnetic region, as shown in Fig. 8b, c. The values of the Curie constant (C), Curie–Weiss constant (θ), and the effective magnetic moment (µeff) are calculated from the 1/χM with T and reported in Table 4 using the following equations [44,45,46]
3.6 Humidity sensing studies
The porosity is an essential parameter for a humidity sensor (HS) and it is a significant feature of nano ferrite. The variation of impedance during exposure to humidity is a basic requirement for the humidity sensor. This disparity depends on the band gaps, surface morphology, size, diffusion rate of gas, and specific surface area (SSA) of the used magnetic materials [47].
As shown in Fig. 9a, b, the variation of impedance in the frequency range of 100–100 kHz as a function of controlled humidity from 20–90 RH% is demonstrated. It is obvious that the impedance experiences no change up to 40%RH, then decreases with increasing testing frequency. At higher frequencies, the impedance variation is insignificant due to the inability of a water molecule to be polarized at higher frequencies [48].
The ultimate variation of the impedance is accomplished at 100 Hz. Consequently, the humidity sensing performance of the Li1.1Co0.3Fe2.1O4 nanocomposite is further evaluated at 100 Hz. Moreover, a humidity level lower than 40% is not recommended to be used as humidity sensing for the studied sample. This is clarified by the physical/chemical adsorption of a water molecule on the surface of the sensor. The response of the humidity sensor exhibited an exponential decrease with an increase in the humidity level, as represented in Fig. 9b. The impedance variation is fitted by an exponential function expressed by the following equation:
where A1, t1, and y0 are constants.
The humidity sensing characteristic of the nanoparticles is elucidated through three sequential processes involving chemisorption, physisorption, and capillary condensation. Initially, at low RH values (less than 40%), water molecules (WMs) are chemically adsorbed on the surface of the sensing materials via double hydrogen bonds and self-ionized to form H+ and OH−. Because of the nature of the double hydrogen bonds, the proton needs more energy to move freely between neighboring OH− groups. At this stage, the OH− groups are hindered, thereby no change in impedance is detected. On the other hand, by increasing the RH level, the second layer of WMs starts to be physically adsorbed on the active sites of the surface of the sensor via a single hydrogen bond. Due to the formation of a single hydrogen bond, the WMs become mobile and behave like those in the bulk liquid. In this mode, the amount of ionized WMs generates a large number of hydronium ions (H3O+) which act as charge carriers. Hence, the required energy for hopping of the generated protons is decreased, enabling them for hopping between adjacent WM, thereby decreasing the sensor impedance. This mechanism is known as the chain reaction or Grotthuss mechanism [49].
The above-described mechanism can be expressed by the following equations:
With further increase in RH level, multiple physiosorbed layers are stacked up and the WMs condenses in the pores causing a further decrease in sensor impedance. The described mechanism is illustrated in Fig. 10.
Hysteresis is one of the essential parameters required to evaluate the reproducibility of the humidity sensor. The manufactured sensor is subjected to a controlled humidification/desiccation regime from 40 up to 90% RH as depicted in Fig. 11. It is ratified that the desorption process is commonly slower than the adsorption process, hence the variation of the impedance is slightly lower during the desorption compared to that in the adsorption process. As demonstrated in Fig. 11, the sensor exhibits less hysteresis, and such a humidity sensor is a favor to be integrated into real-time humidity monitoring devices. More importantly, as shown in Fig. 9b, the response of the Li1.1Co0.3Fe2.1O4 nanocomposite increases exponentially with increasing RH value from 40 to 90%.
The conductivity mechanism of the humidity sensor is studied via Complex Impedance Spectroscopy (CIS) [50]. The Nyquist complex impedance plots for Li1.1Co0.3Fe2.1O4 are estimated over a frequency ranging from 50 Hz up to 5 MHz at various humidity levels from 40 up to 90% as shown in Fig. 12a–d. Under low humidity circumstances, only a nearly straight line is established. A semicircle starts to appear in the humidity level (60—70%).
With a further increment in the RH level, the curvature of the semicircle shrinks due to the interaction between WM and the surface of the sensing material. Finally, at a higher level of humidification (80–90% RH) a semicircle is generated in addition to a straight line in the low-frequency region.
The difference in impedance curves can be associated with the numerous adsorption mechanisms. The intrinsic impedance of the sensor can be identified by the semicircle in the CIS curves. While, the line at low frequency demonstrates the Warburg impedance [51]. A few WM are adsorbed on the surface of the sensor under low humidity situations, and a semicircle or incomplete semicircle occurs in the CIS plot, to curb the movement of the charge carrier. Further increase in humidity level, the Warburg impedance is prevalent, which indicates that a continuous water layer is formed on the surface of the sensor and hence the ionic conduction is initiated. Additionally, the CIS of the examined sample depends on an idealized circuit model. The impedance data are superimposed on an equivalent circuit to achieve a simulation of the prepared sensor. The Nyquist plot (Cole–Cole) can be utilized to identify the data. The equivalent circuit is demonstrated in the inset of Fig. 12c, d. The electric circuit elements have been attained using Z-view software.
Finally, the results clarify that Li1.1Co0.3Fe2.1O4 nano ferrites contain two phases; the grain and the grain boundary. Also, approve that the clarification of the behavior of dielectric parameters and conductivity is on the correct track.
4 Conclusion
The ferrite nano particles Li1.1Co0.3Fe2.1O4 was synthesized in a single-phase cubic spinel structure using the citrate auto combustion technique. The FESEM images illustrated the porous nature and rocky-like shape of the sample. The main conduction mechanism is the electron hopping between the elements at the B site, as Fe2+–Fe3+, and Co2+–Co3+ ions. The maximum impedance value is achieved at 100 Hz; therefore, the humidity sensing behavior of Li1.1Co0.3Fe2.1O4 is evaluated at 100 Hz. The humidity sensing behavior of the nanoparticles has two mechanisms; the chemisorption at low RH values and the physisorption at high RH values. The increase in the RH level decreases the curvature of the semicircle due to the interaction between water molecules and the surface of the sensing material.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References:
S.N. Patil, A.M. Pawar, S.K. Tilekar, B.P. Ladgaonkar, Investigation of magnesium substituted nano particle zinc ferrites for relative humidity sensors. Sens. Actuators, A 244, 35–43 (2016). https://doi.org/10.1016/j.sna.2016.04.019
V. Manikandan et al., Rapid humidity sensing activities of lithium-substituted copper-ferrite (Li–CuFe2O4) thin films. Mater. Chem. Phys. 229, 448–452 (2019). https://doi.org/10.1016/j.matchemphys.2019.03.043
N.A. Roslan et al., Enhancing the performance of vanadyl phthalocyanine-based humidity sensor by varying the thickness. Sens. Actuators, B: Chem. 279(2018), 148–156 (2019)
H. Moustafa, M. Morsy, M.A. Ateia, F.M. Abdel-Haleem, Ultrafast response humidity sensors based on polyvinyl chloride/graphene oxide nanocomposites for intelligent food packaging. Sens. Actuators, A 331, 112918 (2021)
L. Xu et al., Coolmax/graphene-oxide functionalized textile humidity sensor with ultrafast response for human activities monitoring. Chem. Eng. J. 412, 128639 (2021)
S. Yu, H. Zhang, C. Chen, C. Lin, Investigation of humidity sensor based on Au modified ZnO nanosheets via hydrothermal method and first principle. Sens. Actuators B: Chem. 287, 526–534 (2019)
M. Velumani, S.R. Meher, Z.C. Alex, Composite metal oxide thin film based impedometric humidity sensors. Sens. Actuators, B: Chem. 301, 127084 (2019)
R. Cheruku, G. Govindaraj, L. Vijayan, Super-linear frequency dependence of ac conductivity in nanocrystalline lithium ferrite. Mater. Chem. Phys. 146, 389–398 (2014). https://doi.org/10.1016/j.matchemphys.2014.03.043
M.M. Hessien, Synthesis and characterization of lithium ferrite by oxalate precursor route. J. Magn. Magn. Mater. 320(21), 2800–2807 (2008). https://doi.org/10.1016/j.jmmm.2008.06.018
M.H. Abdellatif, Fractal growth of ferrite nanoparticles prepared by citrate-gel auto-combustion method. Silicon 10, 1991–1997 (2018)
E.E. Ateia, M.A. Ateia, M.G. Fayed, S.I. El-Hout, S.G. Mohamed, M.M. Arman, Synthesis of nanocubic lithium cobalt ferrite toward high-performance lithium-ion battery. Appl. Phys. A 128, 483 (2022)
M. Bahgat, F.E. Farghaly, S.A. Basir, O.A. Fouad, Synthesis, characterization and magnetic properties of microcrystalline lithium cobalt ferrite from spent lithium-ion batteries. J. Mater. Process. Technol. 183(1), 117–121 (2007)
J.J. William, I.M. Babu, G. Muralidharan, Lithium ferrite (α-LiFe5 O8) nanorod based battery-type asymmetric supercapacitor with NiO nanoflakes as the counter electrode. New J. Chem. 43(38), 15375–15388 (2019)
V. Manikandan et al., Fabrication and characterization of Ru-doped LiCuFe2O4 nanoparticles and their capacitive and resistive humidity sensor applications. J. Magn. Magn. Mater. 474, 563–569 (2019). https://doi.org/10.1016/j.jmmm.2018.11.072
N. Rezlescu, C. Doroftei, E. Rezlescu, P.D. Popa, Sens. Actuators B Chem. 133, 420 (2008). https://doi.org/10.1016/j.snb.2008.02.047
B. Mali, K. Ashok, H. Sreemoolanadhan, S. Elizabeth, Tuning of magnetic properties in Cr-doped lithium ferrite. J. Alloys Comp. 911, 165036 (2022). https://doi.org/10.1016/j.jallcom.2022.165036
A. Ahniyaz, T. Fujiwara, S.W. Song, M. Yoshimura, Low temperature preparation of β-LiFe5O8 fine particles by hydrothermal ball milling. Solid State Ionics 151(1–4), 419–423 (2002)
S.S. Teixeira, F. Amaral, M.P.F. Graça, L.C. Costa, Comparison of lithium ferrite powders prepared by sol–gel and solid-state reaction methods. Mater. Sci. Eng., B 255, 114529 (2020). https://doi.org/10.1016/j.mseb.2020.114529
B. Randhawa, H. Dosanjh, N. Kumar, Synthesis of lithium ferrite by precursor and combustion methods: a comparative study. J. Radioanal. Nucl. Chem. 274(3), 581–591 (2007)
J.V. Angadi, A.V. Anupama, R. Kumar, H.K. Choudhary, S. Matteppanavar, H.M. Somashekarappa, B. Rudraswamy, B. Sahoo, Composition dependent structural and morphological modifications in nanocrystalline Mn-Zn ferrites induced by high energy gamma-irradiation. Mater. Chem. Phys. 199, 313–321 (2017). https://doi.org/10.1016/j.matchemphys.2017.07.021
H.M. Widatallah, C. Johnson, F.J. Berry, E. Jartych, A.M. Gismelseed, M. Pekala, On the synthesis and cation distribution of aluminum-substituted spinel-related lithium ferrite. J. Grabski. Mater. Lett. 59(2005), 1105–1109 (2005)
B.K. Kuanr, Effect of the strong relaxer cobalt on the parallel and perpendicular pumping spin-wave instability threshold of LiTi ferrites. J. Magn. Magn. Mater. 163, 164–172 (1996). https://doi.org/10.1016/S0304-8853(96)00306-X
G. Mathew, S.N. Swapnw, A.M. John, P.A. Joy, M.R. Anantharaman, Structural, magnetic and electrical properties of the sol–gel prepared Li0.5Fe2.5O4 fine particles. J. Phys. D: Appl. Phys. 39, 900–910 (2006). http://dyuthi.cusat.ac.in/purl/4349. https://doi.org/10.1088/0022-3727/39/5/002
P. Thakur, P. Sharma, J.L. Mattei, P. Queffelec, A.V. Trukhanov, A. Thakur, Influence of cobalt substitution on structural, optical, electrical and magnetic properties of nanosized lithium ferrite. J. Mater. Sci. Mater. Electr. 29(19), 16507–16515 (2018)
E.E. Ateia, M.M. Arman, M. Morsy, Synthesis, characterization of NdCoO3 perovskite and its uses as humidity sensor. Appl. Phys. A 125, 883 (2019). https://doi.org/10.1007/s00339-019-3168-6
E.E. Ateia, M.A. Ateia, M.M. Arman, Assessing of channel structure and magnetic properties on heavy metal ions removal from water. J. Mater. Sci. Mater. Electron. (2021). https://doi.org/10.1007/s10854-021-07008-9
E.E. Ateia, M. Morsy, A.T. Mohamed, Humidity sensor applications based on mesopores—LaCoO3. J. Mater. Sci.: Mater. Electron. 30(21), 19254–19261 (2019). https://doi.org/10.1007/s10854-019-02284-y
M.A. Farrukh, K.M. Butt, K.K. Chong, W.S. Chang, Photoluminescence emission behavior on the reduced band gap of Fe doping in CeO2-SiO2 nanocomposite and photophysical properties. J. Saudi Chem. Soc. 23(5), 561–575 (2019). https://doi.org/10.1016/j.jscs.2018.10.002
E.E. Ateia, M.K. Abdelmaksoud, H. Ismail, A study of the magnetic properties and the magneto-crystalline anisotropy for the nano-composites CoFe2O4/Sm0.7La0.3FeO3. J. Mater. Sci. Mater. Electron. 32(4), 4480–4492 (2021). https://doi.org/10.1007/s10854-020-05189-3
E.E. Ateia, K.K. Meleka, F.Z. Ghobrial, Interplay between cation distribution and magnetic properties for CoAlxFe2−xO4 0.0≤ x ≤0.7 nanoparticles. Appl. Phys. A 127, 831 (2021)
X. Zhao, Fu. Yue, J. Wang, Xu. Yujiao, J.-H. Tian, R. Yang, Ni-doped CoFe2O4 hollow nanospheres as efficient Bi-functional catalysts. Electrochim. Acta 201, 172–178 (2016). https://doi.org/10.1016/j.electacta.2016.04.001
L. Phor, V. Kumar, Structural, thermomagnetic, and dielectric properties of Mn0.5Zn0.5GdxFe2–xO4 (x = 0, 0.025, 0.050, 0.075, and 0.1). J. Adv. Ceram. 9(2), 243–254 (2020). https://doi.org/10.1007/s40145-020-0364-y
M.M. Barakat, M.A. Henaish, S.A. Olofa, A. Tawfik, Sintering behavior of the spinel ferrite system Ni0.65 Zn0.35 Fe2–x Cux O4. J. Therm. Anal. Calorim. 37(2), 241–248 (1991)
L.P. Babu Reddy et al., (2018) Copper ferrite-yttrium oxide (CFYO) nanocomposite as remarkable humidity sensor. Inorg. Chem. Commun. 99, 180–188 (2019)
S. Perveen, M.A. Farrukh, Influence of lanthanum precursors on the heterogeneous La/SnO2–TiO2 nanocatalyst with enhanced catalytic activity under visible light. J. Mater. Sci.: Mater. Electron. 28(15), 10806–10818 (2017). https://doi.org/10.1007/S10854-017-6858-X
K.H. Mahmoud, M.H. Makled, Infrared spectroscopy and thermal stability studies of natural rubber-barium ferrite composites. Adv. Chem. Eng. Sci. 2, 350–358 (2012)
S.R. Naqvi et al., Pyrolysis of high ash sewage sludge: kinetics and thermodynamic analysis using Coats-Redfern method. Renew. Energy 131, 854–860 (2019). https://doi.org/10.1016/j.renene.2018.07.094
R. Yadav, M.K. Yadav, N.K. Singh, Electrocatalytic properties of sol–gel derived spinel CoxFe3−xO4 (0 ≤ x ≤ 1. 5) electrodes for oxygen evolution in Alkaline solution. Int. J. Electrochem. Sci. 8, 6321–6331 (2013). http://electrochemsci.org/papers/vol8/80506321.pdf.
J.S. Kim, H.J. Lee, S.Y. Lee, W. Kim, S.D. Lee, Frequency and temperature dependence of dielectric and electrical properties of radio-frequency sputtered lead-free K0.48Na0.52NbO.3 thin films. Solid Films 518, 6390–6393 (2010). https://doi.org/10.1016/j.tsf.2010.02.078
E.E. Ateia, Effect of gamma irradiation on the structural and electrical properties of Co0.5Zn0.5CeyFe2−yO4. Egypt. J. Solids 29(2), 317–328 (2006)
E.E. Ateia, B. Hussein, C. Singh, M.M. Arman, Multiferroic properties of GdFe0.9M0.1O3 (M = Ag1+, Co2+ and Cr3+) nanoparticles and evaluation of their antibacterial activity. Eur. Phys. J. Plus 137, 443 (2022)
B. Senthilkumar, R.K. Selvan, P. Vinothbabu, I. Perelshtein, A. Gedanken, Structural, magnetic, electrical and electrochemical properties of NiFe2O4 synthesized by the molten salt technique. Mater. Chem. Phys. 130(1–2), 285–292 (2011). https://doi.org/10.1016/j.matchemphys.2011.06.043
A.S. Das, M. Roy, D. Biswas, R. Kundu, A. Acharya, D. Roy, S. Bhattacharya, Ac conductivity of transition metal oxide doped glassy nanocomposite systems: temperature and frequency dependency. Mater. Res. Express. 5, 095201 (2018)
M. Reda, S.I. El-Dek, M.M. Arman, Improvement of ferroelectric properties via Zr doping in barium titanate nanoparticles. J. Mater. Sci. Mater. Electron. 33(21), 16753–16776 (2022). https://doi.org/10.1007/s10854-022-08541-x
M.M. Arman, M.A. Ahmed, Effects of vacancy co-doping on the structure, magnetic and dielectric properties of LaFeO3 perovskite nanoparticles. J. Appl. Phys. 128(7), 1–9 (2022). https://doi.org/10.1007/s00339-022-05623-9
E.E. Ateia, A.T. Mohamed, Nonstoichiometry and phase stability of Al and Cr substituted Mg ferrite nanoparticles synthesized by citrate method. J. Magnet. Magnet. Mater. 426, 217–224 (2017)
E. Rios, J.L. Gautier, G. Poillerat, P. Chartier, Mixed valency spinel oxides of transition metals and electrocatalysis: case of the MnxCo3−xO4 system. Electrochim. Acta 44, 1491–1497 (1998). https://doi.org/10.1016/S0013-4686(98)00272-2
M. Morsy, M.M. Mokhtar, S.H. Ismail, G.G. Mohamed, M. Ibrahim, Humidity Sensing Behaviour of Lyophilized rGO/Fe2O3 Nanocomposite. J. Inorg. Organomet. Polym Mater. 30(10), 4180–4190 (2020). https://doi.org/10.1007/s10904-020-01570-1
P. Chavan, Chemisorption and physisorption of water vapors on the surface of lithium-substituted cobalt ferrite nanoparticles. ACS Omega 6(3), 1953–1959 (2021). https://doi.org/10.1021/acsomega.0c04784
T.Ş Kuru, M. Kuru, S. Bağcı, Structural, dielectric and humidity properties of Al–Ni–Zn ferrite prepared by co-precipitation method. J. Alloys Comp. 753, 483–490 (2018). https://doi.org/10.1016/j.jallcom.2018.04.255
X. Zhao, X. Chen, X. Yu, X. Ding, X.L. Yu, X.P. Chen, Fast response humidity sensor based on graphene oxide films supported by TiO2 nanorods. Diam. Relat. Mater. 109, 108031 (2020)
Acknowledgements
This paper is supported financially by the Academy of Scientific Research and Technology (ASRT), Egypt, under initiatives of Science Up Faculty of Science (Grant No. 6722).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research was funded by Academy of Scientific Research & Technology (No. 6722).
Author information
Authors and Affiliations
Contributions
EEA: experimentation, written the original manuscript, reviewing and editing the final manuscript, supervision. MAA: material preparation, data collection and analysis, Optimum selection of material parameters, experimentation, validation and visualization. MMM: experimentation, written the original manuscript, and editing the final manuscript. MMA: conceptualization, investigation; experimentation, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ateia, M.A., Ateia, E.E., Mosry, M. et al. Synthesis and characterization of non-stoichiometric Li1.1Co0.3Fe2.1O4 ferrite nanoparticles for humidity sensors. Appl. Phys. A 128, 884 (2022). https://doi.org/10.1007/s00339-022-06030-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06030-w