Abstract
Development of improved methods for the synthesis of copper nanoparticles is of high priority for the advancement of material science and technology. Herein, starch-protected zero-valent copper (Cu) nanoparticles have been successfully synthesized by a novel facile route. The method is based on the chemical reduction in aqueous copper salt using ascorbic acid as reducing agent at low temperature (80 °C). X-ray diffraction, scanning electron microscopy and energy-dispersive X-ray spectroscopy measurements were taken to investigate the size, structure and composition of synthesized Cu nanocrystals, respectively. Average crystallite size of Cu nanocrystals calculated from the major diffraction peaks using the Scherrer formula is about 28.73 nm. It is expected that the outcomes of the study take us a step closer toward designing rational strategies for the synthesis of nascent Cu nanoparticles without inert gas protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The controlled fabrication of nanoparticles has propelled nanotechnology into one of today’s most promising and popular fields of scientific research [1]. Potential future advancement requires the ability to prepare nanomaterials in a reproducible and controlled manner [2]. Currently, there is an increasing interest to synthesize metallic copper and copper (I) oxide nanocrystals not only for the expansion of synthetic advancement, but also for the assessment of their electrical, catalytic, sensing and surface properties [3–5]. Metallic copper and copper oxides have been employed as heterogeneous catalysts for numerous environmental progressions, e.g., selective reduction in nitric oxide, oxidation of carbon monoxide and decomposition of nitrogen dioxide [6]. Metallic Cu nanoparticles are striking materials primarily because of their unique properties [3] and low cost compared to other metallic nanomaterials such as gold and silver [7].
The ability to prepare nanomaterial with well-defined morphologies and sharp faces should facilitate the appraisal of their properties [8]. Synthesis of Cu nanoparticles is quiet challenging due to its high tendency for oxidation. It is extremely sensitive to air, and the oxide phases are thermodynamically more stable [9]. The high oxidation rate of Cu nanoparticles may limit their applications [10]. Oxidation of copper nanoparticles can be eliminated if the synthesis is conducted in the presence of CO or H2. On the other hand, handling these gases is rather cumbersome, and use of such gases is avoided when possible [11].
Production of pure copper nanoparticles is rare, unless the whole procedure is carried out under an inert atmosphere [12, 13]. Khanna et al. [14] affirmed the preparation of pure copper nanoparticles by reducing copper salt with sodium formaldehyde sulfoxylate in the presence of carboxylic acids. However, the stability of prepared nanoparticles after they were exposed to air has not been investigated. Cu nanoparticles are usually protected with a capping agent in order to minimize oxidation and control the growth of a crystal by decreasing the surface energies of crystals [10]. However, the capping agents or stabilizers can significantly reduce the oxidation but may not prevent it completely because of their molecular motion [5, 15].
Presently developed synthesis methods for nanoparticles include laser ablation, thermal decomposition, chemical reduction and polyol synthesis. Among these processes, chemical reduction is usually preferred, because this method is easy, cost-effective and efficient, and it can apprehend improved size and size dispersion control by optimizing the experimental factors, for instance the molar ratio of the stabilizer with the precursor salt and the fraction of reducing agent with the precursor salt [16]. The rate of growth of the nanoparticles depends upon various variables, including the concentration of metal ions, the type of reductant, pH and temperature [11]. Time is also a key parameter in nanoparticles synthesis. The availability of enormous number of nuclei at a given time resulted in the decrease in size of nanoparticles, because smaller metal nuclei grow and use metal ions at the same time [16].
A chemical reduction technique typically includes the reduction in metal salts in various solvents and reducing agent [16]. In the present study, a chemical reduction method has been proposed to fabricate starch-stabilized Cu nanoparticles involving chemical reduction in Cu2+ in an aqueous medium without inert gas protection. Cu ions were reduced with ascorbic acid to generate metallic Cu nanoparticles. Ascorbic acid was used as a reducing agent owing to its weak reducing ability. Consequently, the reaction driving force is low and it is not easy for the Cu nanoparticles to aggregate.
Nevertheless, Cu nanoparticles prepared in ambient atmospheric pressure without inert gas protection are prone to oxidation because the oxides of Cu are thermodynamically more stable than pure Cu. In addition, without proper protection copper nanoparticles are found to aggregate rigorously. Starch was used to control the growth of nanoparticles and protect them to avoid oxidation and aggregation. The main objectives of the study were the synthesis of Cu nanoparticles by chemical reduction method and their characterization using X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) techniques.
Experimental
Materials
All the chemicals used in the experiment were of analytical grade. Copper sulphate pentahydrate CuSO4·5H2O (0.1 M), Starch (C6H10O5) n (1.2 %), Ascorbic acid C6H8O6 (0.2 M) and Sodium hydroxide NaOH (1 M) were purchased from Sigma Aldrich. De-ionized water was used for all the experiment.
Synthesis of Cu nanoparticles
The Cu nanoparticles were synthesized by chemical reduction process using copper (II) sulfate pentahydrate as precursor salt and starch as capping agent. The preparation method starts with addition of 0.1 M copper (II) sulfate pentahydrate solution into 120 mL of starch (1.2 %) solution with vigorous stirring for 30 min. In the second step, 50 mL of 0.2 M ascorbic acid solution is added to synthesis solution under continuous rapid stirring. Subsequently, 30 mL of 1 M sodium hydroxide solution was slowly added to the prepared solution with constant stirring and heating at 80 °C for 2 h. The color of the solution turned yellow to ocher. After the completion of reaction, the solution was taken from the heat and allowed to settle overnight and the supernatant solution was then discarded cautiously. The precipitates were separated from the solution by filtration and washed with deionized water and ethanol for three times to take out the excessive starch bound with the nanoparticles. Ocher color precipitates (Fig. 1) obtained are dried at room temperature. After drying, nanoparticles were stored in glass vial for further analysis.
Characterization of nanoparticles
The prepared nanoparticles were characterized using following techniques.
X-ray diffraction analysis
XRD analysis of prepared nanoparticles was performed on a PANlytical X’Pert PRO diffractometer operated at 40 kV and 30 mA with Cu Kα radiation (1.54 Å) as a source. A step scan mode was applied with a step width of 0.02°, sampling time of 0.5 s and measurement temperature of 25 °C. The scanning range of 2θ was between 20° and 80°.
Sample preparation for XRD was quite easy as the nanoparticles were in a powder form. The nanoparticles powder was placed on the top of aluminum slide and spread out to cover up the specified area. This step is important in order to ensure that a large enough area will be exposed to the X-rays during data collection.
The mean size of nanocrystals was measured from the broadening of the diffraction peaks corresponding to the most intensive reflections according to the JCPDS (Joint Committee on Powder Diffraction Standards) database. Scherrer equation was used to determine the crystallite size from XRD diffraction pattern measured for nanoparticles:
where K is the Scherrer constant (shape factor, its value is 0.9), λ is the X-ray wavelength (λ = 0.154 nm), B is the line broadening at half the maximum intensity (FWHM) in radians, θ is the Bragg angle, (the position of the diffraction peak maximum) and d is the averaged dimension of crystallites in nanometers. Lattice parameter is determined through interplanar spacing (d-spacing) of the planes, as follows:
where “a” is lattice parameter or lattice constant in angstrom [Å], d is interplanar spacing or distance in angstrom [Å] and hkl are the Miller indices of the plane of crystal.
Scanning electron microscopy (SEM)–energy-dispersive X-ray spectroscopy (EDX)
The morphology and chemical composition of the synthesized nanoparticles were examined by scanning electron microscopy (SEM, JEOL JSM-6490A) equipped with an energy-dispersive X-ray spectrometer (EDX) (6490 LA). EDX was carried out at an acceleration voltage of 20.0 kV.
Sample was prepared by sprinkling the dispersed nanoparticles onto double-sided adhesive carbon conductive tape which was mounted on a microscopic stub of copper. Then sample is sputter-coated with gold using ion sputtering device (JFC 1500).
Results and discussion
Characterization
X-ray diffraction analysis
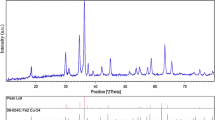
The crystal structure and size of the nanoparticles were verified by XRD analysis. Figure 2 exhibits the XRD pattern of the as-synthesized nanoparticles. Peaks observed at 2θ values of 43.39°, 50.49° and 74.18° correspond to (111), (200) and (220) planes of metallic Cu. These three peaks were quite consistent with those of the standard JCPDS Card No. 04-0836 for the standard spectrum of the pure fcc (face centered cubic) metallic Cu. Besides the metallic Cu peaks, several other diffraction peaks appeared at 29.63°, 36.54°, 42.44°, 61.57°, 73.58° and 77.49° corresponding to (110), (111), (200), (220), (311) and (222) planes of cuprite, respectively, indicate the formation of cubic copper (I) oxide nanocrystals [17, 18]. XRD peaks observed for cuprite was matched well with the standard powder diffraction card of bcc (body centered cubic) cuprite (JCPDS No. 05-667) [19].
The XRD pattern reveals that prepared nanoparticles are a mixture of metallic Cu and copper (I) oxide (Cu2O). The mean size of the crystalline Cu and Cu2O nanoparticles calculated from the major diffractions peaks using the Scherrer formula is about 28.73 and 25.19 nm, respectively. The reflections in the diffractogram confirmed the fcc crystal structure of elemental Cu with lattice constant a = 3.614 Å that match well with standard lattice parameter (a = 3.615 Å, JCPDS Card No. 04-0836) [20]. There are a number of peaks in the XRD pattern that ascribed to the formation of bcc crystal structure of Cu2O with lattice constant a = 4.262 Å, in concord with the standard data (a = 4.269 Å JCPDS Card No. 05-667) [19].
The XRD diffraction pattern showed the coexistence of two crystalline phases, i.e., metallic Cu and Cu2O. This obviously illustrates that the zero-valent copper nanoparticles formed in the chemical reduction stage go through decomposition due to limited stability of Cu [21] and Cu2O might be formed by oxidation [22]. All the nanocubes were indeed Cu and Cu2O; no other phase of copper oxide (CuO) was present. The peak broadening in the XRD pattern indicates the presence of small nanocrystals [23].
Energy-dispersive X-ray spectroscopy
EDX spectroscopy is applied to quantify the elemental composition of the synthesized nanoparticles. The EDX spectrum of the Cu nanoparticles given in Fig. 3 confirmed the existence of Cu, O and C. The peak around 0.5 keV belongs to the binding energy of oxygen (OKα), while peaks located at binding energies of 0.85, 0.94, 8.04 and 8.94 keV correspond to CuL1, CuLα, CuKα and CuKβ, respectively [24]. Additionally, a peak at 0.27 keV corresponding to carbon (CKα) has also been appeared.
The obtained EDX spectrum of Cu nanoparticles is similar to that reported earlier by Kooti and Matouri [18] except for a slightly enhanced peak of carbon. The carbon and oxygen peaks in the samples verified the presence of carbon-based stabilizers [25]. The appearance of carbon peaks in the spectrum may attribute to the carbon tape used to mount the sample on the stub. Also, together with copper carbon and oxygen, gold peak observed at 2.2 keV due to gold coating of sample. The elemental analysis of the prepared Cu nanoparticles is shown in Table 1.
Scanning electron microscopy analysis
SEM analysis was used to study the surface morphology of synthesized nanoparticles. Surface morphology of the Cu nanoparticles is shown in Fig. 4. SEM images demonstrated that the as-prepared Cu and Cu2O nanoparticles are cubic in shape.
Stabilized nanoparticles could also form cluster and rather get close to each other. However, individual nanoparticles are encapsulated from each other by the stabilizing agent and can be re-dispersed. The stabilizers play a crucial role in determining the distribution of particle size and limiting the clustering and flocculation [26]. The present study demonstrates that starch is an effective capping agent that results in the fabrication of small-sized nanoparticles. Competition between various processes such as growth, nucleation, aggregation and adsorption of impurities determines the structure of nanoparticles [27].
Metallic Cu and cuprite nanocrystals have been synthesized by a facile reduction method in an alkaline medium using ascorbic acid as reducing agent and copper sulfate as precursor. In this approach, an organic-based reductant (ascorbic acid) is introduced to the solution containing Cu ions dispersed in starch; the Cu ions are subjected to reduction resulting in the development of elemental Cu nanoparticles [28]. Copper is easily reduced in solution using mild reductant such as ascorbic acid [29]. Addition of sodium hydroxide augmented the rate of reduction [30]. Copper is easily oxidized with a small amount of oxygen present [11]. Consequently, the oxidation of metallic Cu is a key to the production of greater amount of cuprite instead of metallic Cu [17]. The addition of sodium hydroxide is also expected to form Cu(OH)2 which subsequently produce Cu2O nanoparticles upon reduction [31].
Starch serves as a dispersing agent to separate metal ions from each other and hence provides better size control of nanoparticles [11]. The functional groups on the alkyl chains on the surface of the metal cluster take part a very vital role in controlling the conversion of zero-valent copper to their oxides [21]. As metal particles are generated in the aqueous phase, they are unstable by nature, and these metal atoms tend to agglomerate so as to decrease the total surface energy. In addition, some metals serve as nuclei for others to grow on. This agglomeration, which can be caused by attractive van der Waals forces between crystals, should be repressed to limit the final particle size at the nanometric scale [16].
Reaction time of up to 60 min with ascorbic acid leads to well-structured nanoparticles with a decrease in the mean crystal size with time. This implies a homogenization mechanism, which offers a better number of nuclei with time [16]. The size of the produced nanocrystals is relatively small when the rate of reduction is slow; therefore, less powerful reducing agents are favorable for generating smaller size nanoparticles [11] and hence lead to the development of Cu and Cu2O nanoparticles with mean size of 28.73 and 25.19 nm, respectively.
Conclusion
The study demonstrated a promising and generally applicable method to fabricate elemental copper nanoparticles by means of chemical reduction method. XRD results indicated that the starch-stabilized Cu and Cu2O nanoparticles are cubic in shape with mean size of 28.73 and 25.19 nm, respectively. This synthesis pathway is particularly suitable for large-scale synthesis of Cu and Cu2O nanoparticles attributed to its simple process and low cost. It is probable that the development of improved synthetic methods for Cu and Cu2O nanocrystals and more knowledge of their properties should lead to the great advancement in their applications such as catalysis and photoactivated energy conversion.
References
Shenmar, R., Norsten, V.B., Rotello, V.M.: Polymer-mediated nanoparticle assembly: structural control and applications. Adv. Mater. 6, 657–669 (2005)
Balzani, V., Credi, A., Venturi, M.: The bottom-up approach to molecular-level devices and machines. Chem. A Eur. J. 24, 5524–5532 (2002)
Lambert, S., Cellier, C., Gaigneaux, E.M., Pirard, J.P., Heinrichs, B.: Ag/SiO2, Cu/SiO2 and Pd/SiO2 cogelled xerogel catalysts for benzene combustion: relationships between operating synthesis variables and catalytic activity. Catal. Commun. 8, 1244–1248 (2007)
Zhang, H., Zhu, Q., Zhang, Y., Wang, Y., Zhao, L., Yu, B.: One-pot synthesis and hierarchical assembly of hollow Cu2O microspheres with nanocrystals-composed porous multishell and their gas-sensing properties. Adv. Funct. Mater. 17, 2766–2771 (2007)
Liu, Z., Bando, Y.: A novel method for preparing copper nanorods and nanowires. Adv. Mater. 15, 303–305 (2003)
Oritz, J.R., Ogura, T., Medina-Valtierra, J., Acosta-Ortiz, S.E., Bosh, P., De las Reyes, J.A., Lara, V.H.: A catalytic application of Cu2O and CuO films deposited over fiberglass. Appl. Surf. Sci. 174, 177–184 (2001)
Anzlovar, A., Orel, Z.C., Zigon, M.: Copper (I) oxide and metallic copper particles formed in 1, 2-propane diol. Eur. Ceram. Soc. 27, 987–991 (2007)
Kuo, C.H., Chen, C.H., Huang, M.H.: Seed-mediated synthesis of monodispersed Cu2O nanocubes with five different size ranges from 40 to 420 nm. Nano Today 5, 106–3780 (2010)
Jeong, S., Woo, K., Kim, D., Lim, S., Kim, J.S., Shin, H., Xia, Y., Moon, J.: Controlling the thickness of the surface oxide layer on Cu nanoparticles for the fabrication of conductive structures by ink-jet printing. J. Adv. Funct. Mater. 18, 679–686 (2008)
Cushing, B.L., Kolesnichenko, V.L., O’Connor, C.J.: Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 104, 3893–3946 (2004)
Han, K.N., Kim, N.S.: Challenges and opportunities in direct write technology using nano-metal particles. KONA Powder Part J. 27, 73 (2009)
Mott, D., Galkowski, J., Wang, L., Luo, J., Zhong, C.J.: Synthesis of size-controlled and shaped copper nanoparticles. Langmuir 23, 5740–5745 (2007)
Chen, S., Sommers, J.M.: Alkanethiolate-protected copper nanoparticles: spectroscopy, electrochemistry, and solid-state morphological evolution. J. Phys. Chem. B. 105, 8816–8820 (2001)
Khanna, P.K., Gaikwad, S., Adhyapak, P.V., Singh, N., Marimuthu, R.: Synthesis and characterization of copper nanoparticles. Mater. Lett. 61, 4711–4714 (2007)
Kobayashi, Y., Ishida, S., Ihara, K., Yasuda, Y., Morita, T., Yamada, S.: Synthesis of metallic copper nanoparticles coated with polypyrrole. Coll. Polym. Sci. 287, 877–880 (2009)
Dang, T.M.D., Le, T.T.T., Blanc, E.F., Dang, M.C.: Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2, 15009–15012 (2011)
Martis, P., Fonseca, A., Mekhalif, Z., Delhalle, J.: Optimization of cuprous oxide nanocrystals deposition on multiwalled carbon nanotubes. J. Nanopart. Res. 12, 439–448 (2010)
Kooti, M., Matouri, L.: Fabrication of nanosized cuprous oxide using Fehling’s solution. Sci. Iran 17, 73–78 (2010)
Waseda, Y., Matsubara, E., Shinoda, K.: X-ray diffraction crystallography: introduction, examples and solved problems. Springer, Berlin (2011)
Diaz-Droguetta, D.E., Espinozab, R., Fuenzalida, V.M.: Copper nanoparticles grown under hydrogen: study of the surface oxide. Appl. Surf. Sci. 257, 4597–4602 (2011)
Aslam, M., Gopakumar, G., Shoba, T.L., Mulla, I.S., Vijayamohanan, K., Kulkarni, S.K., Urban, J., Vogel, W.: Formation of Cu and Cu2O nanoparticles by variation of the surface ligand: preparation, structure, and insulating-to-metallic transition. J. Coll. Interf. Sci. 255, 79–90 (2002)
Feng, L., Zhang, C., Gao, G., Cui, D.: Facile synthesis of hollow Cu2O octahedral and spherical nanocrystals and their morphology-dependent photocatalytic properties. Nanoscale Res. Lett. 7, 276 (2012)
Murugadoss, G., Rajamannan, B., Madhusudhanan, U.: Synthesis and characterization of water-soluble ZnS: Mn2+ nanocrystals. Chalcogenide Lett. 6, 197–201 (2009)
Khan, M.A.M., Kumar, S., Ahamed, M., Alrokayan, S.A., Al-Salhi, M.S.: Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanoscale Res. Lett. 6, 434 (2011)
Singh, A.K., Raykar, V.S.: Microwave synthesis of silver nanofluids with polyvinylpyrrolidone (PVP) and their transport properties. Coll. Polym. Sci. 286, 1667–1673 (2008)
Qiuli, Z., Zhimao, Y., Bingjun, D., Xinzhe, L., Yingjuan, G.: Preparation of copper nanoparticles by chemical reduction method using potassium borohydride. Trans. Nonferrous Met. Soc. China 20, s240–s244 (2010)
Cornell, R.M., Schwertmann, U.: The iron oxides structure, properties, reactions occurrences and uses. Wiley-VCH, Weinheim (1996)
Wu, S.H., Chen, D.H.: Synthesis of high-concentration Cu nanoparticles in aqueous CTAB solutions. J. Colloid Interf. Sci. 273, 165–169 (2004)
Sau, T.K., Pal, A., Jana, N.R., Wang, Z.L., Pal, T.: Size controlled synthesis of gold nanoparticles using photochemically prepared seed particles. J. Nanopart. Res. 3, 257–261 (2001)
Sahoo, P.K., Kamal, S.S.K., Kumar, T.J., Sreedhar, B., Singh, A.K., Srivastava, S.K.: Synthesis of silver nanoparticles using facile wet chemical route. Def. Sci. J. 549, 447–455 (2009)
Kuo, C.H., Chen, C.H., Huang, M.H.: Seed-mediated synthesis of monodispersed Cu2O nanocubes with five different size ranges from 40 to 420 nm. Adv. Funct. Mater. 17, 3773–3780 (2007)
Acknowledgments
This research is funded by PMAS Arid Agriculture University, Rawalpindi, by Grant ID DRIC-284. The authors gratefully acknowledge Mr. Irfan Sabir for the valuable guidance provided in XRD analysis. In addition, the authors wish to thank Mr. Shams for their assistance during SEM analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Authors declare that they have no competing financial interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Khan, A., Rashid, A., Younas, R. et al. A chemical reduction approach to the synthesis of copper nanoparticles. Int Nano Lett 6, 21–26 (2016). https://doi.org/10.1007/s40089-015-0163-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-015-0163-6