Abstract
Perovskite nanoparticles La1-x□xFe1-y□yO3, (y = x = 0.0, 0.01 and 0.02) were prepared using the citrate combustion method. The samples were crystallized in a single phase orthorhombic structure. The energy dispersive spectroscopy (EDS) confirms that the chemical composition is very close to the nominal one. The particle size values indicated that the samples were prepared in nano scale. The selected area electron diffraction (SAED) pattern shows the good crystallinity of the samples. The magnetic hysteresis loops indicate the antiferromagnetic properties of the samples. The molar magnetic susceptibility (χM) of LaFeO3 sample was enhanced by vacancy doping. The value of Ms of the sample La0.98□0.02Fe0.98□0.02O3 increased by a factor 1.9 than that of sample LaFeO3. The investigated samples have semiconductor-like behavior as clear from the increase in the conductivity with raising the temperature and the values of activation energies. The main conduction mechanisms in the investigated samples are the small polaron tunneling (SP) and the correlated barrier hopping (CBH).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The perovskite materials have a general formula of ABO3, where A is a rare earth element and is coordinated by 12 oxygen anions. While B cation is a 3d transition metal and coordinated to six oxygen anions. Metals with different oxidation states can be replaced by the metal ions at the A and B sites due to the great stability of the ABO3 materials [1]. Thus, novel materials can be designed with various properties for different applications.
Lanthanum orthoferrite (LaFeO3) has a perovskite structure with space group Pbnm and has attracted great interest due to its unique physical and chemical properties. LaFeO3 has G-type antiferromagnetic (AFM) behavior with a Nèel temperature (TN) ~ 730 K [2]. The ordering of ferroelectric or antiferroelectric dipole moments determines the electrical properties of LaFeO3. The < FeO6 > octahedron in LaFeO3 drives the coexisting magnetic and electrical configurations. The perovskite LaFeO3 was synthesized using different methods such as electrochemical [3], solid-state [4], and sol–gel combustion [5, 6] techniques. LaFeO3 was prepared in nano scale easily using the citrate nitrate combustion method [7].
The perovskite structure ABO3 contains an equal amount of A and B site cations (i.e., the A/B cation ratio = 1). When the A/B cation ratio is deviated significantly from unit, the perovskite lattice structure can still be stable. Introduction of the vacancy on both A and B cation sites tilts the < BO6 > octahedron. The octahedral tilting lowers the coordination number of the A cation from 12 to 8 [8], reducing the tension in A–O bonds and increasing lattice energy. The presence of an octahedral tilting distortion affects not only the crystal structure, but also has a profound influence on the physical properties such as electrical conductivity, magnetic superexchange interactions and other electrical properties [9]. The nonstoichiometric A-site cation perovskite ABO3 were developed as cathodes for solid-oxide fuel cells (SOFCs) and improve the oxygen reduction reaction (ORR) activity at high temperatures [10, 11].
Spinicci et al. [12] were prepared La(1−ε)FeO(3−1.5ε) with ε = 0.0, 0.1, 0.2, 0.3 using thermal low-temperature decomposition, a method which ensures a high surface area. La(1−ε)FeO(3−1.5ε) have been used in catalytic methane combustion in the range 300–450 °C. Scafetta et al. [13] have studied the effect of La and Fe deficiency on the c-axis lattice parameter and optical absorption in La1-xFe1-yO3-δ films. Fe vacancies expanded out-of-plane lattice parameters while La deficiency leads to an increase in absorption throughout the spectral range probed. Zhu et al. [14] prepared the A-site cation deficient perovskites (La0.95FeO3-δ) by a simple and cost-effective process. La0.95FeO3-δ samples have highly-efficient bifunctional electrocatalysts for ORR and oxygen evolution reaction (OER) in alkaline solutions.
Arman et al. [15] were investigated the effect of cation deficiency on NdFeO3. The self-doped vacancies on A and B sites of NdFeO3 increased the saturation magnetization (Ms) of the sample Nd0.97□0.03Fe0.97□0.03O3 by 1.5 times more than the parent sample NdFeO3. The antiferromagnetic properties of NdFeO3 perovskite were originated from magnetic interactions such as Nd–Nd, Nd–Fe, and Fe–Fe. While LaFeO3 has Fe–Fe magnetic interaction only. The difference in ionic radii of La3+ ion (1.216 Å) and that of Nd3+ ion (1.163 Å) affects the tolerance factor and tilting of < BO6 > octahedron.
In this present work, we studied the effect of vacancy co-doping on the physical properties such as the magnetic and dielectric properties of LaFeO3 nanoparticles. The substitution of the vacancy on both A and B cation sites simultaneously is influenced by the structure, morphology and all physicochemical properties of the samples. The introduced A and B- site cation vacancies in LaFeO3 enhanced Fe–Fe magnetic interaction and improved the hybridization between the La 3d and O 2p states. Furthermore, the presence of La and Fe defects increases the specific surface area. The vacancy doped samples are more applicable in many applications, such as the heavy metal removal from wastewater, cathode catalysts for lithium-oxygen (Li-O2) batteries [16,17,18].
2 Experimental techniques
The perovskite samples La1-x□xFe1-y□yO3, (0.0 ≤ y, x ≤ 0.02, step 0.01) were prepared by citrate nitrate combustion method [14, 16]. The precursor metal nitrates (purity 99.9%, Sigma–Aldrich) were mixed in stoichiometric ratios with an equivalent molar ratio of citric acid in aqueous media to enhance the homogeneous mixing. To produce La and Fe vacancies, the La(NO3)3∙6H2O and Fe(NO3)3∙9H2O are stoichiometrically decreased by 1% and 2% to generate nonstoichiometric La and Fe defects. The LaFeO3 perovskite has a stable structure upto 30% nonstoichiometric La and Fe defects [12, 16]. To ensure complete complexation, the pH value was adjusted to 7 using the ammonia solution as a fuel. The (citric acid/nitrate) ratio is kept constant for all samples and is equal to 1. The samples were heated on a hot plate to obtain the final product that gives a fluffy powder with a single-phase orthorhombic structure. Thermal dehydration resulted in a highly viscous liquid. The citrate nitrate combustion method is simple, low cost, and quick.
X-ray powder diffraction (XRD) was carried out to study the crystal structure of the samples using a Proker D8 advanced X-ray diffractometer with Cu-Kα radiation (λ = 1.5418 Å). The crystalline phases of the samples were identified using the International Centre for Diffraction Data (ICDD) card number 74–2203.
The samples were characterized by scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) to study the morphology of the samples using OXFORD INCA PentaFETX3-England. The shape and size of the particles were studied using a high-resolution transmission electron microscope (HRTEM) model (JEOl-2100). The magnetic properties of the samples were studied by two techniques: the first is the H-M hysteresis loop using a vibrating sample magnetometer (VSM; 9600–1 LDJ, USA) with a maximum applied field of 20 kOe at room temperature. While the second technique is measurements of dc magnetic susceptibility (χM) using Faraday’s method [19] at different magnetic field intensities as a function of absolute temperature.
The powdered samples were pressed to obtain pellets using a press of value 8 × 105 N/m2 [7]. The two surfaces of each pellet were checked for good conduction by coating the surfaces with silver paste. The electrical properties were measured on pellets of samples using the LCR meter (Hioki model 3532 Japan). The dielectric constant (ε′) and ac conductivity of the samples were measured as a function of temperature in the frequency range 100 Hz to 5 MHz. The data was triplet and taken the average value.
3 Results and discussion
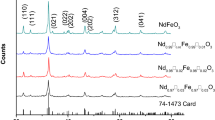
Figure 1 illustrates the XRD patterns of the samples that have the formula of La1-x□xFe1-y□yO3, (0.0 ≤ y, x ≤ 0.02, step 0.01). The prepared samples were crystallized in a single phase orthorhombic structure with space group Pbnm. The data was indexed with ICDD card number 74–2203. The main peak (100%) of LaFeO3 is observed for (200) plane at 2θ = 34.5°. The lattice parameters (a, b and c) were calculated based on orthorhombic unit cell from Eq. (1) and listed in Table 1. The crystallite size (L) was determined using Scherrer’s equation which is given by Eq. (2). [20]
where L is the average crystallite size, θ is the Bragg angle, λ is the wave length of the X-ray radiation (λ = 1.5406 Å), and β is the full width at half maximum intensity of the powder pattern peak. The values of L were listed in Table 1 and indicated that the samples are prepared in nano scale.
The crystallite size (LWH) of the samples and the average lattice strain (ε) were calculated using the well-known Williamson–Hall equations [21]:
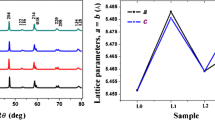
where K is the dimensionless shape factor (K = 0.9) and θ is the Bragg angle. Figure 2 illustrates the relation between βcos\(\theta\) and 4sin\(\theta\). The value of ε was determined from the slope of the plot in Fig. 2 and reported in Table 1. The crystallite size (LWH) was calculated from the intercept of the fitting line with y-axis and its values confirm that the samples were crystallized in nano scale. The W–H plot shows the negative value of the strain and indicates the compressive nature of it which leads to lattice shrinkage [18].
The unit cell volume and the theoretical density were calculated using the following equations, respectively.
where Z (Z = 4) is the number of molecules per unit cell, NA is the Avogadro’s number, M is the molecular weight, and V is the unit cell volume. The values of Dx of the vacancy-doped samples are less than that of LaFeO3 owing to the decrease of La3+ and Fe3+ content with investigated samples. The values of experimental density were calculated using the relation Dexp = \(\frac{m}{V}\) and reported in Table 1. The values of Dexp are smaller than the theoretical density owing to oxygen deficiency.
The tolerance factor was calculated using the following equation [22]:
where rA, rB and rO are the ionic radii of the A, B and oxygen ions respectively. The values of tolerance factors were listed in Table 1. The tolerance factor (t) is less than 1 which indicates that tilting of the < BO6 > octahedra occurs for all the samples. The tolerance factor indicates the orthorhombic structure of the samples La1-x□xFe1-y□yO3 and these results agree with the data obtained from XRD analysis. The values of the tolerance factor decrease with increasing the vacancy content (x and y) at the expense of La3+ and Fe3+ ions, which means that the distortion of the perovskite unit cell increases. This means that the Fe–O–Fe angle decreases with increasing the vacancy content (x and y). On the other hand, the tilting angle decreases. Besides, the values of t (0.9550 < t < 0.9514), indicated that the structure distorts.
To confirm the chemical composition of the samples, the energy dispersive spectroscopy EDS analysis was performed. Table 2 illustrates the EDS data of the investigated samples. The figure shows that the prepared samples are nearly equal to the starting chemical composition without any noticeable impurities. The weight percentage (wt%) of elements (La, Fe, and O) are calculated theoretically from the chemical formula La1-x□xFe1-y□yO3, (0.0 ≤ y, x ≤ 0.02, step 0.01) and that obtained from EDS elemental analysis are shown in Table 2. The results confirm that the chemical composition is very close to the nominal one. The small variation between the values of weight percentage (wt%) (La, Fe, and O) due to oxygen deficiency and converting some Fe3+ to Fe2+.
Figure 3 shows HRTEM images of the samples La1-x□xFe1-y□yO3. The images illustrate the orthorhombic structure of nanocrystalline samples. The values of particle size were listed in Table 1 and indicated that the samples were prepared in nano scale. The values of particle size agree with those obtained from XRD. The particles are agglomerated as shown in Fig. 3 owing to the magnetic properties of the samples. Different brightness from one crystallite to another appears as a result of different orientations of the crystal structure. The insets in Fig. 3 show the d-spacing of the investigated samples with values 0.26, 2.5 and 0.49 nm of the samples La1-x□xFe1-y□yO3, (0.0 ≤ x and y ≤ 0.02, step 0.01), respectively. The selected area electron diffraction (SAED) pattern of the investigated samples is illustrated in the insets in Fig. 3. The patterns show the good crystallinity of the samples.
Figure 4 shows the relation between the molar magnetic susceptibility (χM) and the absolute temperature for LaFeO3 nanoparticles. The sample has antiferromagnetic behavior where the values of χM decreased rapidly with temperature until the Néel temperature then decreased slowly with temperature. The magnetic interactions originated from the superexchange interaction between the Fe3+ ions, while there is no magnetic interaction between La3+ ion and Fe3+ ion because La3+ ion is not magnetic. By increasing the vacancies in the sample, the tilting of < FeO6 > octahedron increases, leading to a weak ferromagnetic ordering along with other crystal directions [23].
The comparative study between the vacancy doped samples in the molar magnetic susceptibility at the magnetic field intensity 1100 Oe is illustrated in Fig. 5. χM increases with increasing the vacancies on the A and B sites. The reasons for the increase of χM are:
-
(1)
Some of the Fe3+ ions convert to Fe4+ ions, which create the three magnetic interactions (antiferromagnetic between Fe3+–Fe3+ and Fe4+–Fe4+, ferromagnetic between Fe3+–Fe4+).
-
(2)
The tilting of < FeO6 > octahedron affects the bond length and strength between Fe–O ions.
The Curie–Weiss law is satisfied in the investigated samples. The straight lines in the paramagnetic region of LaFeO3 sample at 1100 Oe in the temperature range of 850–910 K are shown in Fig. 6.[15] The values of the Curie constant (C), Curie–Weiss constant (θ), and the effective magnetic moment (µeff) are calculated from the reciprocal of magnetic susceptibility with absolute temperature and reported in Table 3 using the following equations:
The presence of a ferromagnetic component in the samples was illustrated by the positive values of θ. The Néel temperature of the samples was determined from the plots of (dM/dT) vs T where M is the magnetization.
Figure 7 illustrates the hysteresis loops of the samples La1-x□xFe1-y□yO3, (0.0 ≤ x, y ≤ 0.02, step 0.01) at room temperature. The S-shape of the hysteresis loops indicates the presence of a weak ferromagnetic component in addition to the main antiferromagnetic order in the samples. The magnetic parameters such as saturation magnetization (Ms) and remnant magnetization (Mr) are listed in Table 4. The value of saturation magnetization Ms of LaFeO3 agrees with the data published by Gabala et al. [24]. The magnetization of the LaFeO3 sample was enhanced by vacancy doping. The value of Ms of the sample La0.98□0.02Fe0.98□0.02O3 increased by the factor 1.9 compared with the sample LaFeO3. The improvement of magnetic parameters for vacancy doped samples can be ascribed to several reasons as follows: (i) The tilting of < FeO6 > induces a variation in the Fe–O–Fe bond angle and distance; (ii) The existence of Fe3+ (HS) (t2g3 eg2) and Fe4+ ions in high spin state (HS) Fe4+ (t2g3 eg1) [7]. The inset in Fig. 7 illustrates the hysteresis loop of the parent sample (LaFeO3) has a clear shift from the origin due to the exchange bias effect. The exchange bias field (HEB) was calculated using Eq. (9):
where HLeft and Hright are the intercept of the magnetization on the −ve and +ve on the H-axis. The values of exchange bias were reported in Table 4. The exchange bias is present due to the exchange coupling between the ferromagnetic-like shell and the antiferromagnetic core in the nanoparticle [25].
Figure 8 illustrates the dependence of the dielectric constant on the absolute temperature of the sample La0.99□0.01Fe0.99□0.01O3. The trend of ε′ of the sample is the general trend of dielectric materials where ε′ increases with increasing the temperature and decreases with increasing the frequency.
Figure 9 shows the dependence of lnσ on the reciprocal of absolute temperature (1000/T) as a function of frequencies for the sample La0.99□0.01Fe0.99□0.01O3. The plot data obeys the well-known Arrhenius equation [26]:
where E is the activation energy, k is the Boltzmann’s constant and T is the absolute temperature. The presence of two different straight lines indicates the presence of two conduction mechanisms in the investigated samples. The activation energies were calculated and reported in Table 5 using the Arrhenius equation at frequency 5 MHz. The activation energy in the high temperature region (EII) is greater than that of low temperature (EI). The presence of two activation energies is due to the presence of two conduction mechanisms, which are the small polaron (SP) tunneling and the correlated barrier hopping (CBH) as shown in the next section.
The samples have semiconducting like behavior as clear from the increase in the conductivity with an increase of temperature. The values of activation energies confirm the semiconducting trend of the samples. The cation deficient in perovskite LaFeO3 played an important role in the enhancement of the conductivity. Introducing of vacancies in LaFeO3 leads to formation oxygen deficiency and converting some of Fe3+ ions to Fe4+ ions (t2g3 eg1 configuration). This will bring a localized electronic structure and lead to an inherent semiconductor nature [16].
Figure 10 illustrates the dependence of lnσ on lnω at different temperatures. The relation between lnσ and lnω obeys the universal power law. [27]
where σac is the ac conductivity, ω = 2πf is the angular frequency, A is a temperature dependent constant and S is the frequency dependent exponent. Figure 11 shows the relation between the frequency exponent factor (S) and T. In the 1st temperature region (300–450 K), the increase of S with T which indicates the small polaron (SP) tunneling in this region. This originated from converting some of the Fe3+ to Fe4+ ions, also, the electron conduction in perovskite-type ferrites takes place through Fe4+–O–Fe3+ [28]. While in the 2nd temperature region (450–800 K), S decreases with T which illustrates that the correlated barrier hopping (CBH) is the main mechanism. The electrons accept enough energy to hop between the O 2p and the Fe 3d orbitals. The hopping of electrons is affected by the Fe–O distance and the Fe–O–Fe angle. The values of the activation energy (EΠ) increased in the off-stoichiometric samples owing to the presence of oxygen vacancies [13].
4 Conclusion
Orthoferrite of formula La1-x□xFe1-y□yO3, (0.0 ≤ y, x ≤ 0.02, step 0.01) were well synthesized using the citrate combustion technique. XRD revealed that the investigated samples crystallized in a single phase orthorhombic structure with space group Pbnm. The particle size of LaFeO3 increased gradually with increasing the vacancy content. The magnetic hysteresis loops indicate the antiferromagnetic properties of the samples. χM of La0.98□0.02Fe0.98□0.02O3 increased by a factor 1.7 times more than of LaFeO3 sample. The investigated samples have semiconducting-like behavior as clear from the increase in the conductivity with the temperature and the values of activation energies. The small polaron tunneling (SP) and the correlated barrier hopping (CBH) are the main conduction mechanisms in the samples.
References
U.F. Vogt, P. Holtappels, J. Sfeir, J. Richter, S. Duval, D. Wiedenmann, A. Züttel, Fuel Cells 9, 899 (2009)
M.A. Ahmed, M.S. Selim, M.M. Arman, Mater. Chem. Phys. 129, 705 (2011)
X.-D. Zhou, J.B. Yang, E.-C. Thomsen, Q. Cai, B.J. Scarfino, Z. Nie, G.W. Coffey, W.J. James, W.B. Yelon, H.U. Anderson, J. Electrochem. Soc. 153, J133 (2006)
M. Idrees, M. Nadeem, M. Mehmood, M. Atif, K.H. Chae, M.M. Hassan, J. Phys. D. Appl. Phys. 44, 105401 (2011)
A.A. Saad, W. Khan, P. Dhiman, A.H. Naqvi, M. Singh, Electron. Mater. Lett. 9, 77 (2013)
M.M. Arman, S.I. El-Dek, J. Phys. Chem. Solids 152, 109980 (2021)
M.M. Arman, M.A. Ahmed, S.I. El-Dek, J. Supercond. Nov. Magn. 31, 1867 (2018)
I.D. Brown, Acta Crystallogr. Sect. B Struct. Sci. 48, 553 (1992)
H.Y. Hwang, T.T.M. Palstra, S.-W. Cheong, B. Batlogg, Phys. Rev. B 52, 15046 (1995)
Y.L. Zhu, Z.-G. Chen, W. Zhou, S.S. Jiang, J. Zou, Z.P. Shao, Chemsuschem 6, 2249 (2013)
F.F. Dong, M. Ni, Y.B. Chen, D.J. Chen, M.O. Tadé, Z.P. Shao, J. Mater. Chem. A 2, 20520 (2014)
R. Spinicci, A. Tofanari, A. Delmastro, D. Mazzab, S. Ronchetti, Mater. Chem. Phys. 76, 20 (2002)
M.D. Scafetta, S.J. May, Phys. Chem. Chem. Phys. 19, 10371 (2017)
Y. Zhu, W. Zhou, J. Yu, Y. Chen, M. Liu, Z. Shao, Chem. Mater. 28, 1691 (2016)
M.M. Arman, M.A. Ahmed, S.I. El-Dek, Appl. Phys. A 126, 27 (2020)
D. Du, R. Zheng, M. He, C. Zhao, B. Zhou, R. Li, H. Xu, X. Wen, T. Zeng, C. Shu, Energy Storage Mater. 43, 293 (2021)
M. M. Arman, J. Supercond. Novel Magn., 1–9 (2022).
E. E. Ateia, M. A. Ateia, M. M. Arman, J. Mater. Sci.: Mater. Electron. 1–12 (2021)
H. Shen, J. Xu, A. Wu, J. Zhao, M. Shi, Mater. Sci. Eng. B 157, 77 (2009)
M.M. Arman, N.G. Imam, R.L. Portales, S.I. El-Dek, J. Magn. Magn. Mater. 513, 167097 (2020)
V.D. Mote, Y. Purushotham, B.N. Dole, J. Theor. Appl. Phys. 6, 1 (2012)
E.E. Ateia, M.M. Arman, M. Morsy, Appl. Phys. A 125, 1 (2019)
H. Shen, G. Cheng, A. Wu, J. Xu, J. Zhao, Phys. Status Solidi A 206, 1420–1424 (2009)
M.A. Gabal, F. Al-Solami, Y.M. Al Angari, A.A. Ali, A.A. Al-Juaid, K.W. Huang, M. Alsabban, Ceram. Int. 45, 160 (2019)
K. Mukhopadhyay, A.S. Mahapatra, P.K. Chakrabarti, J. Magn. Magn. Mater. 329, 133–141 (2013)
R. Ramadan, S.I. El-Dek, M.M. Arman, Appl. Phys. A 126, 900 (2020)
S.R. Elliott, Adv. Phys. 36, 135–218 (1987)
Y. Ren, R. Küngas, R.J. Gorte, C. Deng, Solid State Ion. 212, 47–54 (2012)
Acknowledgements
The authors acknowledge deeply S.I. El-Dek (Faculty of Postgraduate Studies for Advanced Sciences (PSAS), Beni-Suef University, Beni-Suef, Egypt) for her nice cooperation and her kind support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arman, M.M., Ahmed, M.A. Effects of vacancy co‑doping on the structure, magnetic and dielectric properties of LaFeO3 perovskite nanoparticles. Appl. Phys. A 128, 554 (2022). https://doi.org/10.1007/s00339-022-05623-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-05623-9