Abstract
Assessment factors (AFs) are essential in the derivation of occupational exposure limits (OELs) and indoor air quality guidelines. The factors shall accommodate differences in sensitivity between subgroups, i.e., workers, healthy and sick people, and occupational exposure versus life-long exposure for the general population. Derivation of AFs itself is based on empirical knowledge from human and animal exposure studies with immanent uncertainty in the empirical evidence due to knowledge gaps and experimental reliability. Sensory irritation in the eyes and airways constitute about 30–40% of OELs and is an abundant symptom in non-industrial buildings characterizing the indoor air quality and general health. Intraspecies differences between subgroups of the general population should be quantified for the proposal of more ‘empirical’ based AFs. In this review, we focus on sensitivity differences in sensory irritation about gender, age, health status, and vulnerability in people, based solely on human exposure studies. Females are more sensitive to sensory irritation than males for few volatile substances. Older people appear less sensitive than younger ones. However, impaired defense mechanisms may increase vulnerability in the long term. Empirical evidence of sensory irritation in children is rare and limited to children down to the age of six years. Studies of the nervous system in children compared to adults suggest a higher sensitivity in children; however, some defense mechanisms are more efficient in children than in adults. Usually, exposure studies are performed with healthy subjects. Exposure studies with sick people are not representative due to the deselection of subjects with moderate or severe eye or airway diseases, which likely underestimates the sensitivity of the group of people with diseases. Psychological characterization like personality factors shows that concentrations of volatile substances far below their sensory irritation thresholds may influence the sensitivity, in part biased by odor perception. Thus, the protection of people with extreme personality traits is not feasible by an AF and other mitigation strategies are required. The available empirical evidence comprising age, lifestyle, and health supports an AF of not greater than up to 2 for sensory irritation. Further, general AFs are discouraged for derivation, rather substance-specific derivation of AFs is recommended based on the risk assessment of empirical data, deposition in the airways depending on the substance’s water solubility and compensating for knowledge and experimental gaps. Modeling of sensory irritation would be a better ‘empirical’ starting point for derivation of AFs for children, older, and sick people, as human exposure studies are not possible (due to ethical reasons) or not generalizable (due to self-selection). Dedicated AFs may be derived for environments where dry air, high room temperature, and visually demanding tasks aggravate the eyes or airways than for places in which the workload is balanced, while indoor playgrounds might need other AFs due to physical workload and affected groups of the general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure limits for volatile substances should be based on risk assessments considering empirical research (World Health Organization 2010). Such knowledge is a snapshot of current and past research that might be challenged by future and conflicting research results (e.g., based on newly developed research methods). Therefore, exposure limits should be regarded as temporary, since new empirical and reliable research data will require a reassessment.
Risk assessment should consider—on the one hand—the terms and conditions of exposure and—on the other hand—all exposed groups of people. In the worst case, exposure to a volatile substance lasts 24 h a day, seven days a week for a span of life (Rohlman et al. 2008; World Health Organization 2010). Further, one must consider that certain subgroups in the general population (might) differ systematically in sensitivity. Individual differences in sensitivity might be due to internal as well as external factors. Accordant to others (Bell et al. 2013; Hooper and Kaufman 2018; Portier et al. 2010), susceptibility of an individual, here, refers to factors inherent to internal factors (physical predisposition, i.e., internal defects) and vulnerability refers to external factors (e.g., low humidity leads to dry eyes in an otherwise healthy person). Both susceptible and vulnerable conditions could increase the sensitivity to volatile substances. The distinction, however, is far from clear as every perception is the result/interaction of internal and external factors. Susceptibility and vulnerability are often used interchangeable (cf., Merriam-Webster Thesaurus of ‘vulnerability’Footnote 1). Getting back to the general population, i.e., children, older people, or sick people might be more vulnerable or susceptible to certain volatile substances than healthy adults (Rohlman et al. 2008; World Health Organization 2010) and could, therefore, be more sensitive as a group. Such ‘group sensitivities’ must be considered in risk assessment.
Experimental exposure studies with human subjects are considered the gold standard for the derivation of No Observable Adverse Effect Concentrations for sensory irritation (Brüning et al. 2014; Nielsen and Wolkoff 2017). However, as experimental human exposure studies with susceptible or vulnerable people (i.e., children and persons with moderate or severe diseases) are rare from an ethical standpoint, other knowledge must be considered for risk assessment.
Much knowledge about exposure limits was derived from experimental animal models. In the past, animal models have provided information about the ‘mode of action’ (MOA) of different volatile substances (Andersen and Dennison 2001; Bushnell et al. 2007; Clewell 2005). However, the use of data from animal studies for setting human exposure limits is complicated by interspecies differences. Meanwhile, the current strategy in toxicology is to reduce and replace animal testing (National Research Council 2007). Therefore, alternative methods are propagated that use less animals, such as Quantitative Structure Activation Relations (QSAR; OECD 2014; Sullivan et al. 2014), and this also helps to establish a MOA/Adverse Outcome Pathways (AOP) (Organisation for Economic Co-operation and Development; OECD 2014; Patlewicz et al. 2014).
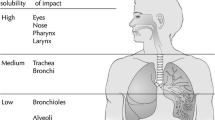
Since about 30–40% of the occupational exposure limits (OELs) (Dick and Ahlers 1998; Paustenbach 2001) of volatile substances are set to avoid irritation of mucous membranes, sensory irritation is an appropriate and sensitive parameter for measuring irritating effects in the respiratory tract (Brüning et al. 2014). Chemical sensitivity spanning chemosensory modalities seems unlikely (Lundström et al. 2012), thus different target sites (Alarie 1973; Arts et al. 2002) must be investigated. Shusterman (2002) described levels of impact on mucous membrane irritation depending on the water solubility of the volatile substance in the airways, and considering that the sites of irritation are concentration dependent, i.e., a nasal irritant at low concentration could readily become a pulmonary irritant at higher concentration (cf., U. S. Environmental Protection Agency 1994):
-
Eyes, nose, pharynx, larynx (high water solubility) – high deposition.
-
Trachea, bronchi (medium water solubility) – medium deposition.
-
Bronchioles, alveoli (low water solubility) – low deposition.
The first sites of contact with a volatile substance in the air are the eyes and the nasal mucosa, which are the focus of most of the methods assessing sensory irritation (Beuerman and Stern 2005; Doty et al. 2004; Kjærgaard et al. 1992). The cornea of the eye is the most innervated tissue in the body (Bonini et al. 2003; Yang et al. 2018; Zander and Weddell 1951). Various stimuli can be transduced by the cornea: thermal, mechanical, and chemical (Beuerman and Stern 2005; Yang et al. 2018). About 70% of the receptors are polymodal nociceptors, which convey inter alia pain in response to chemical stimuli via unmyelinated C-type nerves (Steen and Reeh 1993; Yang et al. 2018). Furthermore, 10% of the receptors in the cornea are Aδ- and C-fiber cold receptors, which may react (amongst others) to environmentally (e.g., dry air) induced tear film desiccation (Acosta et al. 2001; Beuerman and Stern 2005; Yang et al. 2018). Sensory irritation of the upper and lower airways is mediated by the activation of unmyelinated axons of the trigeminal nerve in the nasal mucosa (Alarie 1973) and beneath the epithelium of the lower airways (Benemei et al. 2015; Geppetti et al. 2010; Narula et al. 2014). Chemoreceptors/nociceptors (e.g., transient receptor potential channels; TRP channels) located on free endings of peripheral nerves innervating the airways (Bessac and Jordt 2008) elicit various reflexes or defense mechanisms. Therefore, there are receptors in the eyes, the nose, and lower airways that can be activated by volatile substances (Lehmann et al. 2017), which results in specific perceptions (e.g., burning, stinging; cf., Hummel 2000) and other reflexes and defense mechanisms, e.g., eye blinking (Doty et al. 2004). The response cascade elicited by volatile substances is of special interest because it allows for identifying objective markers of sensory irritation. A scientific approach to describe a response cascade is the concept of AOP (Ankley et al. 2010). Thus, Martinez and Eling (2019) describe such an AOP with a molecular-initiating event (MIE) and three sequential key events (KE) leading to an adverse outcome (sensory irritation).
A prolonged exposure to volatile substances eliciting sensory irritation (so-called local irritants; Schaper 1993) can lead to inflammation and subsequent tissue damage (cytotoxic effect) (Brüning et al. 2014). Thus, physiological indicators of sensory irritation should be avoided for the protection of the organism from chronic health effects such as tissue damage; these indicators are trigeminal-mediated reflexes (e.g., increase of eye blinking frequency) or inflammation (e.g., release of neuropeptides such as substance P) (Brüning et al. 2014). The avoidance of sensory irritation is one aim of setting exposure limits (Brüning et al. 2014; Nielsen and Wolkoff 2017). Thus, the difficulty of distinguishing between genuine sensory irritation-mediated perceptive and objective effects and cytotoxic effects should be emphasized.
The purpose of risk assessment is to identify a level of exposure to volatile substances at which the freedom of health effects is provided with some assurance (health-based standard; Fairhurst 1995). There are several critical outcomes of volatile substances that must be considered in setting exposure limits (World Health Organization 2010). If the database is limited, several (n-fold) assessment factors are used for extrapolation; for example, from animal exposure studies to humans or from young healthy individuals to the general population (Fairhurst 1995). The nomenclature of such factors differs depending on the organization (cf., Dankovic et al. 2015): Assessment factors, extrapolation factors, safety factors, uncertainty factors. This review uses the term assessment factors (AF, according to Vermeire et al. 1999, p. 441) and aims at intra-species extrapolation from young, healthy, mostly male individuals to the general population. Such an AF is’inherently arbitrary, debatable and potentially variable, depending on particular circumstances’ (Fairhurst 1995, p. 379).
While OELs usually are set to protect the worker population at employable age, environmental and indoor exposure limits should protect the general (whole) population including susceptible and vulnerable sub-groups. For the worker population, individual variability in sensitivity to sensory irritation is considered by a maximum AF of 2 if the threshold is based on valid human exposure studies with enough subjects (Brüning et al. 2014; Fairhurst 1995; Nielsen and Wolkoff 2017). Such an AF could be higher for the general population, comprising young and old, females, males, children, individuals with allergy (asthma) and other diseases (which may alter the sensitivity to sensory irritants). One reason is that the relatively low number of subjects in the exposure studies is not representative of the whole spectrum of human variability. Thus, for the general population usually an AF of 10 shall be applied according to ECHA Guidance R8 (Annex R.8–15: Guidance on Derivation of DNEL/DMEL from Human Data).
The assessment/uncertainty factor of 10 for the general population, however, is not dedicated to specific studies (endpoints) that aim to derive such AFs. Thus, the first aim of this study is to identify relevant controlled human exposure studies that could be a platform for derivation of (an) AF(s) for the general population, which is specific for ‘sensory irritation’ in the eyes and airways. The second aim is to propose (an) adequate AF(s) for the general population based on the identified exposure studies. Such a factor still has the disadvantage of AFs per se (cf., Fairhurst 1995), but its derivation is a step towards a more evidence-based approach to risk assessment (for sensory irritation).
Procedure
A literature search was carried out to identify relevant human experimental exposure studies. A total of more than 400 papers on sensory irritation in humans was scanned for information about (human) intraspecies variance in sensitivity to sensory irritation in the eyes and airways. Sensory irritation, here, comprises stimulation of nociceptors located in the upper airways. Such afferent stimulation may cause 'pungent sensations' to limit cytotoxic exposure but may also stimulate protective physiological responses suited to mitigate tissue injury (see discussion by Nielsen and Wolkoff 2017 and OECD 2017).
Grouping factors of the general population
In search of potential sources of intraspecies variability in sensory irritation, the following grouping factors for the general population were considered:
-
(1)
Gender
-
(2)
Age
-
Children.
-
Older people/retired.
-
(3)
Health status
-
People with allergic diseases.
-
Asthmatics.
-
(4)
Vulnerability.Footnote 2
-
(self-reported) multiple chemical sensitivity (MCS).
-
Psychological traits (e.g., affectivity).
Several studies have been identified with respect to the variability of sensory irritation in these sub-groups. The quality of studies was evaluated regarding the criteria for controlled chamber studies described by Nielsen and Wolkoff (2017). The most important criterion is that sensory irritation was appropriately measured, and the volatile substance exposure was well characterized.
Sensory irritation is linked to pungent sensations: stinging, piquancy, burning, tingling, freshness, prickling, irritation, itching, and cooling (Doty et al. 2004). Pungency refers to nasal (and oral) sensations that are mediated by the trigeminal nerve (Doty et al. 2004). As stated above, there are different target sites of sensory irritation in humans (Doty et al. 2004; Shusterman 2002). The following examples (non-exclusive) of markers of irritation are used at these target sites:
-
Eyes: i.e., irritation thresholds, blinking frequency, break-up time, eye redness, biomarkers in tear film, rating of eye irritation.
-
Nose: i.e., irritation thresholds, nasal airflow, changes in secretion, biomarkers in nasal lavage fluid, changes in nasal blood flow, changes in ciliary beat frequency, mucociliary clearance rate, rating of nasal irritation.
-
Pharynx/Larynx; Throat: i.e., changes in blood flow, rating of throat irritation, exhaled nitric oxide (FeNO).
-
Trachea/Bronchi: i.e., coughing, breathing parameters, exhaled nitric oxide (FeNO), urge to cough.
-
Bronchioles/Alveoli: i.e., coughing, breathing parameters, exhaled nitric oxide (FeNO), urge to cough, exhaled breath condensate.
-
The dry sensation in eyes and upper airways is often associated with exposure to some sensory irritants, which may be considered a proto-state of sensory irritation as proposed by Cain et al (2008). However, dry eye sensation may also be induced by other routes, e.g., desiccation of the eye tear film (Wolkoff 2020) or airways (Wolkoff 2018).
Subjective vs. objective markers of sensory irritation
Markers of sensory irritation are not necessarily assigned to only one level of sensory irritation (like breathing parameters). For this study, the level of (subjectively) perceived irritation is of secondary importance. Usually, objective markers are more reliable than ratings (Arts et al. 2006a; Paustenbach et al. 1997; Philpott et al. 2006) as the latter combine sensory and cognitive odor/irritation processing (Cometto-Muñiz and Cain 1991; van Thriel et al. 2008); thus, objective markers are prioritized. For example, the lateralization of chemicals (basis for irritation threshold studies) can only occur if an irritation takes place (e.g., Dalton et al. 2000) and this irritation is due to the volatile substance (chemesthesis). However, other stimuli than sensory irritants may influence sensory irritation. For instance, reflexive changes in eye blinking frequency can also be influenced by mechanical impact, thermal changes, air dryness, visual tasking, and individual factors (Klenø and Wolkoff 2004; Wolkoff 2020, 2018, 2017; Yang et al. 2018). Objective markers may be influenced by other than chemical exposure. Examples are biomarkers in nasal lavage and congestion (i.e., inflammation, allergy; Guilemany et al. 2009), coughing (i.e., air temperature; Benemei et al. 2015), and breathing frequency/depth (i.e., air temperature, COPD; Barry and Annesi-Maesano 2017). Therefore, potential stimuli having an impact on markers of irritation must be controlled in the experimental studies to trace back the sensory irritation to the chemical exposure without invalidating bias. Accordingly, information about different markers of sensory irritation is important.
For subjective measures, the time course of rated sensory irritation provides indirect information of sensory irritation, which takes place, though odor perception may increase the report of symptoms (Nielsen et al. 2007; Wolkoff et al. 2006) as shown by Cometto-Muñiz and Cain (1991). This is because odor thresholds generally are one to three orders of magnitude lower than thresholds for sensory irritation (Cometto-Muñiz and Abraham 2016). Contrary to odor ratings that generally show adaptation over time, ratings of sensory irritation should reveal an over-time increase in perceived irritation to a certain plateau (van Thriel et al. 2002). For eye irritation, many studies indicate that the time course of ratings (over hours) is close to objective markers (i.e., eye blinking frequencies; Juran et al. 2012; Kleinbeck et al. 2017, 2008; Pacharra et al. 2016c). Furthermore, the time course of eye irritation ratings appears plausible in view of pharmacokinetic/pharmacodynamics (PK/PD) modelingFootnote 3 of the rabbit eye (Kleinbeck et al. 2020). Thus, subjective ratings will also be considered with adequate caution, especially in studies where sensory irritation is also measured by objective markers.
There is only one experimental exposure study with children (Hummel et al. 2007); thus, their possible susceptibility or vulnerability for sensory irritation in the eyes and respiratory tract must be assessed by other approaches. Childhood can be conceptualized as a sequence of life stages comprising of windows of susceptibility to environmental agents, which might be enhanced (Firestone et al. 2008; Saadeh and Klaunig 2015; U. S. Environmental Protection Agency 2005). For this reason, developmental aspects of the target organs and their respective innervation will be considered. Potential factors for differential sensitivity in children compared to adults are the eye (precorneal) tear film stability (Borchman et al. 2012; Shrestha et al. 2011; Sledge et al. 2017), intranasal trigeminal function (Hummel et al. 2007), inhalation dosimetry (Foos et al. 2008; Garcia et al. 2009; Ginsberg et al. 2005), specific health effects and risks (Nielsen et al. 2013; Selgrade et al. 2008; Sunderland et al. 2019; Wolkoff and Nielsen 2010), internal dose metrics (Firestone et al. 2008; Valcke and Krishnan 2011), somatosensory processing (Uppal et al. 2016), and AOPs for developmental neurotoxicity (cf., Pistollato et al. 2020).
Manifestations of sensitivity
Sensitivity in sensory irritation to volatile substances has different manifestations. First, sensitivity can refer to sensory irritation at different concentrations of a volatile substance (that is, a lower threshold of sensory irritation means higher sensitivity). Second, the same concentration of a volatile substance can elicit stronger reactions in the sensory system in sensitive than in ‘normal-healthy’ persons (that is, the same concentration leads to a stronger physiological reaction of the sensory system in sensitive people). Third, the same physiological reaction can be perceived differently due to cognitive processing, e.g., odor-mediated. It is unclear whether these manifestations are interrelated (at least, the third seems to be another entity), but it is possible that they are independent. At first glance, the first manifestation of sensitivity might be the most important in deriving exposure limits based on sensory irritation. At long-lasting exposures to volatile substances, however, the other manifestations may become relevant, too. For example, sensitization could occur at lower concentrations in more sensitive people (second manifestation), independently of their sensory irritation threshold. The third manifestation of sensitivity is the most difficult for the derivation of AFs. This manifestation of sensitivity can lead to (reports of) perceptual aspects of sensory irritation in the presence of a volatile substance but without detectable physiological markers of sensory irritation. Though this manifestation of sensitivity is primarily based on cognitive processing it can elicit (psychogenic) symptoms that could be unbearable for an affected person (e.g., with multiple chemical sensitivity (MCS)). An AF for such manifestation of sensitivity is difficult to derive without detailed knowledge about the causality.
Temporal aspects of sensory irritation
Methods to identify differences in sensory irritation in humans are linked to different exposure durations. The toxic load in sensory irritation is a result of both concentration (C) and time (t) of exposure (Cn x t; Pauluhn 2019). While an exposure of a few seconds is reasonable for the assessment of (acute) thresholds, short-term exposure studies usually last minutes to hours, and epidemiological studies refer to years. However, temporal summation and carry-over effects may occur for sensory irritation: across seconds (Wise et al. 2009a, b, 2006, 2005), across minutes (Cain and Cometto-Muñiz 1995), and across hours (Cain et al. 2010; Cain and Cometto-Muñiz 1995; Kleinbeck et al. 2020, 2017).
Though differences in sensitivity might be observed in an exposure over seconds, a temporal summation over minutes or hours might occur at lower concentrations. The trigeminal system seems to act as a mass detector rather than a concentration detector (Frasnelli et al. 2017; Hummel and Frasnelli 2019; Kleinbeck et al. 2020). The detection of the trigeminal impact of a volatile substance may take tens of minutes especially at low concentrations (Cain et al. 2010; Wolkoff and Nielsen 2010). This is due to the latency of response of the responsible chemoreceptors for sensory irritation that could exceed minutes (as in the case of formaldehyde; Tian et al. 2009). Here, the second manifestation of sensitivity (see ‘Manifestations of sensitivity’) may play a role. Studies across days with human subjects are rare. Thus, a carry-over effect from one day to the other could not be found for ethyl acrylate in humans (Kleinbeck et al. 2020); however, signs of sensory irritation could be observed on every single day (increase in eye blinking frequency). Furthermore, Wolkoff et al. (2012) did not find an increase in sensory irritation in mice from day to day when repeatedly exposed to irritating mixtures of ozone-initiated limonene oxidation products over a period of ten days. These studies indicate that clean air between chemical exposures allowed for reversibility between daily impacts. However, the influence of longer continuous exposure periods (days) without recreation phases (which must be considered for indoor exposure limits) has not been studied in human subjects, yet.
Repeated exposure for three weeks to a water aerosol of 76 mg/m3 (31 ppm) acetic acid caused a substance-specific decrease in both the psychophysical and electrophysiological response to sensory irritation (n = 12) (Dalton et al. 2006). The study suggests that sensory irritation at low exposure levels either shows no carry-over effect or a carryover effect may cause desensitization; this is supported by a mice study, cf., Wolkoff et al. (2012), see above.
Epidemiologic studies comprising years were excluded from this review due to multi-factorial influences, and inadequate exposure characterization.
Highest to lowest priority for studies in this analysis of exposure:
-
(1)
across hours.
-
(2)
across minutes.
-
(3)
across seconds (thresholds).
-
(4)
across days.
-
(5)
across years (epidemiological studies) – excluded.
Therefore, studies will, in the first step, be tabulated by exposure duration (pattern) and target organ (cf., Shusterman 2002).
Limitations with respect to volatile substances
Studies with carbon dioxide were excluded for the derivation of an AF due to experimental caveats (e.g., unrealistic high levels) and its acidic nature, though some of these studies show significant differences. However, the caveats hamper a reasonable transfer of results to risk assessment for sensory irritants. Excluded studies concern gender, age, and health status (Acosta et al. 2006; Cometto-Muñiz and Noriega 1985; Feng and Simpson 2003; Kjærgaard et al. 1992; Scheibe et al. 2009; Shusterman and Balmes 1997; Shusterman et al. 2003a).
Influence of grouping factor gender on sensory irritation
Table 1 provides an overview of studies, which included ‘sensory irritation’ in both female and male subjects. Further characteristics of the studies (substances, substance delivery, examined concentrations, water solubility, and measures of sensory irritation) can be found in Sect. ‘Characteristics of the reviewed studies’ (Table 7). Footnote 4 At first glance, most studies either investigate exposure times of seconds (mostly threshold studies) or exposure times of hours. While the first kind of study utilizes acute irritation to determine irritation thresholds, the second kind of study usually uses lower concentrations. Sensory irritation, however, could arise by temporal summation in these studies. Therefore, it is essential to ensure that sensory irritation occurs at all during studies with hours of low exposure concentrations. Therefore, only studies with objective signs of irritation at the highest investigated concentration were analyzed.
Studies investigating ‘seconds’-exposure
Objective measures
Only three studies report gender differences in objective measures.
Claeson and Nordin (2011) found a significant gender effect in the detection of nasal irritation from amyl acetate.Footnote 5 They plotted the detection proportion at seven concentrations for males and females. The exposure concentrations (1592–3849 ppm) were presented in randomized order together with interrandomized exposure to clean air. The slope of the regression differed markedly between females and males and with a steeper regression in females (cf., Fig. 1 in Claeson and Nordin 2011). This indicates that a higher percentage of males detected irritation at lower concentrations, while a lower percentage of males detected irritation at higher concentrations. The P50 (irritation detected by 50%) was 1597 ppm for males and 1509 ppm in females as assessed from the regression line (cf., Table 1 in Claeson and Nordin 2011). Though the detection thresholds of females and males are without statistical significance, Claeson and Nordin (2011) demonstrated that the extrapolated P90 (irritation detected by 90%) is about half for females (3312 ppm) than for males (6678 ppm). Consequently, while around 90% of females would indicate 3300 ppm amyl acetate as irritating less than 75% of males would do so (cf., Table 1 in Claeson and Nordin 2011). An exposure limit, however, should protect the most sensitive individuals. In this case, females appear more sensitive compared to males. However, the sample is too small and too homogenous (mean ± SD age = 25.8 ± 3.6) for generalization to the general population. Further, there might be an odor bias to report nasal irritation in response to clean air, since amyl acetate has a strong odor component (Claeson and Nordin 2011), thus, leading to concern about the presence of sensory irritation at all investigated concentrations. Furthermore, the odor component of amyl acetate might have influenced the irritation ratings more among women than men (Claeson and Nordin 2011). This is possibly due to evolutionary and hormonal explanations (Brand and Millot 2001; Claeson and Nordin 2011; Doty and Cameron 2009). Cognitive and emotional factors may play a role in gender differences (Claeson and Nordin 2011; Lundström et al. 2005; Royet et al. 2003; Thuerauf et al. 2009). Interestingly, subjective ratings of irritation intensity did not elicit a significant gender difference.
Stuck et al. (2006) showed a gender effect in eucalyptolFootnote 6 lateralization. Thus, females demonstrated significantly higher sensitivity than males (35.64 vs. 33.00; higher values mean higher sensitivity). A gender difference was also observed in event-related potential (ERP) analyses. Amplitude and latency of the first negative peak and the second positive peak after a chemosensory event were higher in females compared to males.
It has been demonstrated that the thermal pain threshold is lower in females than in males (Gui et al. 2014). Therefore, males and females might perceive a cooling sensation of certain substances (i.e., menthol) differently leading to a proto-state of sensory irritation. Higher subjective ratings in females, hence, might be due to thermo-sensation. Furthermore, hygroscopic properties of certain substances may lead to a feeling of dryness in the eyes and nose. This perceived dryness might be a reason for ratings of sensory irritation without objective signs of sensory irritation (Dalton et al. 2018; Wolkoff 2018).
Several studies did not report significant gender differences at all, comprising nasal lateralization threshold for methyl isobutyl ketone (Dalton et al. 2000), linalool and menthol (Frasnelli and Hummel 2003), ammonia (Sundblad et al. 2004), benzaldehyde and eucalyptol (Hummel et al. 2003), ethanol (Mattes and DiMeglio 2001), formic acid, acetic acid, propionic acid, cyclohexylamine, dimethylamine, trimethylamine, ethyl formate, ethyl acetate, ethyl acrylate, methylcyclohexanone, cyclohexanone, cyclohexanol, ammonia, and hydrochloric acid (2 gender- and age-stratified samples of 72 non-smoking healthy subjects at two research institutes dividing out the substances; van Thriel et al. 2006), and temporal integration of homologous alcohols (Wise et al. 2007).
Therefore, a general higher sensitivity of females compared to males for acute sensory irritation for most substances seems unlikely based on the above studies. For a few substances, females appeared to be more sensitive; for instance, amyl acetate (Claeson and Nordin 2011) and eucalyptol (Stuck et al. 2006). Other, non-significant hints point in the same direction. However, no study showed a higher sensitivity in men.
Subjective ratings
Subjective ratings of irritation were higher in females compared to males in many studies. Females generally reported a significantly more intense pungency than males when exposed to nine different concentrations (ranging from odor to lateralization threshold) of six substancesFootnote 7 (formic acid, acetic acid, propionic acid, ethyl formate, ethyl acetate, and cyclohexylamine) each (van Thriel et al. 2008).
A significant gender effect was observed in ratings of nasal irritation when exposed to the highest concentration of SO2 Footnote 8 at 9 different concentrations (0.17 mg/m3 to 34 mg/m3; Kleinbeck et al. 2011). However, the authors argue that the dose–effect relationship of nasal irritation rating follows a saturation function that is typical for olfactory ratings, which differs from sensory irritation. However, 10% reduction of the breathing depth (tidal volume) was observed as the first objective and possible sign of sensory irritation at the highest exposure concentration. The responsible site of stimulation is not clear. It cannot be ruled out that the reduction of breathing depth is olfactory-driven. As a single breast belt was used, a change of breast breathing to abdominal breathing could be possible, though implausible in sitting subjects (Kleinbeck et al. 2011).
A gender difference in nasal irritation was not observed by exposure to mentholFootnote 9 by nostril lateralization (Ohla and Lundström, 2013). However, the authors found gender differences in response (event-related potentials) to cineole (higher late positive component (LPC) amplitudes and different temporal patterns in females). Parameters of the EEG were analyzed, i.e., amplitudes, latencies, and more special parameters like the LPC. The LPC proved to be faster and more pronounced in females compared to males in response to cineole.
Other studies did not report gender differences at all in the perception of sensory irritation (Claeson and Nordin 2011; Olofsson and Nordin 2004; van Thriel et al. 2006).
However, as mentioned above, over longer periods (min-hours) even sub-acute concentrations can have a trigeminal impact due to temporal summation (Cain et al. 2010).
Studies investigating ‘minutes’-exposure
Gender effects are not reported after 15 min exposure to 15 ppm acetic acid (Shusterman et al. 2005). Likewise, Yang et al. (2001) reported no gender effects in subjects’ eyes exposed for 5 min to very high concentrations of formaldehyde, a strong sensory irritant, at 1.65, 2.99, and 4.31 ppm. However, in these irritating conditions, no temporal summation took place, but an adaptation/habituation in eye blinking frequency as well as the subjective ratings. As conditions were clearly irritating (increased blinking frequency compared to clean air control condition), temporal summation could take place at lower (sub-acute) concentrations.
Studies investigating ‘hours’-exposures
Objective measures
Only exposure studies with objective signs of irritation are considered. This is due to that objective sensory irritation could be questioned in many studies as markers do not increase even at the highest exposure concentration (Ernstgård et al. 2002; Gminski et al. 2011; Hey et al. 2009; Juran et al. 2012; Kleinbeck et al. 2008; Sundblad et al. 2004; van Thriel et al. 2010; Wålinder et al. 2008). However, Wålinder et al. (2008) reported a significant increase of 2.3 eye blinks/min by two-hour exposure to 10 mg/m3 1-octen-3-ol,Footnote 10 but the eye blinking frequency is still low in the exposure condition (about 8 eye blinks/minute). The change in the eye blinking frequency is probably not due to objective eye irritation but biased by the smell of 1-octen-3-ol, because the exposure concentration is below an estimated LOAEL for sensory irritation (Wolkoff 2013). Furthermore, the eye tear film was unaffected. Ernstgård et al. (2002) reported a gender effect on forced vital capacity 3 h after exposure to 50 ppm m-xylene.Footnote 11 However, this gender difference is unlikely due to acute sensory irritation, as it could not be demonstrated immediately after exposure, and the exposure concentration is far below an estimated LOAEL (Wolkoff 2013).
Objective sensory irritation (as seen in eye blinking frequency) shows up at the end of exposure in studies with significant changes in eye irritation (4 h, 0–10 ppm peaks of ethyl acrylate, Kleinbeck et al. 2017; 4 h, 0–40 ppm ammonia, Pacharra et al. 2017, 4 h, 0–20 ppm propionic acid, Pacharra et al. 2016c; 4 h, 20 ppm 2-ethylhexanol, Schäper et al. 2015; 4 h, 0–10 ppm ethyl acrylate, Sucker et al. 2019). Though eye blinking frequencies also increase with time during the control condition (likely due to visual demands during ‘exposure’), the blinking frequencies are significantly higher at the end of the highest exposure concentration.
Most notably, objective sensory irritation from ethyl acrylate was re-investigated in a similar manner (4 h exposure; 0–10 ppm) (Kleinbeck et al. 2020, 2017; Sucker et al. 2019). A significant increase in the eye blinking frequency (e.g., 5 blinks/min in Kleinbeck et al. 2017) was seen at the highest exposure condition in comparison to the control condition (0 ppm). Neither of the studies found a gender effect on the eye blinking frequency when subjects were exposed to 5 ppm ethyl acrylate (TWA) with peaks of 10 ppm.
Other studies reported objective measures of eye irritation (eye blinking frequency), but no gender effect was observed. For instance, a change in irritation markers was observed for propionic acid (10 ppm with peaks of 20 ppm; Pacharra et al. 2016c), ammonia (20 ppm (TWA) with peaks of 40 ppm; Pacharra et al. 2017), and 2-ethylhexanol (20 ppm; Schäper et al. 2015). All exposures were at or above their estimated threshold for sensory irritation (Wolkoff 2013).
Temporal summation is usually accompanied by the respective perceptual ratings during the exposure condition (i.e., Fig. 4 in Kleinbeck et al. 2017).
Subjective ratings
Significant gender effects could only be observed in studies in which objective irritation is uncertain (Ernstgård et al. 2002; Wålinder et al. 2008).
Pacharra et al. (2016b) analyzed perceptual ratings in nine experiments, in which subjects were exposed to different substances (some of the experiments are described above). The results showed that trait-like modulators (i.e., odor-mediated sensitivity) affected (pungency and burning) ratings by females, but not by males.
Studies investigating ‘days’-exposure
Kleinbeck et al. (2020) and Lang et al. (2008) exposed subjects on subsequent days to ethyl acrylate and formaldehyde, respectively. Lang et al. (2008) used five randomized sequences of 10 exposure conditions on 10 subsequent workdays for two weeks. Therefore, the sequence of exposures is unbalanced and could lead to systematic biases (e.g., systematic desensitization). Day-to-day carry-over effects cannot be excluded and could aggravate the effects of low exposures. Carry-over effects on objective and subjective markers were studied systematically by exposure of the subjects to the same concentration of ethyl acrylate for five subsequent days on objective and subjective markers (Kleinbeck et al. 2020). Carry-over effects were unobservable, neither in males nor in females from day-to-day exposure, though sensory irritation could be seen on each day.
Summary of findings
Ocular irritation
Doughty (2002) reported no gender effects on spontaneous eye blinking but gender differences in the prevalence of reported eye irritation symptoms have been observed (Smith et al. 2007; van Wijk and Kolk 1997; Wolkoff et al. 2003). However, the influence of gender on sensory irritation in the eye due to experimental exposure to volatile substances could not be supported.
A large variance in eye blinking frequency may emerge over different studies due to individual differences, due to different tasks and situational factors, due to the inadequate separation of complete versus incomplete eye blinking, or insufficient data sampling for statistical analysis. Personal and occupational factors may also play a role in sensory irritation at the eye, e.g., use of contact lenses, eye make-up, certain medication, and eye diseases result in a more vulnerable tear film (Alves et al. 2023; Wolkoff 2020).
Nasal irritation
Gender differences only showed up in sensory irritation at the nose. It is surprising that studies that show gender differences in objective markers of sensory irritation report no gender difference in subjective markers or vice versa if both kinds of markers were measured. Differences are visible during short (seconds) exposures.
Irritation in the middle and lower airways
The only study that showed gender differences on objective markers (urge-to-cough; Gui et al. 2014) investigated thermal pain and not exposure to a volatile substance. However, this gender difference might explain influences other than exposure to volatile substances that might trigger or aggravate sensory irritation in females.
Generally, there is a higher incidence of chronic cough in females compared to males in the adult population regardless of exposure context (Morice et al. 2020; Morice and Kastelik 2003; Nasra and Belvisi 2009). Dicpinigaitis and coworkers demonstrated gender effects in urge-to-cough thresholds when subjects inhaled a capsaicin aerosol (non-volatile substance) with higher sensitivity in females (Dicpinigaitis et al. 2012; Dicpinigaitis and Rauf 1998). A similar effect of higher sensitivity was found in females regarding urge-to-cough with nebulized tartaric acid in a physiological saline solution (Fujimura et al. 1990). The fraction of a volatile substance reaching the lower airways is unclear due to the scrubbing effect of the nose in nasal breathing (Brüning et al. 2014; Garcia et al. 2009; Nielsen and Wolkoff 2017; Shusterman 2007). However, oral breathing together with physical exercise may increase the amount of inhaled volatile substances reaching the middle and lower airways.
In summary, no convincing evidence has been identified about a general gender difference for the endpoint ‘sensory irritation’ for most substances. However, for a few substances gender effects could be demonstrated for females, which turned out to be ‘more sensitive’ than males. This is in accordance with Nielsen and Wolkoff (Nielsen and Wolkoff 2017; Wolkoff 2013), who state that gender-related influences are, if observed at all, small. However, the influence of odor perception cannot be ruled out to bias the outcome, cf., Cometto-Muniz and Cain (1991) and van Thriel et al. (2008). Furthermore, climatic conditions (e.g., exposure to dry air) were not considered by Nielsen and Wolkoff (2017). Thus, the observed gender differences reported here may be due to the methodological shortcomings of the reviewed studies.
Nielsen and co-workers (Nielsen and Wolkoff 2017; Nielsen et al. 2007) argued that an AF of about 2 would account for the known differences in sensitivity due to gender (and age, lifestyle, and diseases). Furthermore, they claimed that a data-driven AF requires the evaluation of the effects of age, smoking, gender, and differences between eye and nasal irritation for each specific substance (Nielsen and Wolkoff 2017). Such a data-driven AF also requires consistency across studies (Nielsen and Wolkoff 2017).
In the light of possible gender effects, only six studies reported gender differences in sensory irritation due to volatile substances out of 29 reviewed studies. These have investigated a variety of different substances (See Sect. 'Characteristics of the reviewed studies', Table 7), and of which some outcomes might have been odor-driven (Claeson and Nordin 2011; Wålinder et al. 2008). However, interactions of gender and lifestyle and of gender and diseases (which both are included in the AF of 2 proposed by Nielsen and Wolkoff (2017)) are not reported in this review due to such studies are rare. Most of the reviewed studies investigate first and foremost young, healthy, non-smoking subjects. On that basis, a conservative AF of 2 for gender, age, lifestyle, and diseases is unchallenged. However, for this specific group of the general population and the respective substances, an AF for gender could be lower than 2, in part due to an odor-mediated bias. Consequently, substance-specific AFs are favorable from an economic point of view.
Influence of grouping factor age / older people on sensory irritation
Table 2 shows studies comparing young adults and older adults. Further characteristics of the studies (substances, substance delivery, examined concentrations, and measures of sensory irritation) can be found in Sect. ‘Characteristics of the reviewed studies’ (Table 7). Nearly all studies investigated short exposures (seconds) in the nose. Many studies determine irritation thresholds.
Studies investigating ‘seconds’-exposure
Some studies showed significant age effects in objective measurements. Of these, all thresholds for sensory irritation of volatile substances in older subjects are shown in Table 3.
If there is a significant effect of age, older subjects show lower sensitivity (higher thresholds) than younger subjects.
Subjective ratings do not reflect the objective differences in thresholds (cf., Table 3). It should be considered that age-related pathologies might be responsible for the loss of nasal sensitivity (Nordin et al. 2012; Stevens et al. 1982). Further, higher odor thresholds (lower sensitivity) among older people (Olofsson et al. 2021; Sinding et al. 2014; Stevens and Cain 1987) may also contribute to the overall variance.
Studies investigating ‘hours’-exposure
There is only one study that investigated the age effects of a longer exposure duration of 4 h with 2-ethylhexanolFootnote 12 (Schäper et al. 2015). Sensory irritation could be demonstrated by higher eye blinking frequency at the end of the highest and constant exposure condition of 20 ppm 2-ethylhexanol (temporal summation). However, there is no difference in eye blinking frequency or in eye irritation rating between young (18–35 years) and older subjects (45–67 years). The lack of difference, however, might be due to the relatively low age of the older subjects. Age effects in trigeminally induced eye blinks by fragrances occur mostly in subjects above 60 years (cf., Acosta et al. 2006; Frasnelli and Hummel 2003; Stevens et al. 1982).
Summary of findings
The compiled information about age effects may indicate that older people have higher thresholds. Confounding factors, i.e., (unrecognized) diseases (Rosenkranz et al. 2020), might cause higher prevalence in older age (Nordin et al. 2012). Another confounding factor could be differences in odor thresholds with lower odor sensitivity among older people (Olofsson et al. 2021; Sinding et al. 2014; Stevens and Cain 1987). On the other hand, it is well-known that older people, especially females, have a less stable eye tear film, which might result in elevated sensitivity to sensory irritants in the eyes (Wolkoff 2020).
Hence, the overall picture of possible age effects for the general population, as concluded by Nielsen and Wolkoff (2017), appears less clear, in part, since health status and other confounders may bias the outcome.
Age-related changes in the eye
Physiologically, there is a progressive reduction in nerve density in the human cornea occurring at the age of 70 years and older (He and Bazan 2010). This might be the effect of age-related pathologies (or eye diseases) and not a general decrease associated with age. The reduction might lead to reduced eye blinking in case of chemical exposure. Normal eye blinking has two functions: to restore the tear film and to defend the eye from environmental exposures (Alves et al. 2023; Wolkoff 2020). Therefore, eye blinking activity is essential for a healthy ocular surface; for instance, in maintaining the eye tear film stability (Wolkoff 2020). The loss of sensitivity, therefore, might result in a longer presence of a sensory irritant in the eyes.
Peshori et al. (2001) analyzed eye blinking frequency in human subjects of different ages (20–80 years). They used irritating electrical stimuli of the supraorbital branch of the trigeminal nerve to evoke blinking. The lowest stimulus intensity that reliably evoked blinking was set as threshold. For data collection, the electrical stimulus was the twofold threshold. They demonstrated a significant increase in lid-closing duration, excitability, and latency of the eye blinking in subjects over 60 years compared to younger subjects. A reduced sensitivity and higher latency of eye blinking may lead to slower clearance of the eye tear film in older adults. Therefore, a more efficient clearance may prevent sensory effects in the eyes of young adults but be detrimental to the eyes of older people with a longer clearance time. For example, age is a risk factor for eye symptomatology (Sharma and Hindman 2014; Wolkoff 2020).
Age-related changes in the nose
The odor thresholds among older people are higher (lower sensitivity) than among younger people (Olofsson et al. 2021; Sinding et al. 2014; Stevens and Cain 1987). Whether sensory irritation is affected by odor perception is unclear. However, if sensory irritation ratings were (at least in part) olfactory-driven, older subjects would probably show lower sensitivity. Sunwoo et al. (2006) demonstrated that the mucociliary clearance time (removal of foreign bodies; the saccharin test), which is generally slower in older males (n = 8, mean age: 71.8 years) compared to young male subjects (n = 8; mean age: 21.7 years), is affected by 90 min exposure to low air humidity (10%) only in elderly subjects; however, the difference diminished after 180 min exposure. Ho et al. (2001) measured slower ciliary beat frequency, a higher percent of ciliary cross sections displaying single tubules, and longer mucociliary clearance time in subjects older than 40 years (41–90 years; n = 43) compared to younger subjects (11–40 years; n = 47). Consequently, harmful substances may stay longer in the nose of older people (see further discussion in Wolkoff 2018).
A detrimental effect of delayed protective reflexes in older subjects should come up with longer exposure. Consequently, older people might have higher thresholds (in ‘seconds’ exposure) indicating lower sensitivity but would need, nevertheless, lower exposure levels due to other age-related influences on the susceptibility of sensory irritation at longer exposure periods (i.e., reduced warning signs). There is a small, but demonstrable age-related decline in early warning functions of pain (Gibson and Farrell 2004). For example, Barbariga et al. (2018) found an effect of aging on nerve morphology and substance P expression (loss with age) in human corneas.
Age-related cognitive changes
Influence of expectations among old people cannot be ruled out. Older people might think that their eyes and nose have become less sensitive and therefore reduce their effort during threshold experiments. Miller et al. (2013) investigated the influence of priming elderly stereotypes (schematic images of a person due to group membership) on olfactory tests (Sniffin’ Sticks; Hummel et al. 1997), perceptual ratings, verbal and motor behaviors. Stereotypes were primed by describing a profound age-related decline in olfactory function in primed subjects while control subjects received general information regarding the olfactory system. They did not find an influence of priming on olfactory performance; however, verbal and motor behaviors were altered by priming older people.
Influence of the grouping factor age / children on sensory irritation
Empirical evidence
Hummel et al. (2007) found differences between age groups in the assessment of the trigeminal function (nasal lateralization) of eucalyptol in children in comparison to adults (5–54 years). Thus, lower scores (i.e., lower sensitivity) were observed in the youngest group (5 years) compared to all other, lower scores in subjects at the age of 9 compared to participants at the age of 14, and subjects at the age of 6 years had lower scores compared to’subjects aged 4 [!] and above’. However, the conclusion by Hummel et al. (2007) indicates no significant differences between subjects at age 7 and older subjects (up to 54 years). It remains unclear whether the lower score in younger children (5 years) is due to lower sensitivity or due to the test procedures used for nasal lateralization.
There is no experimental study with children younger than 5 years. Therefore, other sources of information should be used in risk assessment. One must keep in mind that impairments of childhood development may have lifelong consequences on possible effects of sensory irritation in children (Peled 2011).
Other sources of information on sensory irritation susceptivility in children
While fetuses and children are particularly susceptible to neurotoxic effects as the brain is more sensitive during its growth stages, less is known about children’s sensitivity to sensory irritation (Berglund et al. 1992). However, developmental changes in the organs potentially affected by sensory irritation can give indirect hints.
The eye tear film stability is higher in children compared to adults (Borchman et al. 2012). Thus, the meibum of infants and children contains less CH3 and unsaturated C-C groups and an increased aldehyde-to-lipid hydroperoxide ratio (Borchman et al. 2012; Shrestha et al. 2011; Sledge et al. 2017; Wolkoff 2020). Further, the eye blinking frequency in infants is substantially lower (< 1 blinks/min; Mantelli et al. 2007; Zametkin et al. 1979) than in adults (Cruz et al. 2011). Furthermore, the tear break-up time is longer in infants than in adults (Cho and Yap 1993; Mohidin et al. 2002; Ozdemir and Temizdemir 2010). This indicates that infants and children have a more stable and intact eye tear film.
Berglund et al. (1992) consider children’s respiratory system as prone to the effects of indoor air pollutants that might lead to developmental impairment in the lung. Children have faster respiratory (Berglund et al. 1992; Ginsberg et al. 2005) and metabolic rates in comparison to adults (Berglund et al. 1992). However, the nasal contribution to breathing with exercise is lower in children, in part due to oral inhalation, compared to adults (Bennett et al. 2008). Therefore, a sensory irritant might reach the middle and lower respiratory system in children during exercise, while the irritant might be scrubbed in the nose of adults, depending on its water solubility (e.g., Garcia et al. 2009). Furthermore, children breathe 50% more per kg body weight compared to adults (Peled 2011). The lung epithelium, however, is not fully developed during early childhood (Foos et al. 2008; Peled 2011), while the pulmonary surface area per body weight is higher than in adults (Ginsberg et al. 2010). However, growth of the lung is not the only developmental factor (Saadeh and Klaunig 2015). All developmental changes in a child’s body may be crucial in the risk assessment process for children. For example, children’s immune system is also developing (Foos et al. 2008; Peled 2011; Schwartz 2004). All of this may cause more susceptibility in children to the systemic effects of air pollutants. This could be shown by Valcke and Krishnan (2011) for internal dose metrics in neonates compared to adults. Generalization, however, is hampered by many not well-characterized factors, e.g., the relevance of the pollutant polarity, the higher metabolic rate, and the exposed surface in the airways of children versus in the airways of adults (cf., Garcia et al. 2009).
Environmental factors like exposure to indoor and outdoor air pollutants like tobacco smoke or exhaust emissions are responsible for respiratory health effects in children (Kasznia-Kocot et al. 2010; Selgrade et al. 2008). Selgrade et al. (2008) summarized investigations on ozone (a pulmonary irritant) exposure in infant rhesus monkeys compared to clean air exposure. This showed significant changes in airway development, i.e., decreased airway size, increased number of mucous cells, and less innervation. Therefore, pharmacokinetic and pharmacodynamics age- and gender-specific models were developed (Sarangapani et al. 2003; Sweeney et al. 2015) to evaluate the risk of volatiles.
For instance, nasal modeling of airborne formaldehyde uptake (computational fluid dynamics) of human noses (2 children, 5 adults) showed that 90% of inhaled formaldehyde was extracted in the nose of adults as well as children, while a maximum of 10% may pass the nasal cavity and reach the larynx and eventually the lower airways at resting conditions (Garcia et al. 2009). Another nasal modeling showed that airborne formaldehyde-induced DNA protein cross-linking about 1.5 higher in adults than in children (Firestone et al. 2008), indicative of about 50% lower maximum effective dose in children; thus, indicative of less susceptible in children than adults for this highly water-soluble substance.
Developmental influences
For the risk assessment of sensory irritation in children, it is reasonable to consider the development and the number of sensors as well as differences in nervous transfer.
Adverse outcome pathways represent the sequence of biological events that are caused by chemical exposures and disturb homeostasis (Frank et al. 2018). Adverse outcome pathways for sensory irritation have been described (Frank et al. 2018; Martinez and Eling 2019), and AOP key events have been defined that lead to a specific adverse outcome (OECD 2013). Such a sequence begins with MIEs, which describe initial points of interaction resulting in pertubation (OECD 2013). Frank et al. (2018) identified 5 MIEs for sensory airway irritation:
-
(1)
Activation of nociceptors
-
(2)
Peroxidation of membrane lipids
-
(3)
Induction of reactive oxygen species (ROS) and oxidative stress
-
(4)
Abrupt changes in extracellular saline
-
(5)
Phospholipase activation during detergent–membrane interactions
Each of these MIEs is directly induced by irritant exposure and can result in sensory irritation symptoms (Frank et al. 2018). Some studies show developmental aspects of these MIEs, such as nociceptors (increased physiological sensitivity to pain in neonates; Anand and Carr 1989; Bouza 2009), peroxidation of membrane lipids, and ROS and oxidative stress (Auten and Davis 2009).
For volatile substances binding to the TRPA1 receptor, Martinez and Eling (2019) defined 3 KEs leading to the adverse outcome of sensory irritation:
-
(KE 1) Increase in intracellular CA+2 in nerves
-
(KE 2) Trigeminal/vagal nerve activation
-
(KE 3) Neurogenic inflammation (increase of substance P and CGRP)
Age-related differences in KE1 ‘Increase in intracellular CA+2 in nerves’
The increase in intracellular CA+2 is an indicator of receptor (e.g., TRPA1) activation. Banzrai et al. (2016) found developmental differences in sensory axonal excitability in normal mice using threshold tracking. Though, it is not clear whether a transfer to humans’ sensory system is possible. As an indirect hint for developmental differences in receptor activation, the density of receptors is considered as they indicate differences in sensitivity (the higher the sensor density, the higher the chance of receptor activation at a certain concentration).
In eyes, nerve terminals or free endings are responsible for transducing sensory stimuli into nerve signals (Belmonte et al. 2004; He et al. 2010). The numbers of free nerve endings are proportional to corneal sensitivity (Belmonte et al. 2004; He et al. 2010).
The sensory innervation of the cornea is derived from 30–80 stromal nerve trunks (Spadea et al. 2013; Zander and Weddell 1951). A single neuron supports 200–3000 individual corneal nerve endings (Spadea et al. 2013). Developmental aspects of corneal innervation are known for mice (He and Bazan 2016). The innervation of adult mice and adult humans share many common features (He and Bazan 2016, 2010). In mice, the innervation of the cornea still develeops after birth (He and Bazan 2016). The mouse cornea is mature after 8 weeks from birth (He and Bazan 2016). Cornea maturity age in mice corresponds to the human age of 6 years (relating average lifespans of mice and men; Dutta and Sengupta 2016). Between week 1 and week 3 the mouse cornea is mainly innervated by stromal nerves (He and Bazan 2016). Epithelial nerves were short, thin, and extended without a determined direction. Epithelial nerves derive from stromal nerve branches penetrate the subbasal layer of the cornea (He and Bazan 2016). With maturity, those nerves grow to a whorl-like structure. There is a stromal nerve regression when the mouse is mature, which might be due to neurotrophins regulating connections between nerve cells (e.g., nerve growth factor; He and Bazan 2016).
As argued above for older people, less innervation means less sensitivity of children’s eyes, which might lead to less sensory irritation but a higher susceptibility to damage as defense mechanisms (like eye blinking) may be insufficient. Contrary, the tear film stability is higher in children compared to adults (Borchman et al. 2012). Therefore, risk assessment of any volatile substance requires accounting for the complex interplay of different defense mechanisms at different developmental stages in children. It is still not known whether volatile substances might have an impact on sensory development in children at a concentration harmless to adults. Any damage to corneal nerves, however, leads to diminished corneal sensitivity and could lead to long-term alterations in the functional integrity of the ocular surface (Marfurt 2010; Marfurt et al. 2010; Medeiros and Santhiago 2020; Spadea et al. 2013). Although corneal nerves are capable of regeneration, this process is slow and imperfect (Spadea et al. 2013). For example, regeneration after most corneal surgeries is accompanied by reduced nerve density, alterations in nerve architecture, and consequently lower corneal sensitivity (Spadea et al. 2013). In children, any damage to sensory nerves might lead to long-term impairment that must be considered in risk assessment.
Age-related differences in KE2 ‘Trigeminal/vagal nerve activation’
In addition to differences in nerve density, there might be differences in nerve transmission during developmental changes in children. Uppal et al. (2016) investigated the neural dynamics of somatosensory processing of adaptation across childhood via vibrotactile stimuli at the children’s wrist. Differences in somatosensory evoked potentials could be identified in different age groups (6–10, 10–13, and 13–17 years old). The authors conclude that reactivity to tactile stimulation seems to change dramatically during childhood. Uppal et al. (2016) speculate that such difference might be due to the co-working of’immature’ and’mature’ networks; for instance, there could be different configurations of neural generators in somatosensory cortices or developmental changes in the anatomy. Otherwise, it is possible that skull thickness might influence the scalp-reported signal, though the underlying neuronal response was the same (Uppal et al. 2016).
Many populations of neurons in the vertebrate nervous system pass through 4 phases of development (Davies 1988):
-
(1)
Differentiation from progenitor cells
-
(2)
Growth of axons to their target fields
-
(3)
Target field innervation
-
(4)
Remodelling axon connections
Though much of the trigeminal system of rats develops during the embryonic stage (Davies 1988; Stainier and Gilbert 1991) there is also postnatal development of the trigeminal system (Toma et al. 2006). For example, glial elements of root entry zones in the central nervous system are mainly developed postnatally in rats (Toma et al. 2006) until the end of week 2 (corresponding to 1 year in humans, relating average life spans of rats and men; Andreollo et al. 2012). Therefore, the central nervous system parts of mammalian trigeminal systems seem not to be mature at birth. This could lead to stronger adverse perceptions in children at a certain developmental stage exposed to stimulus intensities, which would not be adverse in children at other developmental stages (cf., vibrotactile stimuli in Uppal et al. 2016).
Age-related differences in KE3’Neurogenic inflammation (increase of Substance P and CGRP)’
Distribution of the neural peptides substance P and calcitonin gene-related protein (CGRP) was determined by staining in mice cornea (He and Bazan 2016) with a higher content of CGRP than that of substance P in mice epithelial innervation. However, the authors did not investigate developmental changes in this distribution. In older studies, the pattern of corneal CGRP innervation of rats continuously changes during the first 3 weeks after birth (1.5 years in humans, relating average life spans; Andreollo et al. 2012) and then reaches the adult state (Jones and Marfurt 1991). During week 1, for example, CGRP innervation density of the pericentral corneal epithelium increases relative to the peripheral corneal epithelium, intraepithelial axons become more branched, thinner, more prominently beaded, and the number of free nerve endings increases (Jones and Marfurt 1991). This suggests that less CGRP might be released due to sensory irritation of the eye in children compared to adults due to lower availability. The same might be true for substance P as 99% of all corneal protein gene product (PGP)-p.5-immunoreactive nerves contain both CGRP and substance P in dog corneas (Marfurt et al. 2001).
Risk assessment for children, therefore, requires consideration of developmental changes affecting MIEs and KEs.
Summary of findings
The only experimental study with children demonstrated that 7-year-old children are as sensitive to nasal irritation as adolescents and adults (Hummel et al. 2007). Therefore, some researchers consider that risk assessment of sensory irritation in children might not be of special concern (Dourson et al. 2010). Others conclude that children’s risk assessment requires special consideration due to developmental changes (Saadeh and Klaunig 2015). For example, Hunter et al. (2010) identified postnatal phases of special susceptibility to ozone in rats; however, ozone is not a sensory, but a pulmonary irritant.
Saadeh and Klaunig (2015) conceptualize different risk factors for children, inter alia physiological and immunological development, breathing rate, physical activity, and respiratory system development. They integrate the available methods for children’s inhalational risk assessment by comprehensive PK/PD modeling of different target sites. Such modeling could be used to identify target sites at which children might be more sensitive or obtain a higher dosage compared to young adults. A quantification of differences (if identified) must be the next step.
For occupational risk assessment, Maier et al. (2014) argue that young naïve non-smoking adults represent the most responsive sub-group for sensory irritation in controlled exposure studies. Regarding the argumentation above, this claim cannot be transferred to the general population. Children and older people might suffer more from the same exposure to sensory irritants than young naïve adults. Furthermore, certain (chronic) diseases might influence the sensitivity (even in naïve non-smoking young adults).
Influence of the grouping factor health status on sensory irritation
Different diseases have an impact on the eyes and the airways. For example, inflammatory diseases have a direct impact on the sensors involved in sensory irritation. Furthermore, acute impacts can lead to worsening (exacerbation) of chronic lung diseases (‘acute on chronic’ lung disease; Kreuter and Cottin 2017). Environmental pollution and occupational exposures elicit such exacerbations, for example in asthma and COPD (Eisner et al. 2010; Vogelmeier et al. 2017; Skaaby et al. 2021).
Allergy/asthma
In respiratory allergies, substances that are harmless to the organism can elicit an immune response. The first step in developing a respiratory allergy is the sensitization to a substance (called antigen; Briatico-Vangosa et al. 1994). Such sensitization can be classified into 4 hypersensitivity reactions (Dispenza 2019; Gell and Coombs 1963). The most common type which incorporates hay fever and allergic asthma (cf., prevalence in Germany, Bergmann et al. 2016) is Type 1 allergy. The critical event during sensitization is the development of mast cell binding antibodies (immunoglobulin E; IgE). An individual is sensitized if during an immune response antigen-specific IgE antibodies are produced that ‘prime’ mast cells in various tissues (Briatico-Vangosa et al. 1994). Consequently, repeated exposure to the same or similar substance leads to a hypersensitivity reaction (Briatico-Vangosa et al. 1994). The neuropeptide nerve growth factor is a neurotrophic factor that plays a critical role in hypersensitivity (Päth et al. 2002). A sensitized individual might develop an allergy on subsequent exposure to the antigen. Activated mast cells, other inflammatory cells, and resident cells can stimulate nerve endings and cause long-lasting changes in neuronal excitability (Undem and Taylor-Clark 2014). Such a reaction is an exaggerated response of the sensory system due to interaction with sensory nerves, changes in central nervous processing, and altered transmission in sympathetic, parasympathetic, and enteric autonomic nerves (Undem and Taylor-Clark 2014).
Seasonal allergic rhinitis and asthma are inflammatory diseases affecting the airways, first the upper airways and second the lower respiratory tract. Both are linked by a common pathogenic process with overlapping inflammatory cells, mediators, and cytokines (Bjermer 2007). The bridge between the upper and lower airways seems to be systemic inflammation (Alving and Malinovschi 2010; Bjermer 2007), which might lead to a higher sensitivity against sensory irritants. Therefore, studies on elevated sensitivity in seasonal allergic rhinitis and in asthma were analyzed together.
Furthermore, exposure to some volatile substances by itself might generate an allergic disease (Nurmatov et al. 2015; Tagiyeva and Sheikh 2014), i.e., occupational asthma (Maestrelli et al. 2009) or Reactive Airway Disease Syndrome (RADS; Johnson et al. 2019). Some volatile substances activate the TRPA1 receptor by forming a Michael addition product with a cysteine residue of TRPA1 through covalent protein modification; thus, as a consequence, causing allergic reactions (i.e., isothiocyanate and sulfides; Mihara and Shibamoto 2015).
Many studies have experimentally investigated differences between subjects with allergies and healthy control subjects. All of these have in common that there is a self–selection of participants, i.e., only subjects with mild (or moderate) allergy may participate. In other words, people with severe disease and assumed higher sensitivity probably deselect such exposure studies. Table 4 shows the studies investigating allergy/asthma effects in sensory irritation. Further characteristics of the studies (substances, substance delivery, examined concentrations, and measures of sensory irritation) can be found in Sect. ‘Characteristics of the reviewed studies’ (Table 7).
Studies investigating ‘seconds’-exposure
Shusterman et al. (2003a) demonstrated that rhinitis status could predict VOC localization of n-propanol (indicator of irritation); allergic rhinitis sufferers turned out to be more sensitive than the healthy controls (no numbers given). An odor-driven bias reaction cannot be ruled out.
It was demonstrated that markers of sensory irritation depended on the severity of rhinitis by comparison of exposed subjects with non-active and active rhinitis with healthy controls to mannitol (dry powder mannitol; 200 mg mannitol/mL; Koskela et al. 2000). An increase in 15-hydroxyeicosatetraenoic acid (molar mass = 320.5; an index of epithelial cell activation) correlated with nasal symptoms for itching and burning. Nasal symptoms increased even until 10 min after the challenge in subjects with active allergic rhinitis, while this was not the case in healthy subjects. Furthermore, nasal peak inspiratory flow was reduced after the mannitol challenge in patients with active allergic rhinitis. However, the transfer of these results to volatile substances is not possible.
Petrova et al. (2008) could not identify an elevated sensitivity (ocular threshold, nasal threshold, combined threshold) to ammonia in mild to moderate asthmatics compared to healthy control subjects. However, none of the asthmatics had acute inflammatory or rhinitis symptoms during the test sessions.
Studies investigating ‘minutes’ exposure
Shusterman et al. (2005) exposed subjects with and without seasonal allergic rhinitis to acetic acid (15 ppm) for 15 min. The subjects with rhinitis were tested outside their relevant pollen season. The nasal airway resistance compared to baseline was greater among the subjects with rhinitis compared to those without rhinitis immediately and 15 min after the exposure.
Shusterman et al. (1998, 2003b) exposed subjects with and without seasonal allergic rhinitis to chlorineFootnote 13 (1 ppm; a reactive substance) for 15 min. The nasal airway resistance compared to baseline was greater in the rhinitis group immediately and 15 min after exposure compared to the group without rhinitis. However, the subjective rating of nasal irritation was low (closer to the label ‘none’ than to ‘slight’) so that sensory irritation might not be the reason for this physiological reaction. An odor-mediated bias for both studies cannot be ruled out.
Studies investigating ‘hours’ exposure
Pacharra et al. (2017) describe weak changes in the eye blinking frequency (marker of sensory irritation) by exposure to ammonia (peaks of 40 ppm, 20 ppm TWA). Subjects with seasonal allergic rhinitis did not show a higher sensitivity than healthy control subjects, neither in subjective ratings nor in objective markers of sensory irritation. The study was conducted off-season, subjects with seasonal allergic rhinitis still showed higher fractional exhaled NO (FeNO). There might be a self-selection of subjects with mild allergic rhinitis since those with moderate or severe symptoms are likely to deselect participation in an exposure study.
Sucker et al. (2019) found differences in perceptual ratings only during sham exposure to ethyl acrylateFootnote 14 with higher ratings in atopic subjects compared to healthy control subjects. Moreover, the interaction between atopy and ethyl acrylate exposure was not significant. However, they observed that the eye blinking frequency in all atopics was higher than 20 blinks/min, while the healthy control subjects showed 8 to 37 blinks/min. Yet, the main effect of atopy on eye blinking was only marginal.
Fadeyi et al. (2015) exposed asthmatic and non-asthmatic subjects to a mixture of ozone-limonene reaction products (i.e., formaldehydeFootnote 15) in a field environmental chamber for 3 h. Odor intensity and sensory irritation were rated lower by asthmatic subjects compared to healthy control subjects. Perceived physiological–like symptom ratings (flu, chest tightness, and headache) of asthmatics, however, were often higher compared to the non-asthmatic subjects. It is unclear whether sensory irritation took place as objective markers of sensory irritation were not measured. Although the report of sensory irritation is low among both groups of subjects, the finding is compatible with the fact that asthmatics produce more mucous acting as a scrubber (e.g., Garcia et al. 2009), in agreement with the finding that sensitized mice are less sensitive than normal mice to formaldehyde at low relative humidity (Larsen et al. 2013). In general, no convincing evidence has been found that asthmatics are more sensitive than healthy subjects to formaldehyde (a highly water-soluble substance), as reviewed by Wolkoff and Nielsen (2010); a conclusion later supported by Golden (2011).
Summary of findings
There is inconclusive evidence of higher sensitivity in subjects with allergic diseases. Subjects with seasonal allergic rhinitis were usually investigated outside the pollen season (Pacharra et al. 2017; Shusterman et al. 2005; Sucker et al. 2019). It remains unclear whether the described (lack of) effects could also be seen during pollen season with acute complaints and whether a medication during pollen season has additional effects. Still, a minimal persistent inflammation can also be observed in patients with seasonal allergic rhinitis outside the pollen season (Ricca et al. 2000).
As mentioned by Pacharra et al. (2017) and above, individuals with a severe allergic disease probably deselect participation in such exposure studies. Therefore, other sources of knowledge must be considered.
There are indirect hints that airway inflammation (due to acute allergy) might change the sensitivity to sensory irritants based on animal experiments and knowledge about inflammatory processes during allergic phases in the eyes and airways. For instance, Belvisi (2003) proposed that changes in Aδ- und C-fibers in airway inflammation might lead to exaggerated function in response to the inflammatory process. The next paragraphs discuss such influences.
Potential influences of allergic diseases on the eye
Acosta et al. (2013) investigated keratoconjunctivitis in guinea pigs. They recorded changes in the electric activity of cornea conjunctival sensory nerve fibers in response to CO2 following an ocular allergic challenge provoked by ovalbumin. After repeated exposure to the allergen, the frequency of impulses in polymodal fibers was significantly higher and the impulse latencies were shorter compared to controls. The response to heat, however, was lower after the first allergic challenge but returned to control levels after the repetitive exposures.
Potential influences of allergic diseases on nose/airways
Opiekun et al. (2003) could not identify differences in objective markers of sensory irritation (ocular redness, nasal mucosal swelling) when exposing asthmatics and healthy control subjects to fragrances, though asthmatics reported more nasal symptoms. No sensory irritation could be provoked by the fragrances at the investigated concentrations.
Asthma is frequently cited as an outcome of adverse health events relating to air fresheners (Johnson et al. 2019). Fragrances inhaled in indoor air are usually below thresholds for sensory irritation (Nielsen and Wolkoff 2017). However, asthma symptoms can be triggered by very low doses of the sensitizer and irritants can exacerbate existing asthma (Johnson et al. 2019). Basketter et al. (2019) reviewed the impact of fragrances on asthmatics compared to healthy subjects. They conclude that adverse health effects caused by inhalation of fragrances are uncommon, and a convincing biological and mechanistic basis is lacking.
Volatile substances might cause respiratory allergies (i.e., occupational asthma; Baur et al. 2012; Briatico-Vangosa et al. 1994). Several substances have been identified that can cause hypersensitivity when inhaled (Baur et al. 2012; Briatico-Vangosa et al. 1994; Nurmatov et al. 2015). Work-related asthma can be divided into occupational asthma (new onset asthma) and work-aggravated asthma (Baur et al. 2012). Occupational asthma is elicited by occupational allergens with well-defined etiological role and IgE-mediated patho-mechanisms as well as occupational agents with unknown patho-mechanism (Baur et al. 2012). Atopics with nonspecific bronchial hyper-responsiveness are a susceptible group that is also affected by chronic exposure to relatively low doses of irritant gases, fumes, or aerosols (Baur et al. 2012; Burge et al. 2012; Dykewicz 2009; Kipen et al. 1994). Often, such chronic causative concentrations of irritants are below their OELs or permissible exposure limits (Baur et al. 2012).
Subjects suffering from allergic rhinitis show a greater increase in nasal resistance than healthy subjects when they were exposed to substance P and methacholine (Devillier et al. 1988). This effect might be due to the increased number of basophils and mast cells in and on the nasal epithelium of the allergic patients (Devillier et al. 1988; Nielsen 1991) indicating a higher vegetative reaction capacity.
Oetjen and Kim (2018) describe the reciprocal interactions between the immune and the sensory nervous system. The immune system shapes the scope and intensity of sensory responses by modulating the sensory nervous system. For example, the TRP channels can be activated by volatile substances but also act as a common pathway for many different immune-cell-derived stimuli (Chiu et al. 2012; Oetjen and Kim 2018). These proteins encompass different channels that regulate different sensory responses like nociception and thermo-reception (Bautista et al. 2014; Oetjen and Kim 2018). For example, chronic inflammation can affect neuronal physiology by increasing innervation at sites of inflammation (Jia and Lee 2007; Oetjen and Kim 2018). O'Hanlon et al. (2007) could demonstrate that nerve fibers in the epithelium, subepithelium, and glandular/vascular regions are significantly increased in allergic rhinitis. Nasal sensitivity was correlated with protein gene product 9.5 subepithelial innervation as well in subjects with allergic rhinitis as in healthy subjects (O’Hanlon et al. 2007). Furthermore, Undem et al. (2000) describe changes in neural activity in allergic diseases, thus, allergic inflammation affects inter alia the primary afferent nerve leading to changes in neuronal excitability. Allergic inflammation may cause central sensitization, which might modulate neural reflexes (Undem et al. 2000).
It is hypothesized that even skin allergy might result in higher respiratory responsiveness (Arts et al. 2006b) indicating that skin and respiratory allergy are not separate diseases in animal models (Arakawa et al. 1995; Arts et al. 2006b, 2004, 1998; Blaikie et al. 1995; Botham et al. 1989). However, in humans, contact sensitization of a fragrance mix leads to a higher rate of bronchial hyper-responsiveness in women (Schnabel et al. 2010) and of non-allergic rhinitis (Gelardi et al. 2017). Furthermore, inhalation of fragrances may heighten the risk of developing contact dermatitis in patients with contact allergy (Schnuch et al. 2010). A sensitization of the respiratory tract, therefore, can be facilitated by the skin and inhalative exposure (Kimber et al. 2018). Many volatile substances can migrate across the skin and trigger immune responses (Kimber et al. 2018). A hybrid (combined) AOP for both skin and respiratory sensitization is proposed comprising 4 KEs considering communalities and differences between contact allergens and chemical respiratory allergens (cf., Fig. 2 in Kimber et al. 2018). This hybrid AOP highlights where the pathways of skin sensitization and sensitization of the respiratory tract overlap and where they may diverge.
Allergy in rats and mice
Fujimaki et al. (2004) showed that exposure to formaldehyde (40; 80; 2000 ppb for 12 weeks) can induce differential immunogenic and neurogenic inflammatory responses (Interleukin 1, nerve growth factor, interferon-γ) in an allergic mouse model at different exposure concentrations in comparison to non-allergic mice. The results should be assessed carefully, because the highest level of formaldehyde would have induced stress-related reactions, because of a substantial reduction of the breathing rate in the mice, according to Nielsen et al. (1999).
Morris et al. (2003) investigated breathing responses to irritants in the healthy and allergic airway-diseased mice. Mice with ovalbumin-induced allergic airway disease showed enhanced breathing patterns and/or obstructive responses.
However, Hansen et al. (2016) investigating mice with airway allergy observed no aggravation of allergic symptoms when exposed to ozone or a mixture of ozone and limonene reaction products. They even hypothesized that anti-inflammatory properties of the tested limonene-containing pollutants might have attenuated airway allergy (Hansen et al. 2016).
Neither air humidity nor allergic sensitization (by ovalbumin) had an impact on sensory irritation by 5 ppm formaldehyde in mice (Larsen et al. 2013). This might be due to a more efficient scrubber effect for this highly water-soluble substance caused by increased mucus production in asthmatic animals and in agreement with Garcia et al. (2009).
However, it is undisputed that capsaicin-sensitive sensory nerve mechanisms are upregulated by allergy-caused sensitization and inflammation in the airways (Lundberg 1995; Saria 1988).
Chronic obstructive pulmonary disease (COPD).
Chronic obstructive pulmonary disease (COPD) is ‘a disease state with progressive airflow obstruction.’ (Bose et al. 2015 p. 245). COPD leads to epithelial cell damage, bronchoconstriction, lung parenchymal destruction, and mucus hypersecretion (Bose et al. 2015). There are TRP channels in the lung epithelium that are activated by irritants like chlorine (reactive substance) or aldehydes, which could contribute to COPD exacerbation (Bose et al. 2015). Bose et al. (2015) reviewed the role of oxidative stress and TRP channels in COPD. Inhalation of substances, triggering the redox-sensitive signaling cascades, can cause cough, wheezing, and the exacerbation of airway inflammation, especially in susceptible people with asthma and COPD, children and older people (Zholos 2015). Oxidative stress leads to lipid peroxidation which might result in an inflammatory response (Zholos 2015). Several TRP channels are capable of sensing reactive oxygen species and reactive nitrogen species (Shimizu et al. 2014), i.e., TRPM8 in COPD.
Basoglu et al. (2015) investigated the influence of aerosolized Adenosine 5’-triphospate (ATP) in smokers and patients with COPD compared to non-smoking controls. COPD patients reported the strongest change in dyspnea on a Borg scale (change between pre- and post-provocation with ATP) compared to smokers (lower change) and healthy controls (no change). Because of the inflammatory process, significant amounts of ATP are released from various cells, inter alia by nerve endings (Basoglu et al. 2015). Therefore, the inflammatory response of sensory irritation might aggravate COPD by releasing ATP to extracellular space.
Chronic cough is a symptom of COPD (Nasra and Belvisi 2009). The cough reflex is usually initiated by the activation of airway sensory nerves, i.e., C-fibers and Aδ-fibers (Nasra and Belvisi 2009). However, ‘cough receptors’ do not express TRPV1 receptors or substance P (Mazzone 2004; Ricco et al. 1996). Nevertheless, a lowered threshold to aerosolized citric acid- and capsaicin-induced (non-volatile substances) cough has been demonstrated in patients with asthma or COPD (Doherty et al. 2000; Gatti et al. 2006; Higenbottam 2002; Wong and Morice 1999).
Belvisi et al. (2011) summarize that the activation of the TRPA1 receptor on vagal sensory afferents in the lung by irritant substances could lead to central reflexes (dyspnea, changes in breathing pattern, and cough) which might aggravate symptoms and pathophysiology of respiratory diseases.
Gatti et al. (2006) investigated the mechanism by which chronic inflammation (like in asthma or COPD) leads to a lowered cough threshold in Guinea pigs. They found that protease-activated receptor-2 stimulation exaggerates TRPV1-dependent cough by different mechanisms.
Shapiro et al. (2021) showed that the airway length and the number of branch points are significantly increased in chronic cough compared to healthy subjects in the human epithelium. Influence of age and gender was not found.
Impact of other diseases on the neurosensory system
Benoliel et al. (2006) found changes in the trigeminal neurosensory system following acute and chronic paranasal sinusitis in human subjects. They measured electrical detection thresholds for large myelinated nerve fibers and heat detection thresholds for thin unmyelinated nerve fibers. They observed hypersensitivity of large myelinated nerve fibers in early inflammatory neuritis, while long-lasting processes may result in hyposensitivity as they are, presumably, accompanied by nerve damage (Benoliel et al. 2006).
Auais et al. (2003) observed immunomodulatory effects of sensory nerves in rats during respiratory virus infection. The stimulation with capsaicin led to the recruitment of numerous lymphocytes (CD4 and T-cells) and monocytes in infected rats, while this could not be observed in healthy rats. Virus infection induced overexpression of NK1-receptors for substance P on lymphocyte subpopulations in the lymphoid tissue (Auais et al. 2003).
Eye tear dysfunction is generally associated with tear film instability, hyperosmolarity, and corneal sensitivity leading to eye-irritating symptoms in human subjects (Rahman et al. 2015). Other ocular diseases caused by environmental and occupational conditions further aggravate the tear film stability, which might lead to elevated vulnerability to external stimuli (Wolkoff 2020).
Summary of findings
There is ample evidence that the sensory system responsible for sensory irritation evoked by volatile substances is affected in airway diseases (i.e., TRPV1; Szallasi 2002). Bessac and Jordt (2008) argue that both TRPV1 and TRPA1 sensors, which play a major role in sensory irritation caused by volatile irritants, are also important in airway reflex control. Therefore, they may contribute to chemical hypersensitivity, chronic cough, airway inflammation in asthma, COPD, and reactive airway dysfunction syndrome. TRPV1 and TRPA1 (together with TRPV4 and TRPM8 receptors) are affected by airway diseases (Grace et al. 2014). Thus, there is a complex interplay in diseases, where the sensory system is affected and contributes to diseases in the airways. The same may be true for the sensory system of the eyes.
The quantification of differences between healthy individuals and individuals with diseases is difficult. Johansson et al. (2016) suggested a three-fold higher sensitivity of asthmatics compared to healthy individuals. Even a nine-fold difference in sensitivity was identified for sulfur dioxide. However, this study’s outcome should be considered cautiously because substantial differences between asthmatics and healthy people were only identified for a few substances. Furthermore, the differences could be ascribed to oral inhalation breathing or the substances were pulmonary irritants or both sensory and pulmonary irritants, as for sulfur dioxide, see discussion in Nielsen and Wolkoff (2017). The study does not support a general AF for asthmatics, rather, protection of asthmatics should be based on a substance-by-substance approach.
Furthermore, the perception of sensory irritation depends on personal factors, which are discussed in the next paragraph.
Influence of the grouping factor vulnerability on sensory irritation
Individuals might differ in the amount of ‘adversity’ of perceived sensory irritation. Differences arise from differences in the sensory system itself or from differences in the evaluation of the perception delivered by the sensory and olfactory systems. For example, expectations and beliefs (i.e., health risk perception; Dalton 1999), reactions of co-workers and bystanders, and affective orientation (Watson et al. 1988) influence the perception and report of irritation. Perceived health risk is also able to modulate inflammatory airway response in asthmatics (Jaén and Dalton 2014). Psychosocial factors like personality and stress might lead to building-related complaints and could even exacerbate CI and symptoms (Dalton and Jaén, 2010). External factors may alter the perception of volatile substances. Furthermore, there is a high individual variation in reporting trigeminal sensitivity; consequently, what is irritant to one might barely be noticed by another subject (Dalton and Jaén, 2010). Therefore, exposure to odors and irritants can lead to adverse responses in (vulnerable) people with MCS, which is also known as idiopathic environmental intolerance (Dalton and Jaén, 2010). As there are no uniform diagnostic criteria for MCS (Eis et al. 2008), declarations of prevalence range from 0.5% to 33% (Dantoft et al. 2021; Rossi and Pitidis 2017; Zucco and Doty 2022; highest prevalence in studies without a medical evaluation).
Multiple chemical sensitivity is a syndrome of non-specific multisystem symptoms caused by exposure to common odorous substances at non-toxic levels (Azuma et al. 2015; Cullen 1987; Dalton and Jaén, 2010; Eis et al. 2008; Graveling et al. 1999; Nordin 2020; Rossi and Pitidis 2017; Winder 2002). Exaggerated responses in MCS subjects might be caused by differences in the sensory system or in the evaluation of the perception (Nordin 2020). Neurogenic inflammation can be the result of volatile substances interpreted as threats that act as stressors upon the body (Nordin 2020). Consequently, the threshold for sensory perception in MCS subjects might be lower (Nordin 2020).
Studies investigating the impact of volatile irritants on sensory irritation in MCS patients are shown in Table 5 and associated substances are listed in Sect. 'Characteristics of the reviewed studies' (Table 7). MCS patients were identified by criteria developed by Cullen (1987), while other studies rely on self-reporting using questionnaires (self-reported MCS, sMCS).
Studies investigating ‘seconds’-exposure
Objective markers
Nordin et al. (2005) investigated chemosensory ERPs in self-reported hyper- and hyposensitive subjects (as measured by the Chemical Sensitivity Scale; Nordin et al. 2003). Ratings of malodorous pyridineFootnote 16 exposure were higher in hypersensitive subjects compared to hyposensitive subjects. However, this effect is not reflected in ERP amplitudes or latencies (no group differences; Nordin et al. 2005). The authors argue that the neural cortical basis for this effect might be an uncaptured higher cortical level not acquired by chemosensory ERPs.
Subjective ratings
Female subjects with MCS rated the irritation of the odorant γ-undecalatoneFootnote 17 (no concentration given) higher than female subjects without MCS (Azuma et al. 2016). MCS was diagnosed according to the 1999 consensus criteria (Anonymus 1999). However, there is no indication of any sensory irritation beyond subjective ratings.
In another study, females with MCS rated irritation caused by odorants higher than females without MCS; likewise, the odor was perceived more intense (Alobid et al. 2014).
van Thriel et al. (2008) did a median-split on sMCS rating (based on the chemical and general environmental sensitivity questionnaire; Kiesswetter et al. 1999) on their subjects without a preselection. Nevertheless, when exposed to six different volatile substancesFootnote 18 at nine different concentrations (ranging from odor threshold to nasal irritation threshold) for several seconds (flow olfactometry), subjects with higher sMCS values rated pungency more intense (p = 0.06, n.s.).
Studies investigating ‘minutes’-exposure
Objective markers
Andersson et al. (2015) investigated the breathing rate of 18 MCS subjects (2 males) in response to a low concentration of n-butanolFootnote 19 (3.7 ppm) for 50 min compared to subjects without MCS. MCS was diagnosed according to criteria described by Lacour et al. (2005). No differences in breathing parameters were observed, though subjective ratings of odor intensity and symptoms were higher in the MCS group (even during ‘sham exposure’).
Dantoft et al. (2015) investigated the epithelial lining fluid from the nasal cavity of MCS and healthy control subjects before, during, and after exposure to 3.7 ppm n-butanol for 50 min (different aspect but same experiment as described in Andersson et al. 2015). No abnormal upper airway inflammatory mediator levels in 19 cytokines and chemokines could be observed in the MCS subjects (Dantoft et al. 2015), i.e., no indication of sensory irritation. It should be noted, however, that the n-butanol concentration of 3.7 ppm is a factor of about 50 below an estimated LOAEL for sensory irritation (Wolkoff 2013), and a factor of 100 above its P100 odor threshold (Nagata, 2003).
Subjective ratings
Claeson and Andersson (2017) exposed subjects with or without CI to heptane in combination with 70 µg/m3 acrolein,Footnote 20 a strong sensory irritant. Chemical intolerance was addressed by a single question. Compared to control subjects, subjects with CI reported greater sensory irritation in the eyes, nose, and throat when exposed to heptane-masked acrolein at a concentration considered to be below the sensory irritation threshold. It remains unclear whether heptane has masked the smell of acrolein completely. If not, this would lead to different odor hedonics in the two experimental conditions (heptane vs. heptane + acrolein) that could influence the reported sensory irritation. Furthermore, it is uncertain whether the acrolein concentration is above the threshold for sensory irritation, cf., Dwivedi et al. (2015), Wolkoff (2013).
Studies investigating ‘hours’-exposure
Objective markers
Kiesswetter et al. (2005) investigated eye blinking frequencies in sMCS subjects and control subjects during exposure to 2-ethylhexanol (20 ppm). The eye blinking frequencies of sMCS subjects (identified according to Kiesswetter et al. 1999) did not differ significantly from those of control subjects. No difference was observed between female subjects with generalized self-reported CI (defined by questionnaires, cf., Pacharra et al. 2016a) and female control subjects in nasal inflammatory markers (substance P, TNF-α) after exposure to ascending concentrations steps up to 10 ppm ammonia, a concentration well below the lateralization threshold (Pacharra et al. 2016d; Smeets et al. 2007) for 75 min. The addition of subjects’ olfactory function (measured by Sniffin’ Sticks; Hummel et al. 1997) as a covariate resulted in higher TNF-α in the CI group. However, this effect was not modulated by exposure (pre-post-comparison; Pacharra et al. 2016a).
Subjective ratings
Van Thriel et al. (2005) re-analyzed the subjective ratings of the above study (Kiesswetter et al. 2005). Only ratings of eye irritation symptoms (Swedish Performance Evaluation System, SPES; Iregren 1998)Footnote 21 were higher during constant exposure in the sMCS group compared to the control subjects.
sMCS subjects reported higher ratings of eye and nose irritation (measured by SPES) than healthy controls when exposed to 2-butanoneFootnote 22 and ethyl benzeneFootnote 23 for 4 h not exceeding their OELs at that time (varying concentrations at an average of 189 ppm 2-butanone and 98 ppm ethyl benzene) (van Thriel et al. 2002). While the ratings of sMCS subjects increased over time, the ratings of control subjects stayed the same at a low and constant level of 10 ppm butanone and 10 ppm ethyl benzene.
Seeber et al. (2002) carried out and analyzed 14 experimental inhalation studies with mostly 4 h’ exposures to different volatile substancesFootnote 24 at constant or changing concentrations. Eye and nose irritation was rated by the subjects, some with sMCS. Mean ratings of eye and nose irritation were higher in sMCS subjects than in control subjects during the exposure. The ratings increased over time in sMCS subjects and stayed at a low level in control subjects, as in the study by van Thriel et al. (2002).
Female subjects with CI rated ammoniaFootnote 25 as more unpleasant and more pungent than female control subjects (Pacharra et al. 2016a; see above).
Summary of findings
In studies of ‘hours’ exposure, only subjective ratings increased in sMCS subjects compared to healthy controls. Eye blinking frequency as an objective marker of sensory irritation was unaffected, though an exposure-related increase in blinking was seen at the highest exposure level.
In ‘seconds’ exposure, only MCS subjects reported an increase in ratings, but objective markers of sensory irritation remained unchanged. Only one study showed higher eye blinking frequency for exposure to ethyl acrylate (0–40 ppm) than the control condition (Kiesswetter et al. 2005). However, though an objective marker of sensory irritation (blinking frequency) could be related to chemical exposure, there was no moderating effect in sMCS on sensory irritation. It is possible that health risk perception has an influence on perception in MCS patients, as shown in asthmatics to result in moderate perception and airway response according to Jaén and Dalton (2014). Palmquist and Claeson (2022) argue that subjects with building-related symptoms and CI perceive irritation at lower concentrations than others. They hypothesize that the increased sensitivity is due to altered trigeminal reactivity by inflammation or oxidative stress. It cannot be ruled out that this effect is odor-driven. Stress is known to have an impact (lowering) on the (olfactory) detection level of mercaptoethanol (Pacharra et al. 2015). In a recent review, Viziano et al. (2018) conclude that a combination of neural altered processing of sensorial ascending pathways with peculiar personality traits lead to MCS. The results reported here support the influence of personality traits. Thus, the next paragraph deals with the influence of personality traits (affectivity) on the perception of sensory irritation.
Influence of the grouping factor affectivity on sensory irritation
Affectivity is the ability to experience and cope with affects. Such affects can be positive or negative (Watson et al. 1988). For instance, olfactory stimuli can induce mood changes (Seubert et al. 2009), i.e., there is an affective response to odors. Automatic associations about odors indicate implicit attitudes towards odors (Bulsing et al. 2009, 2007). The implicit association test can diagnose such associations (Bulsing et al. 2009, 2007). A few studies about affectivity and sensory irritation have been identified.
Studies about ‘hours’-exposure
Subjective ratings
Lang et al. (2008) used ‘negative affectivity’ (Positive and negative affect scales, PANAS; Watson et al. 1988) as a covariate in their analysis already referred to in the gender paragraph. They argue that at lower formaldehyde concentrations, negative affectivity had a stronger influence than at high formaldehyde concentrations. However, this study suffers from methodological caveats (unbalanced sequence of exposures and unusually high eye blinking frequencies), which hampers the interpretation; however, negative affectivity seems to affect ratings only at low concentrations.
Ihrig et al. (2006) investigated the influence of personal traits on the complaints about ammonia exposure. Subjects were exposed up to 50 ppm ammonia on five subsequent days in an ascending sequence. Besides the PANAS questionnaire, the Freiburger Persönlichkeits Inventar (FPI; Fahrenberg et al. 2010) was used to assess the affectivity. The study showed that positive affectivity was significantly, negatively correlated to the rating of irritative ocular and nasal symptoms (SPES; Iregren 1998) only at low concentrations (10 and 20 ppm). This shows that the higher positive affectivity, the lower the symptom ratings when exposed to low ammonia concentrations. Subscales ‘Nervousness’ and ‘Depression’ from the FPI had a significant positive correlation with ratings of irritation symptoms at low concentrations. The higher the subscale, the higher the ratings of irritation.
Müller et al. (2013) also used the PANAS questionnaire to characterize their subjects. Furthermore, they identified hypo- and hypersensitive subjects with a median split on the rating of a CO2-exposure. Subjects were exposed to five different concentrations of formaldehyde on five subsequent days for 4 h. The irritation ratings by the hyposensitive subjects compared to hypersensitive subjects did not differ significantly for the eyes and the nose (SPES). However, results about the affectivity were unreported.
Nordin et al. (2017) exposed subjects to odorous substances (limonene as a pleasant odor and pyridine as an unpleasant odor) for 35 min at levels below their thresholds for sensory irritation but above their odor thresholds. They found that negative affectivity led to higher symptom ratings only when exposed to the unpleasant odor.
Pacharra et al. (2016d) used an implicit association test using odor terms (Bulsing et al. 2007) to identify subjects with negative and positive implicit associations towards odor. Furthermore, they divided the subjects into a low and a high olfactory acuity group (based on Sniffin’ Sticks; Hummel et al. 1997). Both dichotomized implicit association towards odor and dichotomized olfactory acuity, were independent factors that resulted in four experimental groups. The exposure scenario (with ammonia) was the same as in Pacharra et al. (2016b; see above). Subjects with strong positive associations towards odor rated ammonia as more pungent than those with weak positive automatic associations towards odor only in the subgroup with low olfactory acuity.
Summary of findings
Both MCS and (negative) affectivity result in higher ratings of irritation at low concentrations. At higher concentrations, the ratings of MCS and negative affectivity subjects and control subjects converge. The exposure concentrations are generally far below the threshold for sensory irritation in most of the studies. In one study differences between MCS and control subjects could not be observed in the eye blinking frequency at the highest exposure (Kiesswetter et al. 2005). Thus, odor perception appears to play a central, but not exclusive role, in reporting irritation.
It is well known that expectations, beliefs, information, and biases have an impact on odor perception and reported irritation symptoms (Dalton and Jaén, 2010; Jaén and Dalton 2014), as previously suggested by Das-Munshi et al. (2006). Several psychological characteristics are found in idiophatic environmental intolerance patients (Papo et al. 2006).
Bornschein et al. (2002) did not observe any deviation from norms between 12 MCS-patients and 12 matched controls in a positron emission tomography study where cerebral glucose metabolism was an indicator of cerebral dysfunction. Chemical intolerance seems to be associated with disease comorbidities (Palmer et al. 2021). However, a causal relationship has not been identified.
The influence of psychological and physiological components on the report of sensory irritation is difficult to assess (Rossi and Pitidis 2017; Zucco and Doty 2022). The influence of expectations and beliefs, however, has not been found in objective markers of sensory irritation. Nevertheless, the perception of strong symptoms even at low concentrations of volatile substances should be considered in risk assessment. Thus, the complex (top-down) psychological effects of odorous (pleasant or unpleasant) substances should be assessed, if complete or partially-complete protection of the general population should be the target. This, however, is challenging since odor thresholds are orders of magnitude lower than thresholds for sensory irritation (Cometto-Muñiz and Abraham 2016). In this case, perceptual ratings, still, are the only possibility to quantify differences in sensitivity for MCS or negative affectivity individuals.
General discussion
In the regulation of volatile substances, sensory irritation is an important endpoint (Brüning et al. 2014; Nielsen and Wolkoff 2017).
There are at least three obvious factors that have an impact on sensory irritation elicited by volatile substances:
-
(1)
volatile substance at a certain concentration
-
(2)
situational context/demands
-
(3)
individual differences
Different volatile substances have different water solubilities (cf., Garcia et al. 2009; Shusterman 2002) and, therefore, different deposition rates along the airways that determine the target site at which sensory irritation takes place. At target sites, different channels (i.e., TRPA, TRPV, GPCR, ASIC; Lehmann et al. 2017; Shusterman 2007) might lead to sensory irritation. High concentrations of substances, however, may reach ‘lower’ target sites depending on the deposition capacity. Furthermore, for lipophilic substances, the protective perceived irritation in the upper airways may be absent. Thus, they can cause injury at high concentrations. Risk assessment should handle such lipophilic substances with special care in a substance-by substance approach.
However, the target site of a volatile substance also depends on situational influences like mode of breathing, i.e., nasal breathing versus oral breathing, e.g., due to the physical workload. Physical workload might lead to more oral breathing resulting in a higher fraction of volatile substances reaching ‘lower’ target sites. Individual variance might be higher or lower for one channel and vice versa.
This manuscript (1) reviewed empirical evidence about differences between groups in the general population and (2) aimed at deriving an AF for the general population. Wherever possible, controlled human exposure studies have been considered. The results of the reviewing process are summarized in Table 6.
Evaluation of differences identified by grouping factors
The first aim of this study is to identify relevant (human exposure) studies that could be a platform for derivation of (an) AF(s), which is specific for sensory irritation in the general population.
Individual differences in sensory irritation depend on the sensory receptors, the transduction, and the strength/evaluation of the perception.
For instance, the density of receptors differs across lifespan (with a maximum in healthy adolescents and at healthy young adulthood). Further, some diseases might influence sensory density (i.e., chronic cough; Shapiro et al. 2021). However, there are the body´s own defense mechanisms (i.e., eye blinking, nasal mucociliary clearance). These also depend on individual differences that might differ over the lifespan and may be hampered by diseases; thus, they may influence sensory irritation.
A first step to capture individual differences in the general population is to take a closer look at sub-groups known to represent such differences, i.e., gender, age groups, patients suffering from diseases, and subjects that consider themselves as sensitive. This first step is carried out in this study.
Gender
The grouping factor gender has the best empirical base of all reviewed grouping factors. The influence of gender on sensory irritation has been investigated on a variety of endpoints at different time scales. Females report more symptoms in the eyes in a few studies. However, no gender differences could be found in objective markers of sensory irritation in the eye. Females seem to be more sensitive than males when exposing the nose for seconds in some studies, but an odor-mediated bias cannot be excluded in every significant study. Therefore, it cannot be ruled out that methodological shortcomings have an impact on the differences. Many studies did not find convincing evidence for gender differences at all. The reviewed studies are unable to disprove an AF of 2 as proposed by Nielsen et al. (2007; Nielsen and Wolkoff 2017) because it must incorporate not only the influence of gender, but also the additional influences of age, lifestyle, and diseases, and in some cases climatic conditions. The reviewed studies are representative of first and foremost young, healthy, non-smoking subjects, and the investigated substances. Therefore, substance-specific AFs (for gender) might be more adequate than a general AF for gender influence.
Age
The influence of age on sensory irritation is ambiguous. Obviously, is it reasonable to distinguish between older people and young children in a risk assessment.
Older people
Based on the reviewed studies (n = 10), older people are less sensitive than young adults. However, it seems that high age is not necessarily a causal influence but could be a correlative effect on sensory (or olfactory) loss in older people. As there is a high variability as well in the young adult groups as in the older groups, age-related pathologies may be responsible for the effect of older age (Stevens et al. 1982). However, older people as a group are less sensitive to sensory irritation. An AF for older age, therefore, seems unnecessary at first glance. Anyway, caution is advised. Lesser sensitivity might result in slower, weaker, or missing physiological defense processes against sensory irritants, i.e., less eye blinking (Sharma and Hindman 2014; Wolkoff 2020, 2017), or lower epithelial beat frequency (Ho et al. 2001). Especially, detrimental effects arising from weaker own-body protection might come up after longer exposures of hours or days (which are rarely/not captured in the reviewed studies). Objective age effects are only seen in threshold studies (seconds exposure) with higher thresholds in older people. Only one study investigated exposures longer than seconds (4 h exposure of 20 ppm 2-ethylhexanol; Schäper et al. 2015). No age effect was observed in eye blinking, though 2-ethylhexanol triggered more blinking during the highest exposure condition (20 ppm) in both young and older subjects (45–67 years). However, it remains unclear whether there could be age effects at longer exposure times, older ages, with other substances or deteriorating climatic conditions. A temporal summation (over longer periods) due to insufficient defense mechanisms in older people cannot be excluded. One long-standing question is whether a less stable eye tear film, as encountered in older people (especially women), is more vulnerable to sensory irritation, i.e., the no-observed-adverse-effect-level for a given irritant would be lower than in younger people with a healthy and stable tear film. Therefore, an empirically based quantification of a risk factor for older people is not possible.
Children
Experimental studies investigating the sensitivity of young children are scarce (n = 1). At the age of 7 years, children show no significantly lower sensitivity than older subjects when lateralizing eucalyptol in the nose. It is not clear whether younger children have a higher sensitivity. As experimental studies are difficult from an ethical perspective, other approaches are necessary to derive AFs for young children. For sensory airway irritation, Frank et al. (2018) identified so-called KEs and MIEs, which describe initial points of interaction with a volatile substance that result in a perturbation / sensory irritation. Several studies have shown developmental changes in processes involved in MIEs.
Therefore, KEs leading to sensory irritation by volatile substances binding to the TRPA1 receptor might be moderated by developmental processes at a young age. Thus, developmental processes might influence the perception of sensory irritation at a given concentration. To make it more complicated, the organism reacts to defend itself against harmful volatile substances; for instance, eye blinking to maintain the tear film stability and to clear the ocular surface and mucus production to remove harmful substances from the airways (mucociliary clearance). Such defense mechanisms allow for coping with volatile substances that could evoke sensory irritation. However, if these mechanisms are impaired, volatile substances could lead to sensory irritation at concentrations that otherwise would be tolerable for young healthy adults. Usually, there is more than one cooperating defense mechanism. For example, infants show lower eye blinking frequency (less than one time a minute). On the other hand, their eye tear film is more stable than in adults. Thus, there is a complex interplay of defense mechanisms involved in coping with sensory irritants. A comprehensive modelling of such mechanisms (i.e., PD/PK models) regarding developmental changes would be helpful for risk assessment as it allows for indirect assessment of exposure effects via ‘critical mass’ at the trigeminal system.
Based on what is known about developmental processes up to now, an AF could not be derived.
Health status
Target sites for volatile irritants are also impaired in several diseases (i.e., allergic rhinitis, asthma, COPD). Diseases might also influence the kinetics and dynamics of the sensory irritant. However, empirical evidence by experimental exposure is limited to diseases. Most likely, patients with severe diseases refrain from participating in exposure studies. Consequently, empirical evidence is limited to patients with mild to moderate course of disease and cannot be generalized. Furthermore, many diseases might have a direct (higher sensitivity at target sites) or indirect impact (hampering or intensifying defense mechanisms) on sensory irritation. The impact of diseases on sensory irritation has only been investigated in a few studies. Again, comprehensive models of impaired target sites and suspended defense mechanisms would be helpful. In the case of allergy, allergen-induced neuromodulations are accurately described (cf., Undem and Taylor-Clark 2014). Furthermore, AOPs of allergy development already exist (i.e., in the case of chemical respiratory allergy/occupational asthma; Kimber et al. 2014). What is missing are models that allow for a quantification of, for example, differences between healthy and asthmatic people in sensory irritation. Ideally, the severity of the disease (i.e., via RAST classes in allergy) should be considered in such models that might be expanded to comorbidities. It would be desirable that such models consider different degrees of impairment at target sites and defense mechanisms (dynamic components), which depend on the diseases. The magnitude of impairment can probably be mapped to the severity of disease. Prevalence of diseases should be considered for the derivation of an AF for the whole population, if possible, even regarding the severity (i.e., RAST classes in allergy). It is worth mentioning that long-term exposure to volatile substances at concentrations not generating sensory irritation might have an adverse impact (exacerbation of asthma / increased probability to develop asthma) in susceptible groups (asthmatics / children) of the general population. In risk assessment, such a potential hazard should be considered for each substance. Again, QSAR models of respiratory sensitizers (i.e., Graham et al. 1997) would be helpful.
On the other hand, the disease asthma appears to exhibit an indirect protection against water-soluble irritants due to the excess mucus production in the nasal cavity and throat (cf., Fadeyi et al. 2015; Golden 2011; Johansson et al. 2017; Larsen et al. 2013; Wolkoff and Nielsen 2010).
Sensory irritants may cause or aggravate asthma/allergy symptoms (Baur et al. 2012; Briatico-Vangosa et al. 1994; Nurmatov et al. 2015). Thus, risk assessment for sensory irritation can be more complicated, even at concentrations far below the irritation threshold among subjects suffering from low affectivity or allergies. Odor-mediated reactions cannot be ruled out, cf., Cometto-Muniz and Cain (1991).
Vulnerability
There is a large individual variety in the perception of volatile substances. The same volatile substance that causes an irritant perception in one person might be barely noticed by another person (Dalton and Jaén, 2010). This difference can be clearly observed in MCS patients (n = 12 studies reviewed). The exposure concentrations are far below sensory irritation but above the threshold for odor perception in most studies about MCS. MCS subjects show higher perceptual and symptom ratings compared to control subjects, but MCS patients often perceive the odor more intensely, while the odor thresholds among MCS patients and healthy people do not differ (We referred to such an effect as the third manifestation of sensitivity in ‘Manifestations of sensitivity’).
Why some odors evoke health symptoms is not completely understood but some processes might be responsible (Schiffman and Williams 2005), like affectivity, modulation of breathing, exacerbation of diseases by inducing stress and impairing mood, and learned associations (Jaén and Dalton 2014). Further factors influencing odor perception were summarized by Wolkoff (2013; cf., Table 3).
It is not possible nor reasonable to develop an AF that accommodates a sensitivity triggered, solely, by odor perception, since odor thresholds generally are orders of magnitude lower than thresholds for sensory irritation (Cometto-Muñiz and Abraham 2016).
Adequate risk communication might mitigate such perceptual differences (Dalton and Jaén, 2010; Jaén and Dalton 2014; Nordin 2020).
AF for the general population
In addition to finding differences in sensitivity to sensory irritation between groups of the general population, the second aim of this study was the derivation of an AF for the general population considering vulnerability and susceptibility factors. This aim could not be achieved due to several reasons:
-
(1)
Lack of an empirical base (children, health status)
-
(2)
Lack of representativity / generalization (health status, children, vulnerability)
-
Self-selection of subjects (health status, MCS, affectivity)
-
(3)
Time aspects (older people)
-
Influence of altered warning signals / clearance (older people, children)
-
Potential of volatile substances to elicit or aggravate diseases (e.g., asthma, allergies)
-
(4)
Absence of objective indicators of sensory irritation in many studies (most notably, vulnerability) or trigger olfactory effects
The investigated groups, however, might differ by other variables than the grouping factors. This may influence the outcome according to the study by Rosenkranz et al. (2020), who demonstrated that a fraction of otherwise ‘healthy’ subjects showed signs of diseases. Thus, study results may be influenced by unrecognized and undiscovered illnesses. Furthermore, psychological effects, inter alia expectations or stress might affect airway physiology (Jaén and Dalton 2014; Pacharra et al. 2015). In addition, methodological shortcomings may underestimate the odor potencies of a substance as they increase the variability and therefore inflate what is assessed as individual differences (Cain and Schmidt 2009).
How to address the identified problems in risk assessment
Variety of target sites
In animal studies, extrapolation factors were used to extrapolate from subacute to subchronic exposures and from subchronic to chronic exposures. For example, a multitude of 226 different extrapolation factors have been identified for 71 volatile substances by extrapolation from subchronic to chronic effects in inhalation (Escher et al. 2020). The variance in these factors shows confounders like the uncertainty of identified no-observed-adverse-effect-levels and differences in study design.
It seems reasonable to establish AFs that are target site specific. If a volatile substance at a certain concentration only affects the eye, gender variance at the nose does not necessarily have to be considered. The same might be true for other target sites.
Therefore, QSARs (Abraham et al. 2016, 2007, 1998, 1996; Alarie et al. 1996; Cometto-Muñiz et al. 1998; Cometto-Muñiz and Abraham 2016; Cronin et al. 2003; Hau et al. 1999) could be a tool to refine grouping of substances (European Chemicals Agency 2021). The consequence of such grouping procedures could be a variety of AFs for substance groups. The derivation of an AF would require knowledge about the concentration/mass of sensory irritant at a specific target site (kinetic processes); and in the end, the defined target of percentage protection of the population.
Individual /intraspecies factors
In animal studies, intraspecies factors are usually not considered at all. Risk assessment for the general population, however, must consider susceptible and vulnerable groups with individual factors, i.e., children or persons suffering from chronic diseases.
This review could propose an AF only for the grouping factor gender. Other grouping factors describe other sensitivities in sensory irritation. Interaction between such grouping factors are also possible. Johansson et al. (2016), for example, identified a ninefold higher sensitivity of asthmatics to sulfur dioxide. However, a general AF of 10, as proposed by the authors, could be valid only for pulmonary irritants (like sulfur dioxide). A substance-specificity in AFs, therefore, is more reasonable from an economic point of view than a general AF. Models must be developed that incorporate differences in susceptible and non-susceptible people exposed to the same concentration of the same volatile substance (QSAR group). It must be emphasized, however, that justifying and deriving AFs for susceptible people would require a detailed analysis of causation at the individual level, cf., Haanes et al. (2020). Furthermore, it must be considered that the efficiency of defense mechanisms (dynamic processes) also could be differential.
Temporal aspects of exposure
The duration of the volatile substance exposure is relevant. Controlled human exposure studies use different experimental situations to investigate individual differences in human sensory irritation, ranging from seconds- to hours-exposure and – rarely—to exposure on subsequent days. However, sensory irritation underlies temporal summation (effects of up to tens of minutes have been observed; Cain et al. 2010). Therefore, it is reasonable to assume that hours-exposures should reveal any sensory irritation effect. In human exposure studies over hours, only a few concentrations can be tested. Due to ethical reasons, the highest concentration is not allowed to exceed legal OEL values. Therefore, harmless concentrations might even be higher (cf., Mangelsdorf et al. 2020) and individual differences would not come into effect. Therefore, seconds- and minutes-exposures are relevant, too.
In risk assessment, exposure effects found in seconds- to hours-exposures must be extrapolated to, in the worst case, lifelong exposure. It is unclear whether defense mechanisms would work properly if they were challenged continuously over the years. If not, it could be speculated that volatile substance concentrations repeatedly over hours might induce sensory irritation after longer-lasting exposures without sufficient exposure-free periods for recovery. Such a situation, however, appears only hypothetical for a major part of the population.
The relationship between defense mechanisms / clearance in response to continuous volatile substance exposure should be further investigated.
The potential of volatile substances to elicit allergies/asthma at low concentrations must be considered in risk assessment. Reliable models of such pathogenesis are necessary.
Situational contexts of exposure
Changing from nasal to oral breathing (dynamic component) leads to a circumvention of the nose and its scrubbing (kinetic) effect. Therefore, there might be special AFs, i.e., for sports halls and playgrounds / schoolyards (regarding diseases and maybe also for hospitals / retirement homes). Furthermore, visual demands might lead to higher vulnerability of dry eyes because of less or incomplete eye blinking (Wolkoff 2020).
Coping with different situational contexts depends on individual prerequisites. Therefore, when considering individual differences in sensory irritation and proposing AFs, substance and situational factors must be assessed, too (cf., Haanes et al. 2020).
Model requirements for risk assessment
Comprehensive modeling with kinetic (delivery to target site) and dynamic (action and reaction at target site) components (i.e., PBPKFootnote 26, PD/PKFootnote 27, TD/TKFootnote 28) could be used for subgroup-specific risk assessment (i.e., Poet et al. 2010). While Cn x t models require a non-interrupted exposure duration (Pauluhn 2019) with a stable influence of dynamic and kinetic processes, realistic exposure can be intermittent. Thus, in some phases, one of the processes will prevail (leading to the summation of substance or clearance). Comprehensive modelling (i.e., PD/PK models) of the concentrations at target sites is required in evidence-based risk assessment for sensory irritation. Such models should consider the kinetic component (delivery to target side) as well as the dynamic component (action at target site and physiological responses like defense mechanisms) in sensory irritation as well as the effects of continuous exposure. For systemic effects, gender and age differences in biochemical processes have been considered, e.g., in PB/PK models of 2-butoxyethanol disposition in rats and mice (Corley et al. 2005).
Similar models for sensory irritation are still lacking. A ‘critical’ mass of substances at the specific receptors that leads to sensory irritation should be identified (trigeminal system as a mass detector rather than a concentration detector; Frasnelli et al. 2017; Hummel and Frasnelli 2019; Kleinbeck et al. 2020). Deliverance to the receptor as well as clearance at the receptors might be influenced by age effects at longer exposure and as a result may influence the critical mass at the receptor. One must keep in mind that not only the receptors but also nerve transmission might be affected (nerve damage in chronic sinusitis, Auais et al. 2003; i.e., developmental changes in children, Uppal et al. 2016). Nevertheless, exposure studies with potentially susceptible subjects are also needed to evaluate the adequacy of the PD/PK modeling and to relate differences in the response of the target site to differences in objective and subjective markers in sensory irritation in vivo.
Conclusion
Though recent publications propose AFs for asthmatics and MCS patients, respectively, (10; Johansson et al. 2016) and the general population (20; Mangelsdorf et al. 2020), this review was unable to identify supporting and meaningful empirical evidence for an AF greater than 2 for protection of the susceptible subgroups of the general population, solely based on human exposure studies. This implies it can be for some substance between 1 and 2. Asthmatics and healthy controls were differently affected only by a few substances in the review by Johansson et al. (2016). However, Johansson et al. considered substances that were either pulmonary irritants or both sensory and pulmonary irritants (e.g., sulfur dioxide), while Mangelsdorf et al. (2020) applied a general precautionary AF of 20 without scientific support. Thus, it is recommended to apply an evidence-based risk assessment approach to be carried out, substance by substance. QSARs can be used to classify substance classes for substance- and pathway-specific AFs (Falk-Filipsson et al. 2007). Furthermore, contexts of exposure must be considered. Other AFs might apply for workplaces where dry air, high room temperature, and visually demanding tasks (e.g., video display unit work) aggravate the eye tear film stability, rather than for places in which workload is balanced. Indoor playgrounds might need other AFs due to physical workload and affected groups of the general population.
Extra emphasis should be put on populations such as neonates and genetically sensitive subgroups, fetuses, and children, because they may be particularly susceptible or vulnerable during development and maturation. Gender differences in sensitivity, deficiencies in the databases, nature of the effect, duration of exposure, and route-to-route extrapolation need also to be considered. Since AFs are used to compensate for lack of knowledge, we consider that it is prudent to adopt a conservative approach. When new empirical knowledge appears that reduces the uncertainties, this should be incorporated into a new risk assessment leading to more evidence-based AFs. While gender differences could be considered by a substance-specific conservative AF of 1–2 (incorporating also influences of age, lifestyle, and diseases, as proposed by Nielsen and Wolkoff 2017), empirical and supporting evidence for the development of other AFs (for children, older persons, patients suffering from diseases) is lacking.
Though older people as a group have higher thresholds for sensory irritation, other factors, like defense mechanisms might be impaired by diseases that could induce more susceptibility to long-term effects of volatile substance exposure.
For children, there might be time slots in their development, in which they might be more susceptible or vulnerable to sensory irritation than adults. However, children might even be less susceptible (cf., higher tear film stability) than adults. Most likely, there is still development in sensory systems from birth to the age of 7 years that might be covered by an AF. However, a quantification based on empirical data is not possible. PD/PK models representing developmental changes could be established for an ‘empirical’ basis of an AF.
For patients suffering from diseases the derivation of an AF from empirical knowledge is also difficult. Many diseases have an impact on sensory systems resulting in sensitization (that might depend on the severity of the disease). On the other hand, increased mucus production due to diseases could even protect the airways from sensory irritation, but such a protective effect of sensory irritation, probably, is an exception for highly water-soluble substances; exaggeration of sensory irritation by disease is more common. Volatile substances might even elicit diseases like allergies/asthma. Again, comprehensive PD/PK models of patients with diseases (representing disease severity) considering the impact of volatile substances in pathogenesis would be desirable to achieve an ‘empirically-justified’ AF.
There is also a lack of knowledge about the impact of affectivity/MCS on sensory irritation (and the inevitable associated olfactory impact). At first glance, both seem to influence the perception of low concentrations of volatile substances. One must keep in mind that the exposure concentrations were far below sensory irritation thresholds in most of the studies. At higher concentrations (still below the sensory irritation threshold) such differences compared to’normal’ subjects decrease. Based on the reviewed studies, a special AF for sensory irritation regarding affectivity/MCS is not justified. Nevertheless, the influence of affectivity/MCS on olfactory loads might be considered in setting exposure limits, however challenging, if possible beyond the individual level. Thus, for OELs perceptual (olfactory) effects of a substance are not health effects. Adverse exposure effects at workplaces require ‘(a) sensory irritation, (b) considerable odor annoyance or (c) in individual cases’odor associated’ symptoms must occur’ (List of MAK and BAT Values 2021, https://series.publisso.de/sites/default/files/documents/series/mak/lmbv/Vol2021/Iss2/Doc002/mbwl_2021_eng.pdf). Therefore, if the personality factor affectivity/MCS elicits ‘considerable odor annoyance’ or ‘odor-associated symptoms’ at lower concentrations than in groups with different personality, this must be dealt with case-by-case at the individual level. However, up to now, MCS is far from being a well-defined symptom class caused by known special properties of volatile substances at specific concentrations. Protection of MCS patients by evidence-based AFs is not possible, and other mitigation strategies should be considered.
It is reasonable to use substance-specific AFs, since differences in sensitivity might be limited to one sensory system and the qualititative and quantitative database for sensory irritation might be different. For example, highly water-soluble substances are scrubbed by the nose and do not reach the middle or lower airways. Further, situational factors might lead to a circumvention of a sensory system (e.g., mouth breathing in a high physical workload). Therefore, it is reasonable to consider situational factors in substance-specific AFs; furthermore, the influence of climatic conditions may be considered.
Comprehensive models of sensory irritation comprising developmental differences, changes caused by diseases, and age-related decline could bridge the gap of knowledge caused by ethical concerns to expose children to volatile substances, experimentally, and by self-selection in experimental studies with patients suffering from diseases. Physiochemical properties like water solubility and QSARs could help to categorize substances eliciting sensory irritation with respect to a target site (e.g., eyes, nose, middle, and lower airways) and mode of action at the target site.
Characteristics of the reviewed studies
See below Table 7.
Notes
The grouping factor is named ‘Vulnerability’ as external factors like chemicals lead to altered perceptions in vulnerable persons. The reason for the altered perception, however, might be an internal factor.
Such models comprise of a kinetic component (delivery to target site) and a dynamic component (action at target site and physiological response) in pharmacology or toxicology (Ashauer and Escher 2010).
Studies are numbered consecutively. In later tables, studies keep their number for reasons of clarity. In Table 7, all reviewed studies are sorted by number.
Water solubility: slightly soluble.
Water solubility: slightly soluble.
Water solubilities: soluble to freely soluble and miscible.
Water solubility: freely soluble.
Water solubility: slightly soluble.
Water solubility: slightly soluble.
Water solubility: very slightly soluble.
Water solubility: very slightly soluble.
Water solubility: slightly soluble.
Water solubility: sparingly soluble.
Water solubility: freely soluble.
Miscible.
Water solubility: very slightly soluble.
Water solubilities: soluble to freely soluble and miscible.
Water solubility: soluble.
Water solubility: freely soluble.
Ratings of different symptoms (inter alia nasal and ocular irritation) were conducted on a categorical 6-level- scale.
Water solubility: freely soluble.
Water solubility: very slightly soluble.
Water solubilities: cf., Table 7.
Water solubility: freely soluble.
Physiology based pharmacodynamic (PBPK) modeling .
Pharmacodynamic, pharmacokinetic (PD/PK) modeling.
Toxicokinetic, toxicodynamic (TK/TD) modeling.
Abbreviations
- AF:
-
Assessment factor
- AOP:
-
Adverse outcome pathway
- ATP:
-
Adenosine triphosphate
- CGRP:
-
Calcitonin gene-related peptide
- CI:
-
Chemical intolerance
- COPD:
-
Chronic obstructive pulmonary disease
- ECHA:
-
European chemicals agency
- ERP:
-
Event-related potentials
- DMEL/DNEL:
-
Derived minimum effect level/derived no effect level
- FPI:
-
Freiburger Persönlickeitsinventar
- KE:
-
Key event
- LOAEL:
-
Lowest observed adverse effect level
- MIE:
-
Molecular initiating event
- MCS:
-
Multiple chemical sensitivity
- MOA:
-
Mode of action
- OA:
-
Occupational asthma
- OECD:
-
Organization for economic co-operation and development
- OEL:
-
Occupational exposure level
- PANAS:
-
Positive affect negative affect scale
- PK/PD:
-
Pharmacokinetic/pharmacodynamics modeling
- PEL:
-
Permissible exposure limit
- QSAR:
-
Quantitative structure–activity relationship
- sMCS:
-
Self-reported multiple chemical sensitivity
- SPES:
-
Swedish performance evaluation system
- TWA:
-
Time weighted average
References
Abraham MH, Andonian-Haftvan J, Cometto-Muñiz JE, Cain WS (1996) An analysis of nasal irritation thresholds using a new solvation equation. Fundam Appl Toxicol 31:71–76. https://doi.org/10.1006/faat.1996.0077
Abraham MH, Kumarsingh R, Cometto-Muñiz JE, Cain WS (1998) Draize eye scores and eye irritation thresholds in man can be combined into one QSAR. Ann N Acad Sci 855:652–656. https://doi.org/10.1111/j.1749-6632.1998.tb10641.x
Abraham MH, Sanchez-Moreno R, Cometto-Muniz JE, Cain WS (2007) A quantitative structure activity analysis on the relative sensitivity of the olfactory and the nasal trigeminal chemosensory systems. Chem Senses 32:711–719. https://doi.org/10.1093/chemse/bjm038
Abraham MH, Gola JM, Cometto-Muñiz JE (2016) An assessment of air quality reflecting the chemosensory irritation impact of mixtures of volatile organic compounds. Env Int 86:84–91. https://doi.org/10.1016/j.envint.2015.07.012
Acosta MC, Tan ME, Belmonte C, Gallar J (2001) Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol vis Sci 42:2063–2067. https://doi.org/10.1016/s0002-9394(01)01279-x
Acosta MC, Alfaro ML, Borras F et al (2006) Influence of age, gender and iris color on mechanical and chemical sensitivity of the cornea and conjunctiva. Exp Eye Res 83:932–938. https://doi.org/10.1016/j.exer.2006.04.018
Acosta MC, Luna C, Quirce S et al (2013) Changes in sensory activity of ocular surface sensory nerves during allergic keratoconjunctivitis. Pain 154:2353–2362. https://doi.org/10.1016/j.pain.2013.07.012
Alarie Y (1973) Sensory irritation by airborne chemicals. CRC Crit Rev Toxicol 2:299–363. https://doi.org/10.3109/10408447309082020
Alarie Y, Schaper M, Nielsen GD, Abraham MH (1996) Estimating the sensory irritating potency of airborne nonreactive volatile organic chemicals and their mixtures. SAR QSAR Environ Res 5:151–165. https://doi.org/10.1080/10629369608032986
Alobid I, Nogué S, Izquierdo-Dominguez A et al (2014) Multiple chemical sensitivity worsens quality of life and cognitive and sensorial features of sense of smell. Eur Arch Otorhinolaryngol 271:3203–3208. https://doi.org/10.1007/s00405-014-3015-5
Alves M, Asbell P, Dogru M et al (2023) TFOS Lifestyle report: impact of environmental conditions on the ocular surface. Ocul Surf 29:1–52. https://doi.org/10.1016/j.jtos.2023.04.007
Alving K, Malinovschi A (2010) Basic aspects of exhaled nitric oxide. Eur Respir Monogr 49:1–31. https://doi.org/10.1183/1025448x.00028509
Anand KJ, Carr DB (1989) The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatr Clin North Am 36:795–822. https://doi.org/10.1016/s0031-3955(16)36722-0
Andersen ME, Dennison JE (2001) Mode of action and tissue dosimetry in current and future risk assessments. Sci Total Env 274:3–14. https://doi.org/10.1016/S0048-9697(01)00744-6
Andersson L, Claeson AS, Dantoft TM et al (2015) Chemosensory perception, symptoms and autonomic responses during chemical exposure in multiple chemical sensitivity. Int Arch Occup Env Health 89:79–88. https://doi.org/10.1007/s00420-015-1053-y
Andreollo NA, dos Santos EF, Araújo MR, Lopes LR (2012) Idade dos ratos versus idade humana: qual é a relação? ABCD Arq Bras Cir Dig São Paulo 25:49–51. https://doi.org/10.1590/S0102-67202012000100011
Ankley GT, Bennett RS, Erickson RJ et al (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741. https://doi.org/10.1002/etc.34
Anonymus (1999) Multiple Chemical Sensitivity: A 1999 Consensus. Arch Environ Health Int J 54:147–149. https://doi.org/10.1080/00039899909602251
Arakawa H, Lötvall J, Kawikova I et al (1995) Airway responses following intradermal sensitization to different types of allergens: ovalbumin, trimellitic anhydride and Dermatophagoides farinae. Int Arch Allergy Immunol 108:274–280. https://doi.org/10.1159/000237164
Arts JH, Kuper CF, Spoor SM, Bloksma N (1998) Airway morphology and function of rats following dermal sensitization and respiratory challenge with low molecular weight chemicals. Toxicol Appl Pharmacol 152:66–76. https://doi.org/10.1006/taap.1998.8504
Arts JH, Mojet J, van Gemert LJ et al (2002) An analysis of human response to the irritancy of acetone vapors. Crit Rev Toxicol 32:43–66. https://doi.org/10.1080/20024091064174
Arts JH, de Koning MW, Bloksma N, Kuper CF (2004) Respiratory allergy to trimellitic anhydride in rats: concentration-response relationships during elicitation. Inhal Toxicol 16:259–269. https://doi.org/10.1080/08958370490427932
Arts JH, de Heer C, Woutersen RA (2006a) Local effects in the respiratory tract: relevance of subjectively measured irritation for setting occupational exposure limits. Int Arch Occup Env Health 79:283–298. https://doi.org/10.1007/s00420-005-0044-9
Arts JH, Mommers C, de Heer C (2006b) Dose-response relationships and threshold levels in skin and respiratory allergy. Crit Rev Toxicol 36:219–251. https://doi.org/10.1080/10408440500534149
Ashauer R, Escher BI (2010) Advantages of toxicokinetic and toxicodynamic modelling in aquatic ecotoxicology and risk assessment. J Env Monit 12:2056–2061. https://doi.org/10.1039/c0em00234h
Auais A, Adkins B, Napchan G, Piedimonte G (2003) Immunomodulatory effects of sensory nerves during respiratory syncytial virus infection in rats. Am J Physiol Lung Cell Mol Physiol 285:L105–L113. https://doi.org/10.1152/ajplung.00004.2003
Auten RL, Davis JM (2009) Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr Res 66:121–127. https://doi.org/10.1203/PDR.0b013e3181a9eafb
Azuma K, Uchiyama I, Tanigawa M et al (2015) Assessment of cerebral blood flow in patients with multiple chemical sensitivity using near-infrared spectroscopy–recovery after olfactory stimulation: a case-control study. Env Health Prev Med 20:185–194. https://doi.org/10.1007/s12199-015-0448-4
Azuma K, Uchiyama I, Tanigawa M et al (2016) Association of odor thresholds and responses in cerebral blood flow of the prefrontal area during olfactory stimulation in patients with multiple chemical sensitivity. PLoS ONE 11:e0168006. https://doi.org/10.1371/journal.pone.0168006
Banzrai C, Nodera H, Higashi S et al (2016) Age-dependent effects on sensory axonal excitability in normal mice. Neurosci Lett 611:81–87. https://doi.org/10.1016/j.neulet.2015.11.032
Barbariga M, Rabiolo A, Fonteyne P et al (2018) The effect of aging on nerve morphology and substance P expression in mouse and human corneas. Invest Ophthalmol vis Sci 59:6026–6026. https://doi.org/10.1167/iovs.18-24707a
Barry M, Annesi-Maesano I (2017) Ten principles for climate, environment and respiratory health. Eur Respir J 50:1701912. https://doi.org/10.1183/13993003.01912-2017
Basketter DA, Huggard J, Kimber I (2019) Fragrance inhalation and adverse health effects: The question of causation. Regul Toxicol Pharmacol 104:151–156. https://doi.org/10.1016/j.yrtph.2019.03.011
Basoglu OK, Barnes PJ, Kharitonov SA, Pelleg A (2015) Effects of aerosolized adenosine 5′-triphosphate in smokers and patients With COPD. Chest 148:430–435. https://doi.org/10.1378/chest.14-2285
Baur X, Bakehe P, Vellguth H (2012) Bronchial asthma and COPD due to irritants in the workplace - an evidence-based approach. J Occup Med Toxicol 7:19. https://doi.org/10.1186/1745-6673-7-19
Bautista DM, Wilson SR, Hoon MA (2014) Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci 17:175–182. https://doi.org/10.1038/nn.3619
Bell ML, Zanobetti A, Dominici F (2013) Evidence on vulnerability and susceptibility to health risks associated with short-term exposure to particulate matter: a systematic review and meta-analysis. Am J Epidemiol 178:865–876. https://doi.org/10.1093/aje/kwt090
Belmonte C, Acosta MC, Gallar J (2004) Neural basis of sensation in intact and injured corneas. Exp Eye Res 78:513–525. https://doi.org/10.1016/j.exer.2003.09.023
Belvisi MG (2003) Sensory nerves and airway inflammation: role of A delta and C-fibres. Pulm Pharmacol Ther 16:1–7. https://doi.org/10.1016/S1094-5539(02)00180-3
Belvisi MG, Dubuis E, Birrell MA (2011) Transient receptor potential A1 channels: insights into cough and airway inflammatory disease. Chest 140:1040–1047. https://doi.org/10.1378/chest.10-3327
Benemei S, Patacchini R, Trevisani M, Geppetti P (2015) TRP channels. Curr Opin Pharmacol 22:18–23. https://doi.org/10.1016/j.coph.2015.02.006
Bennett WD, Zeman KL, Jarabek AM (2008) Nasal contribution to breathing and fine particle deposition in children versus adults. J Toxicol Env Health A 71:227–237. https://doi.org/10.1080/15287390701598200
Benoliel R, Biron A, Quek SY et al (2006) Trigeminal neurosensory changes following acute and chronic paranasal sinusitis. Quintessence Int 37:437–443
Berglund B, Brunekreef B, Knöppel H et al (1992) Effects of indoor air pollution on human health. Indoor Air 2:2–25. https://doi.org/10.1111/j.1600-0668.1992.02-21.x
Bergmann K-C, Heinrich J, Niemann H (2016) Current status of allergy prevalence in Germany. Allergo J Int 25:6–10. https://doi.org/10.1007/s40629-016-0092-6
Bessac BF, Jordt SE (2008) Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiol Bethesda 23:360–370. https://doi.org/10.1152/physiol.00026.2008
Beuerman RW, Stern ME (2005) Neurogenic inflammation: a first line of defense for the ocular surface. Ocul Surf 3:S203–S206. https://doi.org/10.1016/s1542-0124(12)70256-2
Bjermer L (2007) Time for a paradigm shift in asthma treatment: from relieving bronchospasm to controlling systemic inflammation. J Allergy Clin Immunol 120:1269–1275. https://doi.org/10.1016/j.jaci.2007.09.017
Blaikie L, Morrow T, Wilson AP et al (1995) A two-centre study for the evaluation and validation of an animal model for the assessment of the potential of small molecular weight chemicals to cause respiratory allergy. Toxicology 96:37–50. https://doi.org/10.1016/0300-483X(94)03007-O
Bonini S, Rama P, Olzi D, Lambiase A (2003) Neurotrophic keratitis. Eye Lond 17:989–995. https://doi.org/10.1038/sj.eye.6700616
Borchman D, Foulks GN, Yappert MC, Milliner SE (2012) Changes in human meibum lipid composition with age using nuclear magnetic resonance spectroscopy. Invest Ophthalmol vis Sci 53:475–482. https://doi.org/10.1167/iovs.11-8341
Bornschein S, Hausteiner C, Drzezga A et al (2002) PET in patients with clear-cut multiple chemical sensitivity (MCS). Nuklearmedizin 41:233–239. https://doi.org/10.1055/s-0038-1625294
Bose P, Bathri R, Kumar L et al (2015) Role of oxidative stress & transient receptor potential in chronic obstructive pulmonary disease. Indian J Med Res 142:245–260. https://doi.org/10.4103/0971-5916.166529
Botham PA, Rattray NJ, Woodcock DR et al (1989) The induction of respiratory allergy in guinea-pigs following intradermal injection of trimellitic anhydride: a comparison with the response to 2,4-dinitrochlorobenzene. Toxicol Lett 47:25–39. https://doi.org/10.1016/0378-4274(89)90083-0
Bouza H (2009) The impact of pain in the immature brain. J Matern Fetal Neonatal Med 22:722–732. https://doi.org/10.3109/14767050902926962
Brand G, Millot JL (2001) Sex differences in human olfaction: between evidence and enigma. Q J Exp Psychol B 54:259–270. https://doi.org/10.1080/02724990143000045
Briatico-Vangosa G, Braun CL, Cookman G et al (1994) Respiratory allergy: hazard identification and risk assessment. Fundam Appl Toxicol 23:145–158. https://doi.org/10.1006/faat.1994.1093
Brüning T, Bartsch R, Bolt HM et al (2014) Sensory irritation as a basis for setting occupational exposure limits. Arch Toxicol 88:1855–1879. https://doi.org/10.1007/s00204-014-1346-z
Bulsing PJ, Smeets MAM, van den Hout MA (2007) Positive implicit attitudes toward odor words. Chem Senses 32:525–534. https://doi.org/10.1093/chemse/bjm021
Bulsing PJ, Smeets MAM, Van den Hout MA (2009) The implicit association between odors and illness. Chem Senses 34:111–119. https://doi.org/10.1093/chemse/bjn062
Burge PS, Moore VC, Robertson AS (2012) Sensitization and irritant-induced occupational asthma with latency are clinically indistinguishable. Occup Med Lond 62:129–133. https://doi.org/10.1093/occmed/kqr211
Bushnell PJ, Boyes WK, Shafer TJ et al (2007) Approaches to extrapolating animal toxicity data on organic solvents to public health. Neurotoxicology 28:221–226. https://doi.org/10.1016/j.neuro.2006.03.013
Cain WS, Cometto-Muñiz JE (1995) Irritation and odor as indicators of indoor pollution. Occup Med 10:133–145
Cain WS, Schmidt R (2009) Can we trust odor databases? Example of t- and n-butyl acetate. Atmos Environ 43:2591–2601. https://doi.org/10.1016/j.atmosenv.2009.02.024
Cain WS, Jalowayski AA, Schmidt R et al (2008) Chemesthetic responses to airborne mineral dusts: boric acid compared to alkaline materials. Int Arch Occup Env Health 81:337–345. https://doi.org/10.1007/s00420-007-0218-8
Cain WS, Dourson ML, Kohrman-Vincent MJ, Allen BC (2010) Human chemosensory perception of methyl isothiocyanate: chemesthesis and odor. Regul Toxicol Pharmacol 58:173–180. https://doi.org/10.1016/j.yrtph.2010.06.018
Chiu IM, von Hehn CA, Woolf CJ (2012) Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15:1063–1067. https://doi.org/10.1038/nn.3144
Cho P, Yap M (1993) Age, gender, and tear break-up time. Optom vis Sci 70:828–831. https://doi.org/10.1097/00006324-199310000-00009
Claeson AS, Andersson L (2017) Symptoms from masked acrolein exposure suggest altered trigeminal reactivity in chemical intolerance. Neurotoxicology 60:92–98. https://doi.org/10.1016/j.neuro.2017.03.007
Claeson AS, Nordin S (2011) Gender differences in nasal chemesthesis: a study of detection and perceived intensity. Chem Percept 4:25–31. https://doi.org/10.1007/s12078-011-9084-6
Clewell H (2005) Use of mode of action in risk assessment: past, present, and future. Regul Toxicol Pharmacol 42:3–14. https://doi.org/10.1016/j.yrtph.2005.01.008
Cometto-Muñiz JE, Abraham MH (2016) Dose-response functions for the olfactory, nasal trigeminal, and ocular trigeminal detectability of airborne chemicals by humans. Chem Senses 41:3–14. https://doi.org/10.1093/chemse/bjv060
Cometto-Muñiz JE, Cain WS (1991) Nasal pungency, odor, and eye irritation thresholds for homologous acetates. Pharmacol Biochem Behav 39:983–989. https://doi.org/10.1016/0091-3057(91)90063-8
Cometto-Muñiz JE, Noriega G (1985) Gender differences in the perception of pungency. Physiol Behav 34:385–389. https://doi.org/10.1016/0031-9384(85)90200-8
Cometto-Muñiz JE, Cain WS, Abraham MH (1998) Nasal pungency and odor of homologous aldehydes and carboxylic acids. Exp Brain Res 118:180–188. https://doi.org/10.1007/s002210050270
Corley RA, Grant DM, Farris E et al (2005) Determination of age and gender differences in biochemical processes affecting the disposition of 2-butoxyethanol and its metabolites in mice and rats to improve PBPK modeling. Toxicol Lett 156:127–161. https://doi.org/10.1016/j.toxlet.2003.08.013
Cronin MT, Dearden JC, Walker JD, Worth AP (2003) Quantitative structure-activity relationships for human health effects: commonalities with other endpoints. Environ Toxicol Chem 22:1829–1843. https://doi.org/10.1897/01-274
Cruz AA, Garcia DM, Pinto CT, Cechetti SP (2011) Spontaneous eyeblink activity. Ocul Surf 9:29–41. https://doi.org/10.1016/s1542-0124(11)70007-6
Cullen MR (1987) The worker with multiple chemical sensitivities: an overview. Occup Med 2:655–661. https://doi.org/10.1016/s1382-6689(96)00054-3
Dalton P (1999) Cognitive influences on health symptoms from acute chemical exposure. Health Psychol 18:579–590. https://doi.org/10.1037//0278-6133.18.6.579
Dalton PH, Jaén C (2010) Responses to odors in occupational environments. Curr Opin Allergy Clin Immunol 10:127–132. https://doi.org/10.1097/ACI.0b013e3283373470
Dalton PH, Dilks DD, Banton MI (2000) Evaluation of odor and sensory irritation thresholds for methyl isobutyl ketone in humans. Aihaj 61:340–350. https://doi.org/10.1080/15298660008984542
Dalton P, Dilks D, Hummel T (2006) Effects of long-term exposure to volatile irritants on sensory thresholds, negative mucosal potentials, and event-related potentials. Behav Neurosci 120:180–187. https://doi.org/10.1037/0735-7044.120.1.180
Dalton P, Soreth B, Maute C et al (2018) Lack of respiratory and ocular effects following acute propylene glycol exposure in healthy humans. Inhal Toxicol 30:124–132. https://doi.org/10.1080/08958378.2018.1470207
Dankovic DA, Naumann BD, Maier A et al (2015) The scientific basis of uncertainty factors used in setting occupational exposure limits. J Occup Env Hyg 12(Suppl 1):S55-68. https://doi.org/10.1080/15459624.2015.1060325
Dantoft TM, Skovbjerg S, Andersson L et al (2015) Inflammatory mediator profiling of n-butanol exposed upper airways in individuals with multiple chemical sensitivity. PLoS One 10:e0143534. https://doi.org/10.1371/journal.pone.0143534
Dantoft TM, Nordin S, Andersson L et al (2021) Multiple chemical sensitivity described in the Danish general population: Cohort characteristics and the importance of screening for functional somatic syndrome comorbidity—The DanFunD study. PLoS One 16:e0246461. https://doi.org/10.1371/journal.pone.0246461
Das-Munshi J, Rubin GJ, Wessely S (2006) Multiple chemical sensitivities: a systematic review of provocation studies. J Allergy Clin Immunol 118:1257–1264. https://doi.org/10.1016/j.jaci.2006.07.046
Davies AM (1988) The trigeminal system: an advantageous experimental model for studying neuronal development. Development 103(Suppl):175–183. https://doi.org/10.1242/dev.103.Supplement.175
Devillier P, Dessanges JF, Rakotosihanaka F et al (1988) Nasal response to substance P and methacholine in subjects with and without allergic rhinitis. Eur Respir J 1:356–361. https://doi.org/10.1183/09031936.93.01040356
Dick RB, Ahlers H (1998) Chemicals in the workplace: incorporating human neurobehavioral testing into the regulatory process. Am J Ind Med 33:439–453. https://doi.org/10.1002/(SICI)1097-0274(199805)
Dicpinigaitis PV, Rauf K (1998) The influence of gender on cough reflex sensitivity. Chest 113:1319–1321. https://doi.org/10.1378/chest.113.5.1319
Dicpinigaitis PV, Rhoton WA, Bhat R, Negassa A (2012) Investigation of the urge-to-cough sensation in healthy volunteers. Respirology 17:337–341. https://doi.org/10.1111/j.1440-1843.2011.02094.x
Dispenza MC (2019) Classification of hypersensitivity reactions. Allergy Asthma Proc 40:470–473. https://doi.org/10.2500/aap.2019.40.4274
Doherty MJ, Mister R, Pearson MG, Calverley PM (2000) Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax 55:643–649. https://doi.org/10.1136/thorax.55.8.643
Doty RL, Cameron EL (2009) Sex differences and reproductive hormone influences on human odor perception. Physiol Behav 97:213–228. https://doi.org/10.1016/j.physbeh.2009.02.032
Doty RL, Cometto-Muñiz JE, Jalowayski AA et al (2004) Assessment of upper respiratory tract and ocular irritative effects of volatile chemicals in humans. Crit Rev Toxicol 34:85–142. https://doi.org/10.1080/10408440490269586
Doughty MJ (2002) Further assessment of gender- and blink pattern-related differences in the spontaneous eyeblink activity in primary gaze in young adult humans. Optom Vis Sci 79:439–447. https://doi.org/10.1097/00006324-200207000-00013
Dourson ML, Kohrman-Vincent MJ, Allen BC (2010) Dose response assessment for effects of acute exposure to methyl isothiocyanate (MITC). Regul Toxicol Pharmacol 58:181–188. https://doi.org/10.1016/j.yrtph.2010.04.006
Dutta S, Sengupta P (2016) Men and mice: relating their ages. Life Sci 152:244–248. https://doi.org/10.1016/j.lfs.2015.10.025
Dwivedi AM, Johanson G, Lorentzen JC et al (2015) Acute effects of acrolein in human volunteers during controlled exposure. Inhal Toxicol 27:810–821. https://doi.org/10.3109/08958378.2015.1115567
Dykewicz MS (2009) Occupational asthma: current concepts in pathogenesis, diagnosis, and management. J Allergy Clin Immunol 123:519–28. https://doi.org/10.1016/j.jaci.2009.01.061
Eis D, Helm D, Mühlinghaus T et al (2008) The German multicentre study on multiple chemical sensitivity (MCS). Int J Hyg Environ Health 211:658–681. https://doi.org/10.1016/j.ijheh.2008.03.002
Eisner MD, Anthonisen N, Coultas D et al (2010) An official American thoracic Society Public Policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182:693–718. https://doi.org/10.1164/rccm.200811-1757ST
Ernstgård L, Gullstrand E, Löf A, Johanson G (2002) Are women more sensitive than men to 2-propanol and m-xylene vapours? Occup Env Med 59:759–767. https://doi.org/10.1136/oem.59.11.759
Escher SE, Mangelsdorf I, Hoffmann-Doerr S et al (2020) Time extrapolation in regulatory risk assessment: The impact of study differences on the extrapolation factors. Regul Toxicol Pharmacol 112:104584. https://doi.org/10.1016/j.yrtph.2020.104584
European Chemicals Agency (2021) The use of alternatives to testing on animals for the REACH Regulation. European Chemicals Agency
Fadeyi MO, Weschler CJ, Tham KW et al (2013) Impact of human presence on secondary organic aerosols derived from ozone-initiated chemistry in a simulated office environment. Environ Sci Technol 47:3933–3941. https://doi.org/10.1021/es3050828
Fadeyi MO, Tham KW, Wu WY (2015) Impact of asthma, exposure period, and filters on human responses during exposures to ozone and its initiated chemistry products. Indoor Air 25:512–522. https://doi.org/10.1111/ina.12161
Fahrenberg J, Hampel R, Selg H (2010) Freiburger Persönlichkeitsinventar. Primärdaten der Normierungsstichprobe 1999. Psychologisches Datenarchiv PsychData des Leibniz-Zentrums für Psychologische Information und Dokumentation ZPID, Trier
Fairhurst S (1995) The uncertainty factor in the setting of occupational exposure standards. Ann Occup Hyg 39:375–385. https://doi.org/10.1016/0003-4878(95)00007-2
Falk-Filipsson A, Hanberg A, Victorin K et al (2007) Assessment factors–applications in health risk assessment of chemicals. Env Res 104:108–127. https://doi.org/10.1016/j.envres.2006.10.004
Feng Y, Simpson TL (2003) Nociceptive sensation and sensitivity evoked from human cornea and conjunctiva stimulated by CO2. Invest Ophthalmol vis Sci 44:529–532. https://doi.org/10.1167/iovs.02-0003
Firestone M, Sonawane B, Barone S et al (2008) Potential new approaches for children’s inhalation risk assessment. J Toxicol Env Health A 71:208–217. https://doi.org/10.1080/15287390701597905
Foos B, Marty M, Schwartz J et al (2008) Focusing on children’s inhalation dosimetry and health effects for risk assessment: an introduction. J Toxicol Env Health A 71:149–165. https://doi.org/10.1080/15287390701597871
Frank EA, Haber LT, Genter MB, Maier A (2018) Defining molecular initiating events of airway sensory irritation in support of predictive testing approaches. Appl Vitro Toxicol 4:317–331. https://doi.org/10.1089/aivt.2018.0007
Frasnelli J, Hummel T (2003) Age-related decline of intranasal trigeminal sensitivity: is it a peripheral event? Brain Res 987:201–206. https://doi.org/10.1016/s0006-8993(03)03336-5
Frasnelli J, Gingras-Lessard F, Robert J, Steffener J (2017) The effect of stimulus duration on the nostril localization of eucalyptol. Chem Senses 42:303–308. https://doi.org/10.1093/chemse/bjx008
Fujimaki H, Kurokawa Y, Kunugita N et al (2004) Differential immunogenic and neurogenic inflammatory responses in an allergic mouse model exposed to low levels of formaldehyde. Toxicology 197:1–13. https://doi.org/10.1016/j.tox.2003.11.015
Fujimura M, Sakamoto S, Kamio Y, Matsuda T (1990) Sex difference in the inhaled tartaric acid cough threshold in non-atopic healthy subjects. Thorax 45:633–634. https://doi.org/10.1136/thx.45.8.633
Garcia GJ, Schroeter JD, Segal RA et al (2009) Dosimetry of nasal uptake of water-soluble and reactive gases: a first study of interhuman variability. Inhal Toxicol 21:607–618. https://doi.org/10.1080/08958370802320186
Gatti R, Andre E, Amadesi S et al (2006) Protease-activated receptor-2 activation exaggerates TRPV1-mediated cough in guinea pigs. J Appl Physiol 101:506–511. https://doi.org/10.1152/japplphysiol.01558.2005
Gelardi M, Guarino R, Taliente S et al (2017) Allergic and nonallergic rhinitis and skin sensitization to metals: is there a link? Eur Ann Allergy Clin Immunol 49:106–109
Gell PGH, Coombs RRA (1963) The classification of allergic reactions underlying disease. Clin Asp Immunol
Geppetti P, Patacchini R, Nassini R, Materazzi S (2010) Cough: The Emerging Role of the TRPA1 Channel. Lung 188(Suppl 1):S63–S68. https://doi.org/10.1007/s00408-009-9201-3
Gibson SJ, Farrell F (2004) A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain 20:227–239. https://doi.org/10.1097/00002508-200407000-00004
Ginsberg GL, Foos BP, Firestone MP (2005) Review and analysis of inhalation dosimetry methods for application to children’s risk assessment. J Toxicol Env Health A 68:573–615. https://doi.org/10.1080/15287390590921793
Ginsberg G, Foos B, Dzubow RB, Firestone M (2010) Options for incorporating children’s inhaled dose into human health risk assessment. Inhal Toxicol 22:627–647. https://doi.org/10.3109/08958371003610958
Gminski R, Marutzky R, Kevekordes S et al (2011) Chemosensory irritations and pulmonary effects of acute exposure to emissions from oriented strand board. Hum Exp Toxicol 30:1204–1221. https://doi.org/10.1177/0960327110388537
Golden R (2011) Identifying an indoor air exposure limit for formaldehyde considering both irritation and cancer hazards. Crit Rev Toxicol 41:672–721. https://doi.org/10.3109/10408444.2011.573467
Grace MS, Baxter M, Dubuis E et al (2014) Transient receptor potential (TRP) channels in the airway: role in airway disease. Br J Pharmacol 171:2593–2607. https://doi.org/10.1111/bph.12538
Graham C, Rosenkranz HS, Karol MH (1997) Structure-activity model of chemicals that cause human respiratory sensitization. Regul Toxicol Pharmacol 26:296–306. https://doi.org/10.1006/rtph.1997.1170
Graveling RA, Pilkington A, George JP et al (1999) A review of multiple chemical sensitivity. Occup Env Med 56:73–85. https://doi.org/10.1136/oem.56.2.73
Gui P, Ebihara T, Sato R, et al (2014) Gender differences in the effect of urge-to-cough and dyspnea on perception of pain in healthy adults. Physiol Rep https://doi.org/10.14814/phy2.12126
Guilemany JM, Garcia-Pinero A, Alobid I et al (2009) Persistent allergic rhinitis has a moderate impact on the sense of smell, depending on both nasal congestion and inflammation. Laryngoscope 119:233–238. https://doi.org/10.1002/lary.20075
Haanes JV, Nordin S, Hillert L et al (2020) “Symptoms associated with environmental factors” (SAEF) – Towards a paradigm shift regarding “idiopathic environmental intolerance” and related phenomena. J Psychosom Res 131:109955. https://doi.org/10.1016/j.jpsychores.2020.109955
Hansen JS, Norgaard AW, Koponen IK et al (2016) Limonene and its ozone-initiated reaction products attenuate allergic lung inflammation in mice. J Immunotoxicol 13:793–803. https://doi.org/10.1080/1547691X.2016.1195462
Hau KM, Connell DW, Richardson BJ (1999) Quantitative structure-activity relationships for nasal pungency thresholds of volatile organic compounds. Toxicol Sci 47:93–98. https://doi.org/10.1093/toxsci/47.1.93
He J, Bazan HE (2010) Omega-3 fatty acids in dry eye and corneal nerve regeneration after refractive surgery. Prostaglandins Leukot Essent Fatty Acids 82:319–325. https://doi.org/10.1016/j.plefa.2010.02.004
He J, Bazan HE (2016) Neuroanatomy and neurochemistry of mouse cornea. Invest Ophthalmol vis Sci 57:664–674. https://doi.org/10.1167/iovs.15-18019
He J, Bazan NG, Bazan HE (2010) Mapping the entire human corneal nerve architecture. Exp Eye Res 91:513–523. https://doi.org/10.1016/j.exer.2010.07.007
Hey K, Juran S, Schäper M et al (2009) Neurobehavioral effects during exposures to propionic acid-An indicator of chemosensory distraction? Neurotoxicology 30:1223–1232. https://doi.org/10.1016/j.neuro.2009.08.009
Higenbottam T (2002) Chronic cough and the cough reflex in common lung diseases. Pulm Pharmacol Ther 15:241–247. https://doi.org/10.1006/pupt.2002.0341
Ho JC, Chan KN, Hu WH et al (2001) The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med 163:983–988. https://doi.org/10.1164/ajrccm.163.4.9909121
Hooper LG, Kaufman JD (2018) Ambient air pollution and clinical implications for susceptible populations. Ann Am Thorac Soc 15:S64–S68. https://doi.org/10.1513/AnnalsATS.201707-574MG
Hummel T (2000) Assessment of intranasal trigeminal function. Int J Psychophysiol 36:147–155. https://doi.org/10.1016/s0167-8760(99)00108-7
Hummel T, Frasnelli J (2019) The intranasal trigeminal system. Handb Clin Neurol 164:119–134. https://doi.org/10.1016/B978-0-444-63855-7.00008-3
Hummel T, Sekinger B, Wolf SR et al (1997) “Sniffin” sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22:39–52. https://doi.org/10.1093/chemse/22.1.39
Hummel T, Futschik T, Frasnelli J, Huttenbrink KB (2003) Effects of olfactory function, age, and gender on trigeminally mediated sensations: a study based on the lateralization of chemosensory stimuli. Toxicol Lett 140–141:273–280. https://doi.org/10.1016/s0378-4274(03)00078-x
Hummel T, Bensafi M, Nikolaus J et al (2007) Olfactory function in children assessed with psychophysical and electrophysiological techniques. Behav Brain Res 180:133–138. https://doi.org/10.1016/j.bbr.2007.02.040
Hunter DD, Wu Z, Dey RD (2010) Sensory neural responses to ozone exposure during early postnatal development in rat airways. Am J Respir Cell Mol Biol 43:750–757. https://doi.org/10.1165/rcmb.2009-0191OC
Ihrig A, Hoffmann J, Triebig G (2006) Examination of the influence of personal traits and habituation on the reporting of complaints at experimental exposure to ammonia. Int Arch Occup Env Health 79:332–338. https://doi.org/10.1007/s00420-005-0042-y
Iregren A (1998) Computer-assisted testing. In: Costa LG, Manzo L (eds) Occupational Neurotoxicology. CRC Press, Boca Raton, pp 213–232
Jaén C, Dalton P (2014) Asthma and odors: the role of risk perception in asthma exacerbation. J Psychosom Res 77:302–308. https://doi.org/10.1016/j.jpsychores.2014.07.002
Jia Y, Lee LY (2007) Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta 1772:915–927. https://doi.org/10.1016/j.bbadis.2007.01.013
Johansson MK, Johanson G, Oberg M (2016) Evaluation of the experimental basis for assessment factors to protect individuals with asthma from health effects during short-term exposure to airborne chemicals. Crit Rev Toxicol 46:241–260. https://doi.org/10.3109/10408444.2015.1092498
Johansson M, Gustafsson Å, Johanson G, Öberg M (2017) Comparison of airway response in naïve and ovalbumin-sensitized mice during short-term inhalation exposure to chlorine. Inhal Toxicol 29:82–91. https://doi.org/10.1080/08958378.2017.1299260
Johnson MB, Kingston R, Utell MJ et al (2019) Exploring the science, safety, and benefits of air care products: perspectives from the inaugural air care summit. Inhal Toxicol 31:12–24. https://doi.org/10.1080/08958378.2019.1597221
Jones MA, Marfurt CF (1991) Calcitonin gene-related peptide and corneal innervation: a developmental study in the rat. J Comp Neurol 313:132–150. https://doi.org/10.1002/cne.903130110
Juran SA, van Thriel C, Kleinbeck S et al (2012) Neurobehavioral performance in human volunteers during inhalation exposure to the unpleasant local irritant cyclohexylamine. Neurotoxicology 33:1180–1187. https://doi.org/10.1016/j.neuro.2012.06.014
Kasznia-Kocot J, Kowalska M, Gorny RL et al (2010) Environmental risk factors for respiratory symptoms and childhood asthma. Ann Agric Env Med 17:221–229
Khosravi M, Collins PB, Lin RL et al (2014) Breathing hot humid air induces airway irritation and cough in patients with allergic rhinitis. Respir Physiol Neurobiol 198:13–19. https://doi.org/10.1016/j.resp.2014.03.013
Kiesswetter E, Sietmann B, Zupanic M et al (1999) Verhaltenstoxikologische Aspekte der Prävalenz und Ätiologie “multipler chemischer Sensitivität.” Allergologie 22:719–735. https://doi.org/10.5414/ALP22719
Kiesswetter E, van Thriel C, Schäper M et al (2005) Eye blinks as indicator for sensory irritation during constant and peak exposures to 2-ethylhexanol. Environm Toxicol Pharmacol 19:531–541. https://doi.org/10.1016/j.etap.2004.12.056
Kimber I, Dearman RJ, Basketter DA, Boverhof DR (2014) Chemical respiratory allergy: reverse engineering an adverse outcome pathway. Toxicology 318:32–39. https://doi.org/10.1016/j.tox.2014.02.001
Kimber I, Poole A, Basketter DA (2018) Skin and respiratory chemical allergy: confluence and divergence in a hybrid adverse outcome pathway. Toxicol Res 7:586–605. https://doi.org/10.1039/C7TX00272F
Kipen HM, Blume R, Hutt D (1994) Asthma experience in an occupational and environmental medicine clinic. Low-dose reactive airways dysfunction syndrome. J Occup Med 36:1133–1137. https://doi.org/10.1097/00043764-199410000-00017
Kjærgaard S, Pedersen OF, Mølhave L (1992) Sensitivity of the eyes to airborne irritant stimuli: influence of individual characteristics. Arch Env Health 47:45–50. https://doi.org/10.1080/00039896.1992.9935943
Kleinbeck S, Juran SA, Kiesswetter E et al (2008) Evaluation of ethyl acetate on three dimensions: investigation of behavioral, physiological and psychological indicators of adverse chemosensory effects. Toxicol Lett 182:102–109. https://doi.org/10.1016/j.toxlet.2008.09.001
Kleinbeck S, Schäper M, Juran SA et al (2011) Odor thresholds and breathing changes of human volunteers as consequences of sulphur dioxide exposure considering individual factors. Saf Health Work 2:355–364. https://doi.org/10.5491/SHAW.2011.2.4.355
Kleinbeck S, Schäper M, Zimmermann A et al (2017) Prediction of human sensory irritation due to ethyl acrylate: The appropriateness of time weighted average concentration x time models for varying concentrations. Arch Toxicol 91:3051–3064. https://doi.org/10.1007/s00204-017-1934-9
Kleinbeck S, Schäper M, Pacharra M et al (2020) A short-term inhalation study to assess the reversibility of sensory irritation in human volunteers. Arch Toxicol 94:1687–1701. https://doi.org/10.1007/s00204-020-02703-8
Klenø J, Wolkoff P (2004) Changes in eye blink frequency as a measure of trigeminal stimulation by exposure to limonene oxidation products, isoprene oxidation products and nitrate radicals. Int Arch Occup Env Health 77:235–243. https://doi.org/10.1007/s00420-003-0502-1
Koskela H, Di Sciascio MB, Anderson SD et al (2000) Nasal hyperosmolar challenge with a dry powder of mannitol in patients with allergic rhinitis. Evidence for epithelial cell involvement. Clin Exp Allergy 30:1627–1636. https://doi.org/10.1046/j.1365-2222.2000.00923.x
Kreuter M, Cottin V (2017) The threat in chronic lung diseases: acute exacerbations. Eur Respir Rev 26:170075. https://doi.org/10.1183/16000617.0075-2017
Lacour M, Zunder T, Schmidtke K et al (2005) Multiple chemical sensitivity syndrome (MCS) – suggestions for an extension of the US MCS-case definition. Int J Hyg Environ Health 208:141–151. https://doi.org/10.1016/j.ijheh.2005.01.017
Lang I, Bruckner T, Triebig G (2008) Formaldehyde and chemosensory irritation in humans: a controlled human exposure study. Regul Toxicol Pharmacol 50:23–36. https://doi.org/10.1016/j.yrtph.2007.08.012
Larsen ST, Wolkoff P, Hammer M et al (2013) Acute airway effects of airborne formaldehyde in sensitized and non-sensitized mice housed in a dry or humid environment. Toxicol Appl Pharmacol 268:294–299. https://doi.org/10.1016/j.taap.2013.02.006
Lehmann R, Hatt H, van Thriel C (2017) Alternative in vitro assays to assess the potency of sensory irritants-Is one TRP channel enough? Neurotoxicology 60:178–186. https://doi.org/10.1016/j.neuro.2016.08.010
Lundberg JM (1995) Tachykinins, sensory nerves, and asthma–an overview. Can J Physiol Pharmacol 73:908–914. https://doi.org/10.1139/y95-125
Lundström JN, Frasnelli J, Larsson M, Hummel T (2005) Sex differentiated responses to intranasal trigeminal stimuli. Int J Psychophysiol 57:181–186. https://doi.org/10.1016/j.ijpsycho.2005.01.003
Lundström JN, Gordon AR, Wise P, Frasnelli J (2012) Individual differences in the chemical senses: is there a common sensitivity? Chem Senses 37:371–378. https://doi.org/10.1093/chemse/bjr114
Maestrelli P, Boschetto P, Fabbri LM, Mapp CE (2009) Mechanisms of occupational asthma. J Allergy Clin Immunol 123:531–42. https://doi.org/10.1016/j.jaci.2009.01.057
Maier A, Vincent M, Hack E et al (2014) Derivation of an occupational exposure limit for inorganic borates using a weight of evidence approach. Regul Toxicol Pharmacol 68:424–437. https://doi.org/10.1016/j.yrtph.2014.02.001
Mangelsdorf I, Schröder K, Escher SE et al (2020) Risk assessment for irritating chemicals - derivation of extrapolation factors. Int J Hyg Env Health 232:113668. https://doi.org/10.1016/j.ijheh.2020.113668
Mantelli F, Tiberi E, Micera A et al (2007) MUC5AC overexpression in tear film of neonates. Graefes Arch Clin Exp Ophthalmol 245:1377–1381. https://doi.org/10.1007/s00417-007-0602-9
Marfurt CF, Murphy CJ, Florczak JL (2001) Morphology and neurochemistry of canine corneal innervation. Invest Ophthalmol vis Sci 42:2242–2251
Marfurt CF, Cox J, Deek S, Dvorscak L (2010) Anatomy of the human corneal innervation. Exp Eye Res 90:478–492. https://doi.org/10.1016/j.exer.2009.12.010
Marfurt CF (2010) Corneal nerves: anatomy. Encycl Eye. https://doi.org/10.1016/b978-0-12-374203-2.00068-3
Martinez JM, Eling TE (2019) Activation of TRPA1 by volatile organic chemicals leading to sensory irritation. ALTEX 36:572–582. https://doi.org/10.14573/altex.1811012
Mattes RD, DiMeglio D (2001) Ethanol perception and ingestion. Physiol Behav 72:217–229. https://doi.org/10.1016/S0031-9384(00)00397-8
Mazzone SB (2004) Sensory regulation of the cough reflex. Pulm Pharmacol Ther 17:361–368. https://doi.org/10.1016/j.pupt.2004.09.021
Medeiros CS, Santhiago MR (2020) Corneal nerves anatomy, function, injury and regeneration. Exp Eye Res 200:108243. https://doi.org/10.1016/j.exer.2020.108243
Mihara S, Shibamoto T (2015) The role of flavor and fragrance chemicals in TRPA1 (transient receptor potential cation channel, member A1) activity associated with allergies. Allergy Asthma Clin Immunol 11:11. https://doi.org/10.1186/s13223-015-0074-0
Miller SS, Gordon AR, Olsson MJ et al (2013) Mind over age–stereotype activation and olfactory function. Chem Senses 38:167–174. https://doi.org/10.1093/chemse/bjs086
Mohidin N, Bay TC, Yap M (2002) Non-invasive tear break-up time in normal Malays. Clin Exp Optom 85:37–41. https://doi.org/10.1111/j.1444-0938.2002.tb03070.x
Morice AH, Kastelik JA (2003) Cough. 1: Chronic cough in adults. Thorax 58:901–907. https://doi.org/10.1136/thorax.58.10.901
Morice AH, Millqvist E, Bieksiene K et al (2020) ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 55:1901136. https://doi.org/10.1183/13993003.01136-2019
Morris JB, Symanowicz PT, Olsen JE et al (2003) Immediate sensory nerve-mediated respiratory responses to irritants in healthy and allergic airway-diseased mice. J Appl Physiol 94:1563–1571. https://doi.org/10.1152/japplphysiol.00572.2002
Müller JU, Bruckner T, Triebig G (2013) Exposure study to examine chemosensory effects of formaldehyde on hyposensitive and hypersensitive males. Int Arch Occup Env Health 86:107–117. https://doi.org/10.1007/s00420-012-0745-9
Narula M, McGovern AE, Yang SK et al (2014) Afferent neural pathways mediating cough in animals and humans. J Thorac Dis 6:S712–S719. https://doi.org/10.3978/j.issn.2072-1439.2014.03.15
Nasra J, Belvisi MG (2009) Modulation of sensory nerve function and the cough reflex: understanding disease pathogenesis. Pharmacol Ther 124:354–375. https://doi.org/10.1016/j.pharmthera.2009.09.006
National Research Council (2007) Toxicity Testing in the 21st Century. The National Academics Press, Wahington
Nielsen GD (1991) Mechanisms of activation of the sensory irritant receptor by airborne chemicals. Crit Rev Toxicol 21:183–208. https://doi.org/10.3109/10408449109089879
Nielsen GD, Wolkoff P (2017) Evaluation of airborne sensory irritants for setting exposure limits or guidelines: a systematic approach. Regul Toxicol Pharmacol 90:308–317. https://doi.org/10.1016/j.yrtph.2017.09.015
Nielsen GD, Hougaard KS, Larsen ST et al (1999) Acute airway effects of formaldehyde and ozone in BALB/c mice. Hum Exp Toxicol 18:400–409. https://doi.org/10.1191/096032799678840246
Nielsen GD, Wolkoff P, Alarie Y (2007) Sensory irritation: risk assessment approaches. Regul Toxicol Pharmacol 48:6–18. https://doi.org/10.1016/j.yrtph.2006.11.005
Nielsen GD, Larsen ST, Wolkoff P (2013) Recent trend in risk assessment of formaldehyde exposures from indoor air. Arch Toxicol 87:73–98. https://doi.org/10.1007/s00204-012-0975-3
Nordin S (2020) Mechanisms underlying nontoxic indoor air health problems: A review. Int J Hyg Env Health 226:113489. https://doi.org/10.1016/j.ijheh.2020.113489
Nordin S, Millqvist E, Löwhagen O, Bende M (2003) The chemical sensitivity scale: Psychometric properties and comparison with the noise sensitivity scala. J Environ Psychol 23:359–367. https://doi.org/10.1016/S0272-4944(03)00002-1
Nordin S, Martinkauppi M, Olofsson J et al (2005) Chemosensory perception and event-related potentials in self-reported chemical hypersensitivity. Int J Psychophysiol 55:243–255. https://doi.org/10.1016/j.ijpsycho.2004.08.003
Nordin S, Almkvist O, Berglund B (2012) Is loss in odor sensitivity inevitable to the aging individual? A study of “Successfully Aged” elderly. Chemosens Percept 5:188–196. https://doi.org/10.1007/s12078-011-9102-8
Nordin S, Aldrin L, Claeson AS, Andersson L (2017) Effects of negative affectivity and odor valence on chemosensory and symptom perception and perceived ability to focus on a cognitive task. Perception 46:431–446. https://doi.org/10.1177/0301006616686990
Nurmatov UB, Tagiyeva N, Semple S et al (2015) Volatile organic compounds and risk of asthma and allergy: a systematic review. Eur Respir Rev 24:92–101. https://doi.org/10.1183/09059180.00000714
O’Hanlon S, Facer P, Simpson KD et al (2007) Neuronal markers in allergic rhinitis: expression and correlation with sensory testing. Laryngoscope 117:1519–1527. https://doi.org/10.1097/MLG.0b013e3180ca7846
OECD (2013) Revised guidance document on developing and assessing adverse outcome pathways
OECD (2014) Guidance on grouping of chemicals, second edition
OECD (2017) Draft updated guidance document 39 on inhalation toxicity study
Oetjen LK, Kim BS (2018) Interactions of the immune and sensory nervous systems in atopy. FEBS J 285:3138–3151. https://doi.org/10.1111/febs.14465
Ohla K, Lundström JN (2013) Sex differences in chemosensation: sensory or emotional? Front Hum Neurosci 7:607. https://doi.org/10.3389/fnhum.2013.00607
Olofsson JK, Ekström I, Larsson M, Nordin S (2021) Olfaction and aging: a review of the current state of research and future directions. i-Percept 12:204166952110203. https://doi.org/10.1177/20416695211020331
Olofsson JK, Nordin S (2004) Gender differences in chemosensory perception and event-related potentials. Chem Senses 29:629–637. https://doi.org/10.1093/chemse/bjh066
Opiekun RE, Smeets M, Sulewski M et al (2003) Assessment of ocular and nasal irritation in asthmatics resulting from fragrance exposure. Clin Exp Allergy 33:1256–1265. https://doi.org/10.1046/j.1365-2222.2003.01753.x
Österberg K, Persson R, Karlson B, Ørbæk P (2004) Annoyance and performance of three environmentally intolerant groups during experimental challenge with chemical odors. Scand J Work Env Health 30:486–496. https://doi.org/10.5271/sjweh.838
Ozdemir M, Temizdemir H (2010) Age- and gender-related tear function changes in normal population. Eye Lond 24:79–83. https://doi.org/10.1038/eye.2009.21
Pacharra M, Kleinbeck S, Schäper M et al (2016a) Interindividual differences in chemosensory perception: Toward a better understanding of perceptual ratings during chemical exposures. J Toxicol Env Health A 79:1026–1040. https://doi.org/10.1080/15287394.2016.1219547
Pacharra M, Kleinbeck S, Schäper M et al (2016b) Multidimensional assessment of self-reported chemical intolerance and its impact on chemosensory effects during ammonia exposure. Int Arch Occup Env Health 89:947–959. https://doi.org/10.1007/s00420-016-1134-6
Pacharra M, Schäper M, Kleinbeck S et al (2016c) Neurobehavioral effects of exposure to propionic acid revisited-does psychosocial stress interfere with distractive effects in volunteers? Neurotoxicology 55:102–111. https://doi.org/10.1016/j.neuro.2016.05.019
Pacharra M, Schäper M, Kleinbeck S et al (2016d) Olfactory acuity and automatic associations to odor words modulate adverse effects of ammonia. Chem Percept 9:27–36. https://doi.org/10.1007/s12078-016-9202-6
Pacharra M, Kleinbeck S, Schäper M et al (2017) Does seasonal allergic rhinitis increase sensitivity to ammonia exposure? Int J Hyg Env Health 220:840–848. https://doi.org/10.1016/j.ijheh.2017.03.013
Pacharra M, Schäper M, Kleinbeck S et al (2015) Stress lowers the detection threshold for foul-smelling 2-mercaptoethanol. Stress. https://doi.org/10.3109/10253890.2015.1105212
Palmer RF, Walker T, Perales RB et al (2021) Disease comorbidities associated with chemical intolerance. Environ Dis 6:134–141. https://doi.org/10.4103/ed.ed_18_21
Palmquist E, Claeson A-S (2022) Odor perception and symptoms during acrolein exposure in individuals with and without building-related symptoms. Sci Rep 12:8171. https://doi.org/10.1038/s41598-022-12370-7
Papo D, Eberlein-König B, Berresheim HW et al (2006) Chemosensory function and psychological profile in patients with multiple chemical sensitivity: Comparison with odor-sensitive and asymptomatic controls. J Psychosom Res 60:199–209. https://doi.org/10.1016/j.jpsychores.2005.06.075
Päth G, Braun A, Meents N et al (2002) Augmentation of allergic early-phase reaction by nerve growth factor. Am J Respir Crit Care Med 166:818–826. https://doi.org/10.1164/rccm.200202-134OC
Patlewicz G, Ball N, Becker RA, et al (2014) Read-across approaches--misconceptions, promises and challenges ahead. ALTEX 31:387–96. https://doi.org/10.14573/altex.1410071
Pauluhn J (2019) Concentration x time analyses of sensory irritants revisited: weight of evidence or the toxic load approach. That is the question. Toxicol Lett 316:94–108. https://doi.org/10.1016/j.toxlet.2019.09.001
Paustenbach D (2001) Approaches and considerations for setting occupational exposure limits for sensory irritants: report of recent symposia. Aihaj 62:697–704. https://doi.org/10.1080/15298660108984677
Paustenbach D, Alarie Y, Kulle T et al (1997) A recommended occupational exposure limit for formaldehyde based on irritation. J Toxicol Env Health 50:217–263. https://doi.org/10.1080/009841097160465
Peled R (2011) Air pollution exposure: who is at high risk? Atmos Environ 45:1781–1785. https://doi.org/10.1016/j.atmosenv.2011.01.001
Peshori KR, Schicatano EJ, Gopalaswamy R et al (2001) Aging of the trigeminal blink system. Exp Brain Res 136:351–363. https://doi.org/10.1007/s002210000585
Petrova M, Diamond J, Schuster B, Dalton P (2008) Evaluation of trigeminal sensitivity to ammonia in asthmatics and healthy human volunteers. Inhal Toxicol 20:1085–1092. https://doi.org/10.1080/08958370802120396
Philpott CM, Wolstenholme CR, Goodenough PC et al (2006) Comparison of subjective perception with objective measurement of olfaction. Otolaryngol Head Neck Surg 134:488–490. https://doi.org/10.1016/j.otohns.2005.10.041
Pistollato F, de Gyves EM, Carpi D et al (2020) Assessment of developmental neurotoxicity induced by chemical mixtures using an adverse outcome pathway concept. Env Health 19:23. https://doi.org/10.1186/s12940-020-00578-x
Poet TS, Kirman CR, Bader M et al (2010) Quantitative risk analysis for N-methyl pyrrolidone using physiologically based pharmacokinetic and benchmark dose modeling. Toxicol Sci 113:468–482. https://doi.org/10.1093/toxsci/kfp264
Portier C, Tart K, Carter S et al (2010) A human health perspective on climate change: a report outlining research needs on the human health effects of climate change. Environ Health Perspect. https://doi.org/10.1289/ehp.1002272
Rahman EZ, Lam PK, Chu CK et al (2015) Corneal sensitivity in tear dysfunction and its correlation with clinical parameters and blink rate. Am J Ophthalmol. https://doi.org/10.1016/j.ajo.2015.08.005
Ricca V, Landi M, Ferrero P et al (2000) Minimal persistent inflammation is also present in patients with seasonal allergic rhinitis. J Allergy Clin Immunol 105:54–57. https://doi.org/10.1016/s0091-6749(00)90177-5
Ricco MM, Kummer W, Biglari B et al (1996) Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol 496(Pt 2):521–530. https://doi.org/10.1113/jphysiol.1996.sp021703
Rohlman DS, Lucchini R, Anger WK et al (2008) Neurobehavioral testing in human risk assessment. Neurotoxicology 29:556–567. https://doi.org/10.1016/j.neuro.2008.04.003
Rosenkranz D, Bünger J, Hoffmeyer F et al (2020) How healthy is healthy? Comparison between self-reported symptoms and clinical outcomes in connection with the enrollment of volunteers for human exposure studies on sensory irritation effects. Adv Exp Med Biol 1271:49–59. https://doi.org/10.1007/5584_2019_472
Rossi S, Pitidis A (2017) Multiple chemical sensitivity: review of the state of the art in epidemiology, diagnosis and future perspectives. J Occup Env Med 60:138–146. https://doi.org/10.1097/JOM.0000000000001215
Royet JP, Plailly J, Delon-Martin C et al (2003) fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. Neuroimage 20:713–728. https://doi.org/10.1016/S1053-8119(03)00388-4
Saadeh R, Klaunig J (2015) Factors modifying children’s inhalational risk assessment. J Environ Occup Sci 4:56–61. https://doi.org/10.5455/jeos.20150202022401
Sarangapani R, Gentry PR, Covington TR et al (2003) Evaluation of the potential impact of age- and gender-specific lung morphology and ventilation rate on the dosimetry of vapors. Inhal Toxicol 15:987–1016. https://doi.org/10.1080/08958370390226350
Saria A (1988) Neuroimmune interactions in the airways: implications for asthma, allergy, and other inflammatory airway diseases. Brain Behav Immun 2:318–321. https://doi.org/10.1016/0889-1591(88)90034-7
Schaper M (1993) Development of a database for sensory irritants and its use in establishing occupational exposure limits. Am Ind Hyg Assoc J 54:488–544. https://doi.org/10.1080/15298669391355017
Schäper M, Kleinbeck S, Blaszkewicz M, et al (2015) Reizstoffe am Arbeitsplatz: Alterseffekte und olfaktorische Moderatoren. GFA-Press
Scheibe M, Opatz O, Hummel T (2009) Are there sex-related differences in responses to repetitive olfactory/trigeminal stimuli? Eur Arch Otorhinolaryngol 266:1323–1326. https://doi.org/10.1007/s00405-008-0860-0
Schiffman SS, Williams CM (2005) Science of odor as a potential health issue. J Env Qual 34:129–138. https://doi.org/10.2134/jeq2005.0129a
Schnabel E, Schoefer Y, Chen C-M et al (2010) Sensitization to contact allergens and bronchial hyper-responsiveness. Contact Dermatitis 63:157–163. https://doi.org/10.1111/j.1600-0536.2010.01772.x
Schnuch A, Oppel E, Oppel T et al (2010) Experimental inhalation of fragrance allergens in predisposed subjects: effects on skin and airways. Br J Dermatol 162:598–606. https://doi.org/10.1111/j.1365-2133.2009.09510.x
Schwartz J (2004) Air pollution and children’s health. Pediatrics 113:1037–1043. https://doi.org/10.1542/peds.113.4.S1.1037
Seeber A, van Thriel C, Haumann K et al (2002) Psychological reactions related to chemosensory irritation. Int Arch Occup Env Health 75:314–325. https://doi.org/10.1007/s00420-002-0316-6
Selgrade MK, Plopper CG, Gilmour MI et al (2008) Assessing the health effects and risks associated with children’s inhalation exposures–asthma and allergy. J Toxicol Env Health A 71:196–207. https://doi.org/10.1080/15287390701597897
Seubert J, Rea AF, Loughead J, Habel U (2009) Mood induction with olfactory stimuli reveals differential affective responses in males and females. Chem Senses 34:77–84. https://doi.org/10.1093/chemse/bjn054
Shapiro CO, Proskocil BJ, Oppegard LJ et al (2021) Airway sensory nerve density is increased in chronic cough. Am J Respir Crit Care Med 203:348–355. https://doi.org/10.1164/rccm.201912-2347OC
Sharma A, Hindman HB (2014) Aging: a predisposition to dry eyes. J Ophthalmol 2014:781683. https://doi.org/10.1155/2014/781683
Shimizu S, Takahashi N, Mori Y (2014) TRPs as Chemosensors (ROS, RNS, RCS, Gasotransmitters). In: Nilius B, Flockerzi V (eds) Mammalian Transient Receptor Potential (TRP) Cation Channels, vol II. Springer International Publishing, Cham, pp 767–794
Shrestha RK, Borchman D, Foulks GN et al (2011) Analysis of the composition of lipid in human meibum from normal infants, children, adolescents, adults, and adults with meibomian gland dysfunction using (1)H-NMR spectroscopy. Invest Ophthalmol vis Sci 52:7350–7358. https://doi.org/10.1167/iovs.11-7391
Shusterman D (2002) Review of the upper airway, including olfaction, as mediator of symptoms. Env Health Perspect 110(Suppl 4):649–653. https://doi.org/10.1289/ehp.02110s4649
Shusterman D (2007) Trigeminally-mediated health effects of air pollutants: sources of inter-individual variability. Hum Exp Toxicol 26:149–157. https://doi.org/10.1177/0960327107070550
Shusterman D, Balmes J (1997) Measurement of nasal irritant sensitivity to pulsed carbon dioxide: a pilot study. Arch Env Health 52:334–340. https://doi.org/10.1080/00039899709602208
Shusterman DJ, Murphy MA, Balmes JR (1998) Subjects with seasonal allergic rhinitis and nonrhinitic subjects react differentially to nasal provocation with chlorine gas. J Allergy Clin Immunol 101:732–740. https://doi.org/10.1016/S0091-6749(98)70302-1
Shusterman D, Murphy MA, Balmes J (2003a) Differences in nasal irritant sensitivity by age, gender, and allergic rhinitis status. Int Arch Occup Env Health 76:577–583. https://doi.org/10.1007/s00420-003-0459-0
Shusterman D, Murphy MA, Balmes J (2003b) Influence of age, gender, and allergy status on nasal reactivity to inhaled chlorine. Inhal Toxicol 15:1179–1189. https://doi.org/10.1080/08958370390229852
Shusterman D, Tarun A, Murphy MA, Morris J (2005) Seasonal allergic rhinitic and normal subjects respond differentially to nasal provocation with acetic acid vapor. Inhal Toxicol 17:147–152. https://doi.org/10.1080/08958370590904508
Sinding C, Puschmann L, Hummel T (2014) Is the age-related loss in olfactory sensitivity similar for light and heavy molecules? Chem Senses 39:383–390. https://doi.org/10.1093/chemse/bju004
Skaaby S, Flachs EM, Lange P et al (2021) Occupational exposures and exacerbations of asthma and COPD—a general population study. PLoS One 15:e0243826. https://doi.org/10.1371/journal.pone.0243826
Sledge S, Henry C, Borchman D et al (2017) Human meibum age, lipid-lipid interactions and lipid saturation in meibum from infants. Int J Mol Sci 18:1862. https://doi.org/10.3390/ijms18091862
Smeets MA, Bulsing PJ, van Rooden S et al (2007) Odor and irritation thresholds for ammonia: a comparison between static and dynamic olfactometry. Chem Senses 32:11–20. https://doi.org/10.1093/chemse/bjl031
Smith JA, Albeitz J, Begley C et al (2007) The epidemiology of dry eye disease: report of the epidemiology subcommittee of the international dry eye WorkShop (2007). Ocul Surf 5:93–107. https://doi.org/10.1016/S1542-0124(12)70082-4
Spadea L, Salvatore S, Vingolo EM (2013) Corneal sensitivity in keratoconus: a review of the literature. Scient World J 2013:683090. https://doi.org/10.1155/2013/683090
Stainier DY, Gilbert W (1991) Neuronal differentiation and maturation in the mouse trigeminal sensory system, in vivo and in vitro. J Comp Neurol 311:300–312. https://doi.org/10.1002/cne.903110210
Steen KH, Reeh PW (1993) Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett 154:113–116. https://doi.org/10.1016/0304-3940(93)90184-m
Stevens JC, Cain WS (1986) Aging and the perception of nasal irritation. Physiol Behav 37:323–328. https://doi.org/10.1016/0031-9384(86)90241-6
Stevens JC, Cain WS (1987) Old-age deficits in the sense of smell as gauged by thresholds, magnitude matching, and odor identification. Psychol Aging 2:36–42. https://doi.org/10.1037/0882-7974.2.1.36
Stevens JC, Plantinga A, Cain WS (1982) Reduction of odor and nasal pungency associated with aging. Neurobiol Aging 3:125–132. https://doi.org/10.1016/0197-4580(82)90008-2
Stuck BA, Frey S, Freiburg C et al (2006) Chemosensory event-related potentials in relation to side of stimulation, age, sex, and stimulus concentration. Clin Neurophysiol 117:1367–1375. https://doi.org/10.1016/j.clinph.2006.03.004
Sucker K, Hoffmeyer F, Monsé C et al (2019) Ethyl acrylate: influence of sex or atopy on perceptual ratings and eye blink frequency. Arch Toxicol 93:2913–2926. https://doi.org/10.1007/s00204-019-02568-6
Sullivan KM, Manuppello JR, Willett CE (2014) Building on a solid foundation: SAR and QSAR as a fundamental strategy to reduce animal testing. SAR QSAR Environ Res 25:357–365. https://doi.org/10.1080/1062936X.2014.907203
Sundblad BM, Larsson BM, Acevedo F et al (2004) Acute respiratory effects of exposure to ammonia on healthy persons. Scand J Work Env Health 30:313–321. https://doi.org/10.5271/sjweh.800
Sunderland EM, Hu XC, Dassuncao C et al (2019) A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Env Epidemiol 29:131–147. https://doi.org/10.1038/s41370-018-0094-1
Sunwoo Y, Chou C, Takeshita J et al (2006) Physiological and subjective responses to low relative humidity in young and elderly men. J Physiol Anthr 25:229–238. https://doi.org/10.2114/jpa2.25.229
Sweeney LM, Kester JE, Kirman CR et al (2015) Risk assessments for chronic exposure of children and prospective parents to ethylbenzene (CAS No. 100–41-4). Crit Rev Toxicol 45:662–726. https://doi.org/10.3109/10408444.2015.1046157
Szallasi A (2002) Vanilloid (capsaicin) receptors in health and disease. Am J Clin Pathol 118:110–121. https://doi.org/10.1309/7AYY-VVH1-GQT5-J4R2
Tagiyeva N, Sheikh A (2014) Domestic exposure to volatile organic compounds in relation to asthma and allergy in children and adults. Expert Rev Clin Immunol 10:1611–1639. https://doi.org/10.1586/1744666X.2014.972943
Thuerauf N, Reulbach U, Lunkenheimer J et al (2009) Emotional reactivity to odors: olfactory sensitivity and the span of emotional evaluation separate the genders. Neurosci Lett 456:74–79. https://doi.org/10.1016/j.neulet.2009.03.096
Tian LJ, Du YR, Xiao Y et al (2009) Mediating roles of the vanilloid receptor TRPV1 in activation of rat primary afferent nociceptive neurons by formaldehyde. Sheng Li Xue Bao 61:404–416
Toma JS, McPhail LT, Ramer MS (2006) Comparative postnatal development of spinal, trigeminal and vagal sensory root entry zones. Int J Dev Neurosci 24:373–388. https://doi.org/10.1016/j.ijdevneu.2006.06.001
U. S. Environmental Protection Agency (2005) Guidelines for carcinogen risk assessment
U. S. Environmental Protection Agency (1994) Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry. EPA/600/8–90/066F. Washington (DC): United States Environmental Protection Agency. https://www.epa.gov/sites/default/files/2014-11/documents/rfc_methodology.pdf
Undem BJ, Taylor-Clark T (2014) Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol 133:1521–1534. https://doi.org/10.1016/j.jaci.2013.11.027
Undem BJ, Kajekar R, Hunter DD, Myers AC (2000) Neural integration and allergic disease. J Allergy Clin Immunol 106:S213–S220. https://doi.org/10.1067/mai.2000.110153
Uppal N, Foxe JJ, Butler JS et al (2016) The neural dynamics of somatosensory processing and adaptation across childhood: a high-density electrical mapping study. J Neurophysiol 115:1605–1619. https://doi.org/10.1152/jn.01059.2015
Valcke M, Krishnan K (2011) Assessing the impact of the duration and intensity of inhalation exposure on the magnitude of the variability of internal dose metrics in children and adults. Inhal Toxicol 23:863–877. https://doi.org/10.3109/08958378.2011.609918
van Wijk CM, Kolk AM (1997) Sex differences in physical symptoms: the contribution of symptom perception theory. Soc Sci Med 45:231–246. https://doi.org/10.1016/s0277-9536(96)00340-1
van Thriel C, Haumann K, Kiesswetter E et al (2002) Time courses of sensory irritations due to 2-butanone and ethyl benzene exposure: influences of self-reported multiple chemical sensitivity (sMCS). Int J Hyg Env Health 204:367–369. https://doi.org/10.1078/1438-4639-00112
van Thriel C, Kiesswetter E, Schäper M et al (2005) An integrative approach considering acute symptoms and intensity ratings of chemosensory sensations during experimental exposures. Env Toxicol Pharmacol 19:589–598. https://doi.org/10.1016/j.etap.2004.12.024
van Thriel C, Schäper M, Kiesswetter E et al (2006) From chemosensory thresholds to whole body exposures-experimental approaches evaluating chemosensory effects of chemicals. Int Arch Occup Env Health 79:308–321. https://doi.org/10.1007/s00420-005-0057-4
van Thriel C, Kiesswetter E, Schäper M et al (2008) Odor annoyance of environmental chemicals: sensory and cognitive influences. J Toxicol Env Health A 71:776–785. https://doi.org/10.1080/15287390801985596
van Thriel C, Schäper M, Kleinbeck S et al (2010) Sensory and pulmonary effects of acute exposure to sulfur dioxide (SO2). Toxicol Lett 196:42–50. https://doi.org/10.1016/j.toxlet.2010.03.013
Vermeire T, Stevenson H, Pieters MN et al (1999) Assessment factors for human health risk assessment: a discussion paper. Crit Rev Toxicol 29:439–490. https://doi.org/10.1080/10408449991349249
Viziano A, Micarelli A, Pasquantonio G et al (2018) Perspectives on multisensory perception disruption in idiopathic environmental intolerance: a systematic review. Int Arch Occup Env Health 91:923–935. https://doi.org/10.1007/s00420-018-1346-z
Vogelmeier C, Criner G, Martinez F et al (2017) Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Respirology. https://doi.org/10.1111/resp.13012
Wålinder R, Ernstgård L, Norbäck D et al (2008) Acute effects of 1-octen-3-ol, a microbial volatile organic compound (MVOC)–an experimental study. Toxicol Lett 181:141–147. https://doi.org/10.1016/j.toxlet.2008.07.013
Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54:1063–1070. https://doi.org/10.1037//0022-3514.54.6.1063
Winder C (2002) Mechanisms of multiple chemical sensitivity. Toxicol Lett 128:85–97. https://doi.org/10.1016/s0378-4274(01)00536-7
Wise PM, Canty TM, Wysocki CJ (2005) Temporal integration of nasal irritation from ammonia at threshold and supra-threshold levels. Toxicol Sci 87:223–231. https://doi.org/10.1093/toxsci/kfi229
Wise PM, Canty TM, Wysocki CJ (2006) Temporal integration in nasal lateralization of ethanol. Chem Senses 31:227–235. https://doi.org/10.1093/chemse/bjj023
Wise PM, Toczydlowski SE, Wysocki CJ (2007) Temporal integration in nasal lateralization of homologous alcohols. Toxicol Sci 99:254–259. https://doi.org/10.1093/toxsci/kfm144
Wise PM, Toczydlowski SE, Zhao K, Wysocki CJ (2009a) Temporal integration in nasal lateralization of homologous propionates. Inhal Toxicol 21:819–827. https://doi.org/10.1080/08958370802555880
Wise PM, Zhao K, Wysocki CJ (2009b) Dynamics of nasal chemesthesis. Ann N Acad Sci 1170:206–214. https://doi.org/10.1111/j.1749-6632.2009.03912.x
Wolkoff P (2013) Indoor air pollutants in office environments: assessment of comfort, health, and performance. Int J Hyg Env Health 216:371–394. https://doi.org/10.1016/j.ijheh.2012.08.001
Wolkoff P (2017) External eye symptoms in indoor environments. Indoor Air 27:246–260. https://doi.org/10.1111/ina.12322
Wolkoff P (2018) The mystery of dry indoor air - an overview. Env Int 121:1058–1065. https://doi.org/10.1016/j.envint.2018.10.053
Wolkoff P (2020) Dry eye symptoms in offices and deteriorated work performance - a perspective. Build Environ 172:106704. https://doi.org/10.1016/j.buildenv.2020.106704
Wolkoff P, Nielsen GD (2010) Non-cancer effects of formaldehyde and relevance for setting an indoor air guideline. Env Int 36:788–799. https://doi.org/10.1016/j.envint.2010.05.012
Wolkoff P, Skov P, Franck C, Petersen LN (2003) Eye irritation and environmental factors in the office environment–hypotheses, causes and a physiological model. Scand J Work Env Health 29:411–430. https://doi.org/10.5271/sjweh.748
Wolkoff P, Wilkins CK, Clausen PA, Nielsen GD (2006) Organic compounds in office environments - sensory irritation, odor, measurements and the role of reactive chemistry. Indoor Air 16:7–19. https://doi.org/10.1111/j.1600-0668.2005.00393.x
Wolkoff P, Clausen PA, Larsen ST et al (2012) Airway effects of repeated exposures to ozone-initiated limonene oxidation products as model of indoor air mixtures. Toxicol Lett 209:166–172. https://doi.org/10.1016/j.toxlet.2011.12.008
Wong CH, Morice AH (1999) Cough threshold in patients with chronic obstructive pulmonary disease. Thorax 54:62–64. https://doi.org/10.1136/thx.54.1.62
World Health Organization WHO (2010) WHO Guidelines for indoor air quality: selected pollutants
Wysocki CJ, Cowart BJ, Radil T (2003) Nasal trigeminal chemosensitivity across the adult life span. Percept Psychophys 65:115–122. https://doi.org/10.3758/bf03194788
Yang X, Zhang YP, Chen D et al (2001) Eye irritation caused by formaldehyde as an indoor air pollution–a controlled human exposure experiment. Biomed Environ Sci 14:229–236
Yang AY, Chow J, Liu J (2018) Corneal innervation and sensation: the eye and beyond. Yale J Biol Med 91:13–21
Zametkin AJ, Stevens JR, Pittman R (1979) Ontogeny of spontaneous blinking and of habituation of the blink reflex. Ann Neurol 5:453–457. https://doi.org/10.1002/ana.410050509
Zander E, Weddell G (1951) Observations on the innervation of the cornea. J Anat 85:68–99
Zholos AV (2015) TRP channels in respiratory pathophysiology: the role of oxidative, chemical irritant and temperature stimuli. Curr Neuropharmacol 13:279–291. https://doi.org/10.2174/1570159x13666150331223118
Zucco GM, Doty RL (2022) Multiple chemical sensitivity. Brain Sci 12:46. https://doi.org/10.3390/brainsci12010046
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PW declares no financial conflict of interest. PW was not financed by external funding. SK declares no conflict of interest. SK received external funding from the European Panel Federation (EPF).
Ethical statements
The authors thank Dr. Marlene Pacharra for helpful comments on a former version of the paper. The authors also appreciate comments from Dr. E. Leibold and Dr. M. Dunky on a former version.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kleinbeck, S., Wolkoff, P. Exposure limits for indoor volatile substances concerning the general population: The role of population-based differences in sensory irritation of the eyes and airways for assessment factors. Arch Toxicol 98, 617–662 (2024). https://doi.org/10.1007/s00204-023-03642-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-023-03642-w