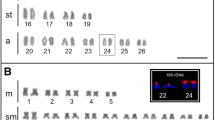
Abstract
Chromosomes of Japanese quail (Coturnix coturnix japonica, 2n=78), a galliform domestic species closely related to chicken, possess multiple heterochromatic segments. Due to the difficulties in careful analysis of such heterochromatic regions, there is a lack of data on their DNA composition, epigenetic status, as well as spatial distribution in interphase nucleus. In the present study, we applied giant lampbrush chromosome (LBC) microdissection for high-resolution analysis of quail centromeric regions of macrochromosomes and polymorphic short arms of submetacentric microchromosomes. FISH with the dissected material on mitotic and meiotic chromosomes indicated that in contrast to centromeres of chicken macrochromosomes, which are known to harbor chromosome-specific and, in some cases, tandem repeat-free sequences, centromeres of quail macroautosomes (CCO1–CCO11) have canonical organization. CCO1–CCO11 centromeres possess massive blocks of common DNA repeats demonstrating transcriptional activity at LBC stage. These repeats seem to have been subjected to chromosome size-correlated homogenization previously described primarily for avian microchromosomes. In addition, comparative FISH on chicken chromosomes supported the previous data on centromere repositioning events during galliform karyotype evolution. In interphase nucleus of different cell types, repetitive elements specific for microchromosome short arms constitute the material of prominent centrally located chromocenters enriched with markers of constitutive heterochromatin and rimmed with clusters of microchromosomal centromeric BglII-repeat. Thus, clustering of such repeats is responsible for the peculiar architecture of quail interphase nucleus. In contrast, centromere repeats of the largest macrochromosomes (CCO1 and CCO2) are predominantly localized in perinuclear heterochromatin. The possible involvement of the isolated repeats in radial genome organization is discussed.







Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- BAC:
-
Bacterial artificial chromosome
- CCO:
-
Japanese quail (Coturnix coturnix japonica) chromosomes
- CNM:
-
Chicken nuclear-membrane-associated repeat
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DOP-PCR:
-
Degenerate oligonucleotide-primed PCR
- FISH:
-
Fluorescence in situ hybridization
- GGA:
-
Chicken (Gallus gallus) chromosome
- LBC:
-
Lampbrush chromosome
- LTRs:
-
Long terminal repeats
- P-arm:
-
short arm of submetacentric microchromosomes
- QEF:
-
Quail embryonic fibroblasts
- SSC:
-
Saline-sodium citrate buffer
References
Berchtold D, Fesser S, Bachmann G, Kaiser A, Eilert JC, Frohns F, Sadoni N, Muck J, Kremmer E, Eick D, Layer PG, Zink D (2011) Nuclei of chicken neurons in tissues and three-dimensional cell cultures are organized into distinct radial zones. Chromosom Res 19:165–182. https://doi.org/10.1007/s10577-010-9182-3
Bloom SE (1974) Current knowledge about the avian W chromosome. Bioscience 24:340–344
Bloom SE, Bacon LD (1985) Linkage of the major histocompatibility (B) complex and the nucleolar organizer in the chicken. Assignment to a microchromosome. J Hered 76:146–154
Burt A, Trivers RL (2006) Genes in conflict: the biology of selfish genetic elements
Chen C-C, Balaban E, Jarvis ED (2012) Interspecies avian brain chimeras reveal that large brain size differences are influenced by cell-interdependent processes. PLoS One 7:e42477. https://doi.org/10.1371/journal.pone.0042477
Chen H, Comment N, Chen J, Ronquist S, Hero A, Ried T, Rajapakse I (2015) Chromosome conformation of human fibroblasts grown in 3-dimensional spheroids. Nucleus 6:55–65. https://doi.org/10.1080/19491034.2014.1003745
Comings DE, Mattoccia E (1970) Studies of microchromosomes and a G-C rich DNA satellite in the quail. Chromosoma 30:202–214
Cournac A, Koszul R, Mozziconacci J (2016) The 3D folding of metazoan genomes correlates with the association of similar repetitive elements. Nucleic Acids Res 44:245–255. https://doi.org/10.1093/nar/gkv1292
Daks AA, Deriusheva SE, Krasikova AV, Zlotina AM, Gaginskaia ER, Galkina SA (2010) Lampbrush chromosomes of the Japanese quail (Coturnix coturnix japonica): a new version of cytogenetic maps. Genetika 46:1335–1338
de la Sena CA, Fechheimer NS, Nestor KE (1991) Variability of C-banding patterns in Japanese quail chromosomes. Genome 34:993–997
Delany ME, Robinson CM, Goto RM, Miller MM (2009) Architecture and organization of chicken microchromosome 16: order of the NOR, MHC-Y, and MHC-B subregions. J Hered 100:507–514. https://doi.org/10.1093/jhered/esp044
Deryusheva S, Krasikova A, Kulikova T, Gaginskaya E (2007) Tandem 41-bp repeats in chicken and Japanese quail genomes: FISH mapping and transcription analysis on lampbrush chromosomes. Chromosoma 116:519–530. https://doi.org/10.1007/s00412-007-0117-5
Eberhart A, Kimura H, Leonhardt H, Joffe B, Solovei I (2012) Reliable detection of epigenetic histone marks and nuclear proteins in tissue cryosections. Chromosom Res 20:849–858. https://doi.org/10.1007/s10577-012-9318-8
Falk M, Feodorova Y, Naumova N et al (2018) Heterochromatin drives organization of conventional and inverted nuclei. Available from: https://www.biorxiv.org/content/early/2018/01/09/244038
Freshney RI (ed) (2005) Culture of animal cells: a manual of basic technique, 5th edn. John Wiley & Sons, New York
Gaginskaya E, Kulikova T, Krasikova A (2009) Avian lampbrush chromosomes: a powerful tool for exploration of genome expression. Cytogenet Genome Res 124:251–267. https://doi.org/10.1159/000218130
Galkina S, Deryusheva S, Fillon V, Vignal A, Crooijmans R, Groenen M, Rodionov A, Gaginskaya E (2006) FISH on avian lampbrush chromosomes produces higher resolution gene mapping. Genetica 128:241–251. https://doi.org/10.1007/s10709-005-5776-7
Griffin DK, Haberman F, Masabanda J, O’Brien P, Bagga M, Sazanov A, Smith J, Burt DW, Ferguson-Smith M, Wienberg J (1999) Micro- and macrochromosome paints generated by flow cytometry and microdissection: tools for mapping the chicken genome. Cytogenet Cell Genet 87:278–281. https://doi.org/10.1159/000015449
Guenatri M, Bailly D, Maison C, Almouzni G (2004) Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J Cell Biol 166:493–505. https://doi.org/10.1083/jcb.200403109
Habermann FA, Cremer M, Walter J, Kreth G, von Hase J, Bauer K, Wienberg J, Cremer C, Cremer T, Solovei I (2001) Arrangements of macro- and microchromosomes in chicken cells. Chromosom Res 9:569–584
Hori T, Suzuki Y, Solovei I, Saitoh Y, Hutchison N, Ikeda JE, Macgregor H, Mizuno S (1996) Characterization of DNA sequences constituting the terminal heterochromatin of the chicken Z chromosome. Chromosom Res 4:411–426
Ishishita S, Tsuruta Y, Uno Y, Nakamura A, Nishida C, Griffin DK, Tsudzuki M, Ono T, Matsuda Y (2014) Chromosome size-correlated and chromosome size-uncorrelated homogenization of centromeric repetitive sequences in New World quails. Chromosom Res 22:15–34. https://doi.org/10.1007/s10577-014-9402-3
Itoh Y, Mizuno S (2002) Molecular and cytological characterization of SspI-family repetitive sequence on the chicken W chromosome. Chromosom Res 10:499–511. https://doi.org/10.1023/A:1020944414750
Kayang BB, Fillon V, Inoue-Murayama M, Miwa M, Leroux S, Fève K, Monvoisin JL, Pitel F, Vignoles M, Mouilhayrat C, Beaumont C, Ito S', Minvielle F, Vignal A (2006) Integrated maps in quail (Coturnix japonica) confirm the high degree of synteny conservation with chicken (Gallus gallus) despite 35 million years of divergence. BMC Genomics 7:1–18. https://doi.org/10.1186/1471-2164-7-101
Koshida Y, Kosin IL (1967) Intra-nuclear sex dimorphism in the growing feathers of six species of Galliformes. Cytologia (Tokyo) 33:230–240
Krasikova AV, Gaginskaia ER (2010) Organization of centromere regions of chromosomes in the lampbrush phase. Tsitologiia 52:515–533
Krasikova AV, Kulikova TV (2017) Distribution of heterochromatin markers in lampbrush chromosomes in birds. Russ J Genet 53:1022–1029. https://doi.org/10.1134/S1022795417090071
Krasikova A, Deryusheva S, Galkina S, Kurganova A, Evteev A, Gaginskaya E (2006) On the positions of centromeres in chicken lampbrush chromosomes. Chromosom Res 14:777–789. https://doi.org/10.1007/s10577-006-1085-y
Krasikova A, Daks A, Zlotina A, Gaginskaya E (2009) Polymorphic heterochromatic segments in Japanese quail microchromosomes. Cytogenet Genome Res 126:148–155. https://doi.org/10.1159/000245914
Krasikova A, Fukagawa T, Zlotina A (2012) High-resolution mapping and transcriptional activity analysis of chicken centromere sequences on giant lampbrush chromosomes. Chromosom Res 20:995–1008. https://doi.org/10.1007/s10577-012-9321-0
Kress C, Montillet G, Jean C, Fuet A, Pain B (2016) Chicken embryonic stem cells and primordial germ cells display different heterochromatic histone marks than their mammalian counterparts. Epigenetics Chromatin 9:5. https://doi.org/10.1186/s13072-016-0056-6
Lampbrush chromosomes. Home page: http://projects.exeter.ac.uk/lampbrush/index.htm. Accessed 16 Oct 2018
Le Douarin N (1973a) A biological cell labeling technique and its use in experimental embryology. Dev Biol 30:217–222. https://doi.org/10.1016/0012-1606(73)90061-4
Le Douarin N (1973b) A Feulgen-positive nucleolus. Exp Cell Res 77:459–468
Le Douarin N, Dieterlen-Lievre F, Creuzet S, Teillet M-A (2008) Quail-chick transplantations. Methods Cell Biol 87:19–58. https://doi.org/10.1016/S0091-679X(08)00202-1
Li J, Leung FC (2006) A CR1 element is embedded in a novel tandem repeat (HinfI repeat) within the chicken genome. Genome 49:97–103. https://doi.org/10.1139/g05-090
Maslova A, Zlotina A, Kosyakova N, Sidorova M, Krasikova A (2015) Three-dimensional architecture of tandem repeats in chicken interphase nucleus. Chromosom Res 23:625–639. https://doi.org/10.1007/s10577-015-9485-5
Matzke MA, Varga F, Berger H, Schernthaner J, Schweizer D, Mayr B, Matzke AJM (1990) A 41-42 bp tandemly repeated sequence isolated from nuclear envelopes of chicken erythrocytes is located predominantly on microchromosomes. Chromosoma 99:131–137
Matzke AJ, Varga F, Gruendler P et al (1992) Characterization of a new repetitive sequence that is enriched on microchromosomes of turkey. Chromosoma 102:9–14
Maya-Mendoza A, Bartek J, Jackson DA, Streuli CH (2016) Cellular microenvironment controls the nuclear architecture of breast epithelia through beta1-integrin. Cell Cycle 15:345–356. https://doi.org/10.1080/15384101.2015.1121354
McPherson MC, Robinson CM, Gehlen LP, Delany ME (2014) Comparative cytogenomics of poultry: mapping of single gene and repeat loci in the Japanese quail (Coturnix japonica). Chromosom Res 22:71–83. https://doi.org/10.1007/s10577-014-9411-2
Mirre C, Stahl A (1978) Peripheral RNA synthesis of fibrillar center in nucleoli of Japanese quail oocytes and somatic cells. J Ultrastruct Res 64:377–387
Nishida C, Ishijima J, Ishishita S, Yamada K, Griffin DK, Yamazaki T, Matsuda Y (2013) Karyotype reorganization with conserved genomic compartmentalization in dot-shaped microchromosomes in the Japanese mountain hawk-eagle (Nisaetus nipalensis orientalis, Accipitridae). Cytogenet Genome Res 141:284–294. https://doi.org/10.1159/000352067
Politz JCR, Scalzo D, Groudine M (2013) Something silent this way forms: the functional organization of the repressive nuclear compartment. Annu Rev Cell Dev Biol 29:241–270. https://doi.org/10.1146/annurev-cellbio-101512-122317
Politz JCR, Scalzo D, Groudine M (2016) The redundancy of the mammalian heterochromatic compartment. Curr Opin Genet Dev 37:1–8. https://doi.org/10.1016/j.gde.2015.10.007
Ragoczy T, Telling A, Scalzo D, Kooperberg C, Groudine M (2014) Functional redundancy in the nuclear compartmentalization of the late-replicating genome. Nucleus 5:626–635. https://doi.org/10.4161/19491034.2014.990863
Saitoh Y, Mizuno S (1992) Distribution of XhoI and EcoRI family repetitive DNA sequences into separate domains in the chicken W chromosome. Chromosoma 101:474–477
Sasaki M, Nishida C (1980) C-banded heteromorphism in the Z chromosome of the Japanese quail, Coturnix c. japonica. Chrom Inf Serv 29:21–22
Schmid M, Enderle E, Schindler D, Schempp W (1989) Chromosome banding and DNA replication patterns in bird karyotypes. Cytogenet Cell Genet 52:139–146. https://doi.org/10.1159/000132864
Shang W-H, Hori T, Toyoda A, Kato J, Popendorf K, Sakakibara Y, Fujiyama A, Fukagawa T (2010) Chickens possess centromeres with both extended tandem repeats and short non-tandem-repetitive sequences. Genome Res 20:1219–1228. https://doi.org/10.1101/gr.106245.110
Shibusawa M, Minai S, Nishida-Umehara C, Suzuki T, Mano T, Yamada K, Namikawa T, Matsuda Y (2001) A comparative cytogenetic study of chromosome homology between chicken and Japanese quail. Cytogenet Cell Genet 95:103–109
Shibusawa M, Nishibori M, Nishida-Umehara C, Tsudzuki M, Masabanda J, Griffin DK, Matsuda Y (2004) Karyotypic evolution in the Galliformes: an examination of the process of karyotypic evolution by comparison of the molecular cytogenetic findings with the molecular phylogeny. Cytogenet Genome Res 106:111–119. https://doi.org/10.1159/000078570
Skinner BM, Volker M, Ellis M, Griffin DK (2009) An appraisal of nuclear organisation in interphase embryonic fibroblasts of chicken, turkey and duck. Cytogenet Genome Res 126:156–164. https://doi.org/10.1159/000245915
Solovei I (2010) Fluorescence in situ hybridization (FISH) on tissue cryosections. Methods Mol Biol 659:71–82. https://doi.org/10.1007/978-1-60761-789-1_5
Solovei I, Gaginskaya E, Hutchison N, Macgregor H (1993) Avian sex chromosomes in the lampbrush form: the ZW lampbrush bivalents from six species of bird. Chromosom Res 1:153–166. https://doi.org/10.1007/BF00710769
Stefos K, Arrighi FE (1971) Heterochromatic nature of W chromosome in birds. Exp Cell Res 68:228–231
Stock AD, Bunch TD (1982) The evolutionary implications of chromosome banding pattern homologies in the bird order Galliformes. Cytogenet Cell Genet 34:136–148. https://doi.org/10.1159/000131802
Tanaka K, Suzuki T, Nojiri T, Yamagata T, Namikawa T, Matsuda Y (2000) Characterization and chromosomal distribution of a novel satellite DNA sequence of Japanese quail (Coturnix coturnix japonica). J Hered 91:412–415
Trofimova I, Krasikova A (2016) Transcription of highly repetitive tandemly organized DNA in amphibians and birds: a historical overview and modern concepts. RNA Biol 13:1246–1257. https://doi.org/10.1080/15476286.2016.1240142
Valente GT, Nakajima RT, Fantinatti BEA, Marques DF, Almeida RO, Simões RP, Martins C (2017) B chromosomes: from cytogenetics to systems biology. Chromosoma 126:73–81. https://doi.org/10.1007/s00412-016-0613-6
Wicker T, Robertson JS, Schulze SR, Feltus FA, Magrini V, Morrison JA, Mardis ER, Wilson RK, Peterson DG, Paterson AH, Ivarie R (2005) The repetitive landscape of the chicken genome. Genome Res 15:126–136. https://doi.org/10.1101/gr.2438004
Yamada K, Shibusawa M, Tsudzuki M, Matsuda Y (2002a) Molecular cloning and characterization of novel centromeric repetitive DNA sequences in the blue-breasted quail (Coturnix chinensis, Galliformes). Cytogenet Genome Res 98:255–261. https://doi.org/10.1159/000071044
Yamada K, Nishida-Umehara C, Matsuda Y (2002b) Characterization and chromosomal distribution of novel satellite DNA sequences of the lesser rhea (Pterocnemia pennata) and the greater rhea (Rhea americana). Chromosom Res 10:513–523
Yamada K, Nishida-Umehara C, Ishijima J, Murakami T, Shibusawa M, Tsuchiya K, Tsudzuki M, Matsuda Y (2006) A novel family of repetitive DNA sequences amplified site-specifically on the W chromosomes in Neognathous birds. Chromosom Res 14:613–627. https://doi.org/10.1007/s10577-006-1071-4
Yang F, Trifonov V, Ng B et al (2009) Generation of paint probes by flow-sorted and microdissected chromosomes. In: Liehr T (ed) Fluorescence in situ hybridization (FISH)—application guide. Berlin, pp 35–52
Zlotina A, Galkina S, Krasikova A, Crooijmans RPMA, Groenen MAM, Gaginskaya E, Deryusheva S (2010) Precise centromere positioning on chicken chromosome 3. Cytogenet Genome Res 129:310–313. https://doi.org/10.1159/000314923
Zlotina A, Galkina S, Krasikova A, Crooijmans RPMA, Groenen MAM, Gaginskaya E, Deryusheva S (2012) Centromere positions in chicken and Japanese quail chromosomes: de novo centromere formation versus pericentric inversions. Chromosom Res 20:1017–1032. https://doi.org/10.1007/s10577-012-9319-7
Zlotina A, Kulikova T, Kosyakova N, Liehr T, Krasikova A (2016) Microdissection of lampbrush chromosomes as an approach for generation of locus-specific FISH-probes and samples for high-throughput sequencing. BMC Genomics 17:1–15. https://doi.org/10.1186/s12864-016-2437-4
Acknowledgements
The authors sincerely thank Felix A. Habermann (Ludwig-Maximilians-University of Munich, Germany) for providing the chicken chromosome-specific paint F12, Richard Crooijmans and Martin Groenen (Wageningen University, The Netherlands) for providing the chicken BAC clone WAG29F23, and Svetlana Galkina for providing the oligonucleotide probe to BglII-repeat.
The research of AZ, AM, and AK was supported by a grant of the President of the Russian Federation (MK-1630.2017.4). The research of NK, ABHA-R, and TL was supported by the DAAD University Partnership Programme between Friedrich Schiller University (Jena, Germany) and Saint Petersburg State University (Saint Petersburg, Russia). The work was partially performed using experimental equipment of the Research Resource Center “Molecular and Cell Technologies” of St. Petersburg State University.
Author information
Authors and Affiliations
Contributions
AZ designed the study, carried out the principal molecular-cytogenetic experiments, and drafted the manuscript. AM carried out 3D molecular-cytogenetic experiments on somatic cells and tissues and drafted the manuscript. NK, ABHA-R, AZ, and TL performed microdissection procedure. AK designed the study, coordinated the project, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
None of the authors have any competing interests in the manuscript.
Ethical statement
All international, national, and institutional guidelines for the care and use of laboratory and farm animals were followed (institutional Ethical Committee approval no. 131-03-2, 14 March 2016).
Additional information
Responsible Editor: Fengtang Yang
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 13744 kb)
Rights and permissions
About this article
Cite this article
Zlotina, A., Maslova, A., Kosyakova, N. et al. Heterochromatic regions in Japanese quail chromosomes: comprehensive molecular-cytogenetic characterization and 3D mapping in interphase nucleus. Chromosome Res 27, 253–270 (2019). https://doi.org/10.1007/s10577-018-9597-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-018-9597-9