Abstract
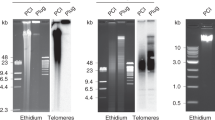
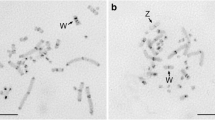
To study whether specific DNA sequences are associated with nuclear membranes, residual DNA was extracted from DNase-treated nuclear envelopes prepared from erythrocytes of adult chickens (Gallus domesticus). This DNA was then blunt-end ligated into a bacterial plasmid vector. DNA blot analysis and nucleotide sequence determination revealed that approximately 30% of the cloned fragments consisted of different multiples of a 41–42 bp tandemly repeated, partially symmetrical sequence. In situ hybridization to chicken chromosomes demonstrated that the sequence was located primarily on microchromosomes, although some hybridization was also observed to macrochromosomes 7 and 8. Digestion of chicken DNA with any of a number of restriction enzymes did not completely reduce the intensity of a high molecular weight band to which the repeated sequence hybridized. These results, along with those obtained from in situ hybridization, suggested that many copies of this sequence are organized into large tandem arrays, and are not dispersed in many shorter repetitive blocks throughout the chicken genome. Although the repetitive sequence constituted approximately 10% of the chicken genome, it did not hybridize to quail or turkey DNA.
Similar content being viewed by others
References
Auer H, Mayr B, Lambrou M, Schleger W (1987) An extended chicken karyotype, including the NOR chromosome. Cytogenet Cell Genet 45:218–221
Beug H, Doederlein G, Freudenstein C, Graf T (1982) Erythroblast cell lines transformed by a temperature-sensitive mutant of avian erythroblastosis virus: a model system to study erythroid differentiation in vitro. J Cell Physiol Suppl 1:195–207
Blin N, Stafford DW (1976) A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res 3:2303–2308
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Carlenius C, Ryttman H, Tegelström H, Jansson H (1981) R-, G- and C-banded chromosomes in the domestic fowl (Gallus domesticus). Hereditas 94:61–66
Cook PR (1988) The nucleoskeleton: artefact, passive framework or active site? J Cell Sci 90:1–6
Franke WW (1966) Isolated nuclear membranes. J Cell Biol 31:619–623
Franke WW (1977) Structure and function of nuclear membranes. Biochem Soc Symp 42:125–135
Franke WW, Deumling B, Ermen B, Jarasch E-D, Kleinig H (1970) Nuclear membranes from mammalian liver. I. Isolation procedure and general characterization. J Cell Biol 46:379–395
Franke WW, Deumling B, Zentgraf H, Falk H, Rae PMM (1973) Nuclear membranes from mammalian liver. IV. Characterization of membrane-attached DNA. Exp Cell Res 81:365–392
Franke WW, Scheer U, Krohne G, Jarasch E-D (1981) The nuclear envelope and the architecture of the nuclear periphery. J Cell Biol 91:39s–50s
Jackson RC (1976) Polypeptides of the nuclear envelope. Biochemistry 15:5641–5651
Jackson DA, Cook PR (1988) Visualization of a filamentous nucleoskeleton with a 23 nm axial repeat. EMBO J 7:3667–3677
Kaspar CB (1974) Isolation and properties of the nuclear envelope. Methods Enzymol XXXI:279–292
Kodama H, Saitoh H, Tone M, Kuhara S, Sakaki Y, Mizuno S (1987) Nucleotide sequences and unusual electrophoretic behavior of the W chromosome-specific repeating units of the domestic fowl,Gallus gallus domesticus. Chromosoma 9:18–25
Labarca C, Paigen K (1980) A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 102:344–352
Lafond RE, Woodcock CLF (1983) Status of the nuclear matrix in mature and embryonic chick erythrocyte nuclei. Exp Cell Res 147:31–39
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Matzke MA, Matzke AJM (1985) Potential difference across the nuclear membrane: a regulator of gene expression? J Bioelect 4:461–479
Maxam A, Gilbert W (1977) A new method for sequencing DNA. Proc Natl Acad Sci USA 74:560–564
McLaughlin S (1989) The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem 18:113–136
Meehan RR, Lewis JD, McKay S, Kleiner EL, Bird AP (1989) Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell 58:499–507
Mirkovitch J, Mirault M-E, Laemmli UK (1984) Organization of the higher-order chromatin loop: specific DNA attachment sites on the nuclear scaffold. Cell 39:223–232
Monneron A, Blobel G, Palade G (1972) Fractionation of the nucleus by divalent cations. J Cell Biol 55:104–125
Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu J-R (1988) A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA 85:6622–6626
Neumann E (1981) Principles of electric field effects in chemical and biological systems. Top Bioelectrochemistry Bioenergetics 4:113–160
Neumann E (1986) Elementary analysis of chemical electric field effects in biological macromolecules. In: Gutmann F, Keyzer H (eds) Modern Bioelectrochemistry. Plenum, New York, pp 97–175
Newport JW, Forbes DJ (1987) The nucleus: structure, function and dynamics. Annu Rev Biochem 56:535–564
Philipp E-K, Franke WW, Keenan TW, Stadler J, Jarasch E-D (1976) Characterization of nuclear membranes and endoplasmic reticulum isolated from plant tissue. J Cell Biol 68:11–29
Pinkel D, Straume T, Gray JW (1986) Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA 83:2934–2938
Porschke D (1985) Effects of electric fields on biopolymers. Annu Rev Phys Chem 36:159–178
Stavy R, Ben-Shaul Y, Galun E (1973) Nuclear envelope isolation in peas. Biochim Biophys Acta 323:167–177
Tone M, Sakaki Y, Hashiguchi T, Mizuno S (1984) Genus specificity and extensive methylation of the W chromosome-specific repetitive sequences from the domestic fowl,Gallus gallus domesticus. Chromosoma 89:228–235
Verheijen R, Van Venrooij W, Ramackers F (1988) The nuclear matrix: structure and composition. J Cell Sci 90:11–36
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matzke, M.A., Varga, F., Berger, H. et al. A 41–42 bp tandemly repeated sequence isolated from nuclear envelopes of chicken erythrocytes is located predominantly on microchromosomes. Chromosoma 99, 131–137 (1990). https://doi.org/10.1007/BF01735329
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01735329