Abstract
Background
Despite Vast improvements in technology and surgical technique in total knee arthroplasty (TKA), approximately 15–25% TKAs, have suboptimal subjective clinical outcomes. Our study sought to evaluate if sensor-guided balancing improves postoperative clinical outcomes compared to a conventional gap balancing technique.
Methods
We searched Web of Science, Embase, PubMed, Cochrane Controlled Trials Register, Cochrane Library, Highwire, CBM, CNKI, VIP, and Wanfang database in March 2022 to identify studies involving sensor-guided balancing versus conventional gap balancing technique in TKA. Finally, we identified 2147 knees assessed in nine studies.
Results
Compared with manual gap balancing, Sensor-guided gap balancing resulted in less rate of Manipulation under anesthesia (MUA) (P = 0.02), however more rate of intraoperative additional procedures (P = 0.0003). There were no significant differences in terms of KSS (P = 0.21), KSS Function score (P = 0.36), OKS (P = 0.61), KOOS (P = 0.78), operative time (P = 0.17), Mechanical axis (P = 0.69) and rate of reoperation between two groups.
Conclusion
Compared with conventional manual gap balancing techniques, sensors have more balancing procedures being performed. However, it did result in a reduction in the rate of MUA. More extensive, high-quality RCTs are required to verify our findings further.
Similar content being viewed by others
Introduction
Total knee arthroplasty (TKA) has proven to be a successful operation in significantly reducing osteoarthritic knee pain and is more cost-effective than prolongation with nonsurgical treatments [1]. However, 15–25% of patients undergoing TKA report dissatisfaction after their procedure [2], and this dissatisfaction occasionally be due to soft tissue imbalance [2, 3]. Proper soft tissue balancing is the most critical contributor to improved outcomes after TKA [3, 4]. It was estimated that soft tissue imbalance causes up to 35% of early TKA revisions., manifesting as stiffness, instability, or tibiofemoral incongruency [5,6,7,8]. Although soft tissue balancing is essential, it is often determined by the surgeon's subjective "feel" of the local ligamentous tension. It typically depends on operative experience [9, 10]. To address this problem and to make ligament balancing less operator dependent, newer technologies such as patient-specific instrumentation [11, 12], computer-assisted surgery [13], and intraoperative pressure sensors [14] have been developed over the past decades. Intraoperative pressure sensors were introduced in TKA surgery to quantify compartmental pressures through a range of motion and determine tibiofemoral congruence. Several studies have shown improved early results with sensors [15,16,17,18], whereas others have failed to demonstrate a clinical benefit compared with the conventional gap balancing technique [19,20,21,22,23]. However, no meta-analysis studies have compared sensor-guided gap balancing with traditional manual knee balancing. Hence, this meta-analysis aims to determine whether sensor-guided gap balancing confers a clinical benefit compared with conventional manual gap balancing, as determined based on improved postoperative clinical outcomes.
Methods
The study was conducted by the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [24]. The protocol for this study was registered at PROSPERO (the International Prospective Register of Systematic Reviews), and the registration number was CRD 42021262271.
Search strategy
We searched Web of Science, Embase, PubMed, Cochrane Controlled Trials Register, Cochrane Library, Highwire, CBM, CNKI, VIP, and Wanfang database in March 2022 to identify studies involving sensor-guided balancing versus conventional gap balancing technique in TKA. The keywords used were "total knee arthroplasty," "total knee replacement," "gap balancing," "sensor," sensor-guided," "manual in conjunction with Boolean operators, "AND" or "OR." Review Manager Software was used to perform the meta-analysis.
Inclusion criteria
We identified and included all randomized controlled trials (RCTs) and non-randomized controlled trials (non-RCTs) comparing sensor-guided gap balancing (SB) and manual gap balancing (MB) in primary TKA in the search strategy. Studies were included for further assessment if they satisfied the following criteria: (1) The TKA procedure was performed for the first time. (2) sensor-guided gap balancing was involved. (3) The comparator was manual gap balancing in the comparative study. (4) At least one of the following indexes was reported: Knee society score (KSS); Knee society function score (KSFS); Oxford knee assessment (OKS); knee injury and osteoarthritis score (KOSS); Operative time; Mechanical axis; Intraoperative additional procedures (additional soft tissue releases or bone recuts); Manipulation under anesthesia (MUA); Reoperation. We also excluded: (1) studies that revision of TKA was performed. (2) unclear or incomplete sample data were available.
Data extraction process
All RCTs and n RCTs comparing SB and MB in primary TKA were identified and included in the search strategy. Two independent investigators screened each of the studies for inclusion in the meta-analysis, and they independently extracted the available data from each study. Data were extracted based on the following: (1) research features (i.e., authors, type of study, year of publication), (2) population information (i.e., gender, body mass index [BMI], age), (3) outcome. We will contact the authors by email or other means to obtain more data if the necessary results are omitted.
Assessment of studies
To assess the methodological quality, we evaluated the non-randomized studies using the nine-star Newcastle–Ottawa Scale (NOS), a validated tool suitable for evaluating the quality of non-randomized studies21. The methodological quality and basis of the RCTs were assessed according to the Cochrane Handbook for Systematic Reviews of Interventions. Two independent investigators assessed the quality of each study, and a third investigator resolved any discrepancies.
Statistical analysis
We used the I2 and Q test to evaluate the heterogeneity between studies. P ≤ 0.1 or I2 value > 50% suggested high heterogeneity; thus, we used the randomized-effects model. Otherwise, we used the fixed-effects model20. In each study, we used the odds ratio (OR) and relevant 95% confidence interval (CI) to measure dichotomous variables such as rates of intraoperative additional procedures, MU, and reoperation. Reported OR was supposed to approximate RR (relative risk) based on Cornfield's rare disease outcome assumption because the outcome is rare23. We used the mean difference (MD) or standard MD to assess continuous outcomes such as KSS, KSS function, OKS, KOOS, ROM, Operative time, and mechanical axis with a 95% confidence interval (CI). We used some statistical algorithms to estimate the standard deviation for those studies that provided only continuous variables for means and range24. We considered the results a statistically significant difference if P values were less than 0.05. Sensitivity analysis was used to assess the stability of the results (if necessary). We performed all statistical analyses with Review Manager (version 5.4 for MAC, the Cochrane Collaboration, Copenhagen).
Results
Search results
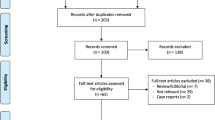
The literature search and selection process are shown in Fig. 1. Finally, nine publications from 2016 to 2022 were included in our meta-analysis. The detailed literature screening process is shown as the PRISMA flow diagram in Fig. 1. 209 relevant citations were identified from the databases according to the literature search strategy described earlier. After deleting 140 duplicates, we obtained 69 articles. Upon review of titles and abstracts of the 69 remaining articles, 53 irrelevant clinical studies were excluded. By reading the 16 full-text articles, we excluded another seven articles for the following reasons: systematic reviews, no compare groups, cadaver researches, and no useful outcome data. The remaining nine articles were deemed appropriate. Finally, we identified 2147 patients (2147 knees) assessed in (4 RCTs [16, 18, 22, 23] and 5 non-RCTs [15, 17, 19,20,21]). All the articles were published in English and Chinese.
Study characteristics and quality
We presented detailed baseline characteristics and general intervention information in Tables 1, 2 and 3. All the articles were published in English and Chinese between 2016 and March 2022.
Risk of bias assessment
The methodological quality of the involved studies ranged from six to seven (Table 4). The risk of bias summary and risk of bias graph for RCTs are shown in Table 4. As a result, the overall quality of the included studies was considered adequate.
KSS
Four studies reported KSS; The pooled data showed that the KSS was not significantly different between the two groups (MD = 0.8 95% CI [− 0.46, 2.07], P = 0.21; Fig. 2).
KSS function score
Two studies reported the KSS function score. The forest plot revealed that both groups experienced similar KSS function scores (MD = 0.89, 95% CI [− 1.02, 2.81], P = 0.36; Fig. 3).
OKS score
Two studies reported the OKS. The forest plot revealed that both groups experienced similar OKS scores (MD = − 0.32, 95% CI [− 1.57, 0.93], P = 0.61; Fig. 4).
KOOS
Two studies reported the KOOS. The forest plot revealed that both groups experienced similar KOOS scores (MD = 0.42, 95% CI [− 2.48, 3.31], P = 0.78; Fig. 5).
Operative time
Three studies reported the operative time. The forest plot revealed that both groups experienced a similar operative time (MD = 13.68, 95% CI [− 5.94, 33.31], P = 0.17; Fig. 6).
Mechanical axis
Two studies reported the mechanical axis. The forest plot revealed that the mechanical axis was not significantly different between the two groups (MD = − 0.07, 95% CI [− 0.43, 0.28], P = 0.69; Fig. 7).
Intraoperative additional procedures
Five studies reported intraoperative additional procedures. The forest plot revealed that the rate of intraoperative additional procedures was significantly more when the sensor was applied (OR = 16.54, 95% CI [3.6, 75.91], P = 0.0003; Fig. 8).
Rate of MUA
Six studies reported the rate of MUA. The forest plot revealed that the rate of MUA was significantly less when the sensor was applied (OR = 0.51, 95% CI [0.28, 0.91], P = 0.02; Fig. 9).
Rate of reoperation
Three studies reported the rate of reoperation. The forest plot revealed that both groups experienced a similar rate of reoperation (RD = − 0.01, 95% CI [− 0.02, 0.01], P = 0.4; Fig. 10).
Discussion
There is uncertainty and controversy about the influence of sensor-guided knee gap balancing and conventional gap balancing techniques on clinical outcomes following primary TKA. We sought to evaluate the body of evidence linking sensor-guided knee gap balancing versus conventional gap balancing technique following primary TKA, carrying out a comprehensive systematic review of RCTs and observational studies. To our knowledge, this is the first meta-analysis comparing the sensor-guided knee gap balancing and conventional manual gap balancing technique in primary TKA. This study showed that the use of an intraoperative sensing technology during primary TKA was not related to a statistically significant improvement in KSS, KSS Function score, OKS, KOOS, operative time, Mechanical axis, and rate of reoperation when comparing conventional soft tissue balancing. The most relevant finding was that compared with conventional manual gap balancing techniques, sensors have more balancing procedures (soft tissue releases or bone recuts) being performed. However, it did result in a reduction in the rate of MUA.
Continued advancements in technology and improvement in surgical techniques have made TKA surgery a very successful operation [25]. However, approximately one-third of early TKA revisions are related to unbalanced soft tissue presenting as stiffness, instability, or early component loosening [8, 26,27,28,29,30,31]. The ligament balancing "feeling" is affected by factors such as patient generalized laxity, degree of joint contracture, BMI, gender, the depth of anesthesia, surgical experience, and even the surgeon's favorite method of balancing(spacer blocks or ligament tensioners) [32, 33]. Optimizing soft-tissue balance in TKA is considered an essential surgical prerequisite to improve clinical outcomes. The laxities of the native knee are not uniform throughout the arc of motion [34], further suggesting that manual gap balancing techniques may not adequately address imbalances, thus emphasizing the need for a fresh look at soft tissue balancing [35]. Navigation provides data on a numerical gap, measured laterally and medially in flexion and extension before cuts are made, usually displayed in millimeters [36]. During a robotic-assisted procedure, the leg is physically manipulated to stress the collaterals in flexion and extension to assess the gap balancing. It is difficult to apply a valgus/varus stress test in flexion due to the inability to control hip rotation and assess it accurately [37]. Sensors offer surgeons different information from traditional navigation or robotic systems. The wireless, intraoperative sensor tibial insert consisting of two microelectronic sensors embedded into the tibial tray has been designed to provide intraoperative real-time feedback. After the tibial and femoral cuts are completed. The capsule is closed by a few stitches. The surgeon holds the leg in a neutral position, and the sensor tibial insert monitors the medial and lateral loading forces from full extension to full flexion. The sensor describes quantitative loads at major contact points in both compartments and peak center of load location during both TKA trials and final implant positioning [14]. The quantitative balance has been defined as a mediolateral intercompartmental loading difference of fewer than 15 pounds [14]. Using this technology, the surgeon receives real-time feedback of the loading in the knee and can adjust any imbalance with soft tissue corrections or additional bony resections [38, 39].
Several authors described the necessity of additional soft tissue release or bone recut to obtain the desired intra-articular loads [19]. In our study, there were more intraoperative additional procedures in the sensor groups, and thus theoretically should increase the patient clinical outcomes. But our meta-analysis didn't verify this hypothesis. We found no significant difference in KSS, KSS Function score, OKS, KOOS, operative time, mechanical axis, and rate of reoperation between two groups at short-term follow-up. However, our meta-analysis found that using the sensor did result in a reduction in MUA rate. Stiffness or arthrofibrosis is one of the most common complications associated with joint arthroplasty surgery [40]. Arthrofibrosis may occur due to several factors, including those on the part of the surgeon and patient [40, 41]. MUA is a therapeutic procedure to treat stiffness or arthrofibrosis [42]. The surgeon can loosen adhesions to reduce joint arthrofibrosis. In our meta-analysis, we chose to focus on MUA as one primary outcome of the utility of the sensor-enabled device. The emotional burden of arthrofibrosis or stiffness on the patient can be difficult, with many patients never obtaining an optimal clinical outcome. In addition, the cost burden cannot be overlooked. Based on 2014 Center's for Medicare & Medicaid Services (CMS) data, The average cost of MUA was close to $1200 per case [17]. Additionally, a majority (62%) of cases were within a 90-day postoperative window [19].
Since complications and readmissions before a 90-day threshold will result in a financial burden to the medical resource, sensor use demonstrates a potential to decrease the incidence of financial loss by mitigating early MUA.
Our findings should be considered with an understanding of the critical limitations of the data set. Firstly, we only included four randomized controlled trials; the other five studies were observational studies, which may have reduced the quality of the evidence for this meta-analysis. Although we have included all related studies thus far and tried to collect more data to make this meta-analysis and assess its effect, more prospective randomized trials investigating other clinical parameters are needed to confirm the results and conclusions. Secondly, there was an essential variability between the studies with respect to the different operating surgeons, different levels of TKA constraint (PCL substituting versus PCL retaining), the patient population, follow-up period, the cohorts evaluated, and the analyses performed. Thirdly, The follow-up period for these studies remains short, principally because this system is so new. Studies with longer follow-up and well-defined groups randomized to surgery performed with or without a sensor would provide valuable data for analysis. Furthermore, most of our included articles studied a specific type of sensor (Verasense) and these results may not be universally applicable to other sensor technologies on the market (e.g. Omnibot). The OMNIBot (Corin Ltd, Rayham, MA) has been shown to be a reliable tool for delivering different alignment philosophies as well as planning and achieving tibio-femoral coronal balancing [43, 44]. The utility of the system is increased when the robot is used in conjunction with a soft-tissue tensioning device—the BalanceBot. So, unlike the Verasense sensor, The BalanceBot device is used in conjunction with the robot. It is inappropriate to include these two types of sensors together in our meta-analysis. There is also no article comparing OMNIBotics and BalanceBot device versus manual gap balance which could meet Inclusion criteria in our meta-analysis.
Conclusion
Even though the use of intraoperative sensor technology was not related to an improvement in KSS, KSS Function score, OKS, KOOS, ROM, operative time, Mechanical axis, and rate of reoperation, the current studies showed that the Sensor use did result in a reduction in the rate of MUA. Given the relevant possible biases in our meta-analysis, more adequately powered and well-designed prospective studies with long-term follow-up were required to determine whether the application of the sensor technology for TKA will have clinical benefits and improve the survival of prostheses.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CIs:
-
Confidence intervals
- RCTs:
-
Randomized controlled trials
- RR:
-
Risk ratio
- OR:
-
Odds ratio
- VMD:
-
Weighted mean difference
- TKA:
-
Total knee arthroplasty
- BMI:
-
Body Mass Index
- KSS:
-
Knee society score
- KSFS:
-
Knee Society Function Score
- OKS:
-
Oxford Knee Assessment
- ROM:
-
Range of motion
- MUA:
-
Manipulation under anesthesia
- EMBASE:
-
Excerpta medica database
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- CNKI:
-
China National Knowledge Infrastructure
- RCT:
-
Randomized controlled trial
References
Dejour DH, Müller JH, Saffarini M, Timoteo M, Chambat P, Deschamps G, Bonnin MP. Implant survival of 3rd-condyle and post-cam posterior-stabilised total knee arthroplasty are comparable at follow-up > 10 years: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2021;30:1001.
Rodriguez-Merchan EC. Patient satisfaction following primary total knee arthroplasty: contributing factors. Arch Bone Jt Surg. 2021;9(4):379–86.
Gustke KA, Golladay GJ, Roche MW, Elson LC, Anderson CR. A new method for defining balance: promising short-term clinical outcomes of sensor-guided TKA. J Arthroplasty. 2014;29(5):955–60.
Gustke KA, Golladay GJ, Roche MW, Elson LC, Anderson CR. Primary TKA patients with quantifiably balanced soft-tissue achieve significant clinical gains sooner than unbalanced patients. Adv Orthop. 2014;2014:628695.
Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315–8.
Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper: Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13.
Kuster MS, Stachowiak GW. Factors affecting polyethylene wear in total knee arthroplasty. Orthopedics. 2002;25(2 Suppl):s235-242.
Huang Z, Sun C. Causes of failure after total knee arthroplasty. Zhonghua Yi Xue Za Zhi. 2015;95(20):1606–8.
Järvelin J, Häkkinen U, Rosenqvist G, Remes V. Factors predisposing to claims and compensations for patient injuries following total hip and knee arthroplasty. Acta Orthop. 2012;83(2):190–6.
Law TY, Marshall L, Rosas S, Vakharia RM, Toma JJ. Sensor-guided knee surgery provides improved patient outcomes and cost savings in a 90-day bundle. Surg Technol Int. 2020;37:327–30.
Batailler C, Swan J, Sappey Marinier E, Servien E, Lustig S. New technologies in knee arthroplasty: current concepts. J Clin Med. 2020;10(1):47.
Kim KK, Howell SM, Won YY. Kinematically aligned total knee arthroplasty with patient-specific instrument. Yonsei Med J. 2020;61(3):201–9.
Han S, Rodriguez-Quintana D, Freedhand AM, Mathis KB, Boiwka AV, Noble PC. Contemporary robotic systems in total knee arthroplasty: a review of accuracy and outcomes. Orthop Clin North Am. 2021;52(2):83–92.
Batailler C, Swan J, Marinier ES, Servien E, Lustig S. Current role of intraoperative sensing technology in total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141:2255.
Chow JC, Breslauer L. The use of intraoperative sensors significantly increases the patient-reported rate of improvement in primary total knee arthroplasty. Orthopedics. 2017;40(4):e648–51.
Elmallah RK, Mistry JB, Cherian JJ, Chughtai M, Bhave A, Roche MW, Mont MA. Can we really “feel” a balanced total knee arthroplasty? J Arthroplasty. 2016;31(9 Suppl):102–5.
Geller JA, Lakra A, Murtaugh T. The use of electronic sensor device to augment ligament balancing leads to a lower rate of arthrofibrosis after total knee arthroplasty. J Arthroplasty. 2017;32(5):1502–4.
Xia L. Application research of improved gap balance technique in total knee arthroplasty. Xinxiang Xinxiang Medical University; 2019.
Cochetti A, Ghirardelli S, Iannotti F, Giardini P, Risitano S, Indelli PF. Sensor-guided technology helps to reproduce medial pivot kinematics in total knee arthroplasty. J Orthop Surg (Hong Kong). 2020;28(3):2309499020966133.
Livermore AT, Erickson JA, Blackburn B, Peters CL. Does the sequential addition of accelerometer-based navigation and sensor-guided ligament balancing improve outcomes in TKA? Bone Joint J. 2020;102-b:24–30.
MacDessi SJ, Bhimani A, Burns AWR, Chen DB, Leong AKL, Molnar RB, Mulford JS, Walker RM, Harris IA, Diwan A, et al. Does soft tissue balancing using intraoperative pressure sensors improve clinical outcomes in total knee arthroplasty? A protocol of a multicentre randomised controlled trial. BMJ Open. 2019;9(5):e027812.
Song SJ, Kang SG, Lee YJ, Kim KI, Park CH. An intraoperative load sensor did not improve the early postoperative results of posterior-stabilized TKA for osteoarthritis with varus deformities. Knee Surg Sports Traumatol Arthrosc. 2019;27(5):1671–9.
Wood TJ, Winemaker MJ, Williams DS, Petruccelli DT, Tushinski DM, de Beer JV. Randomized controlled trial of sensor-guided knee balancing compared to standard balancing technique in total knee arthroplasty. J Arthroplasty. 2021;36(3):953–7.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
Chen C, Shi Y, Wu Z, Gao Z, Chen Y, Guo C, Bao X. Long-term effects of cemented and cementless fixations of total knee arthroplasty: a meta-analysis and systematic review of randomized controlled trials. J Orthop Surg Res. 2021;16(1):590.
Gould D, Dowsey MM, Spelman T, Jo O, Kabir W, Trieu J, Bailey J, Bunzli S, Choong P. Patient-related risk factors for unplanned 30-day hospital readmission following primary and revision total knee arthroplasty: a systematic review and meta-analysis. J Clin Med. 2021;10(1):134.
Jasper LL, Jones CA, Mollins J, Pohar SL, Beaupre LA. Risk factors for revision of total knee arthroplasty: a scoping review. BMC Musculoskelet Disord. 2016;17:182.
Siqueira MB, Klika AK, Higuera CA, Barsoum WK. Modes of failure of total knee arthroplasty: registries and realities. J Knee Surg. 2015;28(2):127–38.
Mathis DT, Lohrer L, Amsler F, Hirschmann MT. Reasons for failure in primary total knee arthroplasty: an analysis of prospectively collected registry data. J Orthop. 2021;23:60–6.
Han SB, Song SY, Shim JH, Shin YS. Risk of a complete exchange or failure in total knee arthroplasty and unicompartmental knee arthroplasty: a nationwide population-based cohort study from South Korea. Arch Orthop Trauma Surg. 2021;141(3):477–88.
Yong TM, Young EC, Molloy IB, Fisher BM, Keeney BJ, Moschetti WE. Long-Term implant survivorship and modes of failure in simultaneous concurrent bilateral total knee arthroplasty. J Arthroplasty. 2020;35(1):139–44.
Heesterbeek PJC, Haffner N, Wymenga AB, Stifter J, Ritschl P. Patient-related factors influence stiffness of the soft tissue complex during intraoperative gap balancing in cruciate-retaining total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25(9):2760–8.
Wyss TF, Schuster AJ, Münger P, Pfluger D, Wehrli U. Does total knee joint replacement with the soft tissue balancing surgical technique maintain the natural joint line? Arch Orthop Trauma Surg. 2006;126(7):480–6.
Roth JD, Howell SM, Hull ML. Native knee laxities at 0°, 45°, and 90° of flexion and their relationship to the goal of the gap-balancing alignment method of total knee arthroplasty. J Bone Joint Surg Am. 2015;97(20):1678–84.
Delanois RE, Elmallah RK. A fresh look at soft-tissue balancing: commentary on an article by Joshua D. Roth, MS, et al: Native knee laxities at 0°, 45°, and 90° of flexion and their relationship to the goal of the gap-balancing alignment method of total knee arthroplasty. J Bone Joint Surg Am. 2015; 97(20):e69.
Saiki Y, Ojima T, Kabata T, Hayashi S, Tsuchiya H. Accuracy of different navigation systems for femoral and tibial implantation in total knee arthroplasty: a randomised comparative study. Arch Orthop Trauma Surg. 2021;141:2267.
Elliott J, Shatrov J, Fritsch B, Parker D. Robotic-assisted knee arthroplasty: an evolution in progress: a concise review of the available systems and the data supporting them. Arch Orthop Trauma Surg. 2021;141:2117.
Golladay GJ, Bradbury TL, Gordon AC, Fernandez-Madrid IJ, Krebs VE, Patel PD, Suarez JC, Higuera Rueda CA, Barsoum WK. Are patients more satisfied with a balanced total knee arthroplasty? J Arthroplasty. 2019;34(7s):S195-s200.
Sabatini L, Bosco F, Barberis L, Camazzola D, Bistolfi A, Risitano S, Massè A, Indelli PF. Kinetic sensors for ligament balance and kinematic evaluation in anatomic bi-cruciate stabilized total knee arthroplasty. Sensors (Basel). 2021;21(16):5427.
Archunan M, Swamy G, Ramasamy A. Stiffness after total knee arthroplasty: prevalence and treatment outcome. Cureus. 2021;13(9):e18271.
Zaffagnini S, Di Paolo S, Meena A, Alesi D, Zinno R, Barone G, Pizza N, Bragonzoni L. Causes of stiffness after total knee arthroplasty: a systematic review. Int Orthop. 2021;45(8):1983–99.
Chalmers BP, Goytizolo E, Mishu MD, Westrich GH. Manipulation under anaesthesia after primary total knee arthroplasty : minimal differences in intravenous sedation alone versus neuraxial anaesthesia. Bone Joint J. 2021;103-b:126–30.
Keggi JM, Wakelin EA, Koenig JA, Lawrence JM, Randall AL, Ponder CE, DeClaire JH, Shalhoub S, Lyman S, Plaskos C. Impact of intra-operative predictive ligament balance on post-operative balance and patient outcome in TKA: a prospective multicenter study. Arch Orthop Trauma Surg. 2021;141(12):2165–74.
Wakelin EA, Shalhoub S, Lawrence JM, Keggi JM, DeClaire JH, Randall AL, Ponder CE, Koenig JA, Lyman S, Plaskos C. Improved total knee arthroplasty pain outcome when joint gap targets are achieved throughout flexion. Knee Surg Sports Traumatol Arthrosc. 2022;30(3):939–47.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
CS, XC contributed to conceptualization. CS, WGL contributed to data curation. CS, QM, XZ contributed to formal analysis. CS contributed to investigation. ZZ, XC contributed to supervision. CS contributed to validation. JZ contributed to visualization. CS contributed to writing—original draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval is not required because this study is based on existing literature.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, C., Zhao, Z., Lee, W.G. et al. Sensor-guided gap balance versus manual gap balance in primary total knee arthroplasty: a meta-analysis. J Orthop Surg Res 17, 243 (2022). https://doi.org/10.1186/s13018-022-03129-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-03129-x