Abstract
The programmed cell death 1 (PD-1) signaling pathway, a key player in immune checkpoint regulation, has become a focal point in cancer immunotherapy. In the context of cancer, upregulated PD-L1 on tumor cells can result in T cell exhaustion and immune evasion, fostering tumor progression. The advent of PD-1/PD-L1 inhibitor has demonstrated clinical success by unleashing T cells from exhaustion. Nevertheless, challenges such as resistance and adverse effects have spurred the exploration of innovative strategies, with bispecific antibodies (BsAbs) emerging as a promising frontier. BsAbs offer a multifaceted approach to cancer immunotherapy by simultaneously targeting PD-L1 and other immune regulatory molecules. We focus on recent advancements in PD-1/PD-L1 therapy with a particular emphasis on the development and potential of BsAbs, especially in the context of solid tumors. Various BsAb products targeting PD-1 signaling are discussed, highlighting their unique mechanisms of action and therapeutic potential. Noteworthy examples include anti-TGFβ × PD-L1, anti-CD47 × PD-L1, anti-VEGF × PD-L1, anti-4-1BB × PD-L1, anti-LAG-3 × PD-L1, and anti-PD-1 × CTLA-4 BsAbs. Besides, we summarize ongoing clinical studies evaluating the efficacy and safety of these innovative BsAb agents. By unraveling the intricacies of the tumor microenvironment and harnessing the synergistic effects of anti-PD-1/PD-L1 BsAbs, there exists the potential to elevate the precision and efficacy of cancer immunotherapy, ultimately enabling the development of personalized treatment strategies tailored to individual patient profiles.
Similar content being viewed by others
Background
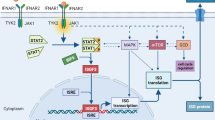
Programmed cell death 1 (PD-1) signaling acts as a fundamental immune checkpoint mechanism, downregulating inflammatory responses and maintaining immune homeostasis [1]. Key structures within PD-1, namely the immune receptor tyrosine-based inhibitory/switch motif (ITIM/ITSM), facilitate signal transduction and recruit phosphatases (SHP1/2) within the cell [2]. The PD-1/PD-L1 signaling not only serves as a crucial pathway for preventing autoimmune diseases, but also significantly influences the delicate balance between tumor immune surveillance and immune tolerance [3]. Increased PD-L1 on tumor cells or infiltrating lymphocytes can result in T cell exhaustion, dampening tumor-specific immunity and promoting tumor progression [4]. PD-1/PD-L1 inhibitors have emerged as a groundbreaking therapeutic approach by blocking the negative regulatory signals, effectively releasing T cells from their exhausted state [5]. Since the approval of the first anti-PD-1 antibody (pembrolizumab) by the FDA in 2014, PD-1/PD-L1 blockade therapies have revolutionized clinical practice, exhibiting potent and durable antitumor effects, particularly in refractory tumors [5,6,7,8,9,10].
PD-1/PD-L1 inhibitors function by disrupting the immunosuppressive signals that tumors exploit, allowing immune cells to recognize and kill cancer cells more effectively [11]. The clinical successes of anti-PD-1/PD-L1 agents have underscored the importance of immune checkpoint blockade in cancer therapeutics [12,13,14,15,16,17]. However, challenges such as resistance, limited response rates, and adverse effects have prompted the exploration of innovative strategies to optimize and broaden the therapeutic impact [18, 19]. In tandem with these developments, bispecific antibodies have emerged as a promising frontier in cancer immunotherapy [20]. By simultaneously targeting PD-L1 and other key molecules involved in immune regulation, bispecific antibodies (BsAbs) offer a multifaceted approach to enhance antitumor immune responses [21,22,23]. This review delves into the recent advancements in PD-1/PD-L1 blockade and explores the potential of bispecific antibodies, with a focus on their development and application in solid tumors. By elucidating the advances in anti-PD-L1 BsAb development, especially those tailored for solid tumors, this review aims to contribute to the evolving understanding of cancer immunotherapy and pave the way for more effective and personalized treatment strategies.
The advances of BsAb
The development of BsAb
The success of monoclonal antibodies targeting tumor-associated antigens (TAAs), such as Her2 or EGFR, in breast and lung cancer therapy has led to the exploration of innovative approaches, including the development of BsAbs [24]. BsAbs, introduced in the 1980s, have garnered considerable attention for their potential in cancer treatment [25]. Functionally, BsAbs serve as effective linkages between immune effector cells and tumor cells, or concurrently block two different oncogenic molecules [26]. Besides, some BsAbs enhance tumor killing by guiding various effector cells to tumor cells in a non-MHC-restricted manner (Fig. 1) [26]. Advancements in technology have resulted in various BsAb formats, classified based on the Fc domain into non-IgG-format and IgG-format. IgG-like agents retain Fc-mediated antibody effector functions, while Fc-free BsAbs lack these functions [26]. Bispecific T cell engagers (BiTEs) and Triomabs are prominent BsAb formats [27, 28]. BiTEs, lacking Fc domains, exhibit short serum half-lives, limiting their clinical application [28, 29]. Triomabs, with an IgG-like structure, show slower clearance but face challenges of immunogenicity and compromised permeability due to the Fc domain (Fig. 2) [30].
Bispecific antibodies (BsAbs) enhance tumor killing by guiding various effector cells to tumor cells in a non-MHC-restricted manner. BsAbs facilitate the interaction between T cells and tumor cells, triggering a sequence of events leading to T cell activation. The primary mechanism employed by activated T cells in cancer cell lysis involves Granzyme-B and perforin (Adapted from “Bispecific Antibody Mechanism of Action”, by BioRender 2023)
The tumor-killing mechanisms of blinatumomab and TrioMabs. Blinatumomab is an anti-CD3 × CD19 bispecific T-cell engager (BiTE) antibody. Blinatumomab is designed to bind to both CD19 of B cells and CD3 of T cells. By linking these two cell types, blinatumomab helps facilitate the T cell response against cancer cells, leading to the destruction of B-cell leukemia cells. Catumaxomab is an anti-CD3 × EpCAM BsAb based on TrioMabs technique, binding to EpCAM of cancer cells and CD3 of T cells. Notably, the Fc domain could bind to Fcγ receptor of effector cells including NK cells, macrophages, and dendritic cells, triggering antibody-dependent cell cytotoxicity or phagocytosis, and complement-dependent cytotoxicity against cancer cells (Adapted from “Bispecific Antibody Design”, by BioRender 2023)
In the last decade, the development of bispecific antibodies has been dominated by BiTEs. These antibodies, which simultaneously bind CD3 of T cells and TAAs of tumor cells, activate T-cell signaling cascades and initiate target-dependent tumor cell killing [26]. Unlike checkpoint inhibitors, BiTEs overcome major histocompatibility complex (MHC) restrictions of the T-cell receptor (TCR), presenting a breakthrough validated in the clinic with FDA approvals for blinatumomab (anti-CD3 × CD19) [31,32,33]. Besides, the anti-CD3 × CD20 BsAb mosunetuzumab has been approved for refractory or relapsed follicular lymphoma as well [34, 35]. However, despite the promising outcomes observed in hematological malignancies, the therapeutic effects of bispecific antibodies in solid tumors, which constitute 90% of all cancers, remain a challenge, primarily due to the suppressive tumor microenvironment (TME) impairing T-cell activity and fostering immune deficiency [33, 36,37,38].
Another avenue of BsAb investigation involves simultaneously targeting two epitopes on tumor cells or cytokines in the TME (Fig. 3). In contrast to BiTEs, these bispecific antibodies aim to block two protumor signaling pathways, generating synergistic anti-cancer effects or minimizing drug resistance [39]. For instance, bifunctional antibody M7824, targeting PD-L1 and TGFβ, has exhibited significant clinical efficacy in non-small cell lung cancer (NSCLC) patients [40]. Besides, Although BsAb clinical outcomes are less satisfying in solid tumors compared to hematologic malignancies, ongoing studies and clinical trials, particularly focusing on commonly expressed antigens (e.g. EpCAM, HER2, PSMA, and CEA), demonstrate the great potential of BsAb in cancer immunotherapy [41]. Recently, BsAbs simultaneously targeting PD-L1 and other immunoinhibitory molecules have been developed. These BsAbs show potent antitumor activity in preclinical and clinical studies, regarded as the next generation of immune checkpoint inhibitors (ICIs) [42,43,44].
BsAbs simultaneously targeting two immunoinhibitory molecules on tumor cells or cytokines in the TME. In contrast to BiTEs, these bispecific antibodies aim to block two immunoinhibitory signaling pathways (except 4-1BB agonist antibodies), generating synergistic anti-cancer effects or minimizing drug resistance (Created with Biorender)
The challenges for BsAb in solid tumors
In addressing solid tumor malignancies, BsAbs encounter significant hurdles that impede their clinical success. Predominantly, these challenges include managing adverse reactions associated with treatment, mitigating both on-target and off-target toxicities, and navigating the intricacies of the immunosuppressive TME [41]. A critical issue associated with BsAbs, especially those with intact Fc domains, is the risk of off-target toxicity, exemplified by Cytokine Release Syndrome (CRS) [45]. CRS is a systemic inflammatory reaction characterized by a spectrum of clinical manifestations, from mild symptoms to severe, potentially fatal conditions, often marked by laboratory signs such as cytopenia [46]. The pathophysiology of CRS involves an immune cascade triggered by IFN-γ release from activated T cells, which subsequently prompts macrophages to produce an excess of inflammatory cytokines [41]. A significant contributor to this issue is the inadvertent T-cell activation, which can occur through mechanisms like FcγR binding on non-target cells [47].
The standard mitigation strategy involves corticosteroid pretreatment and optimized dosing [48]. Furthermore, targeting IL-6, a key cytokine in CRS pathogenesis, with antagonists like tocilizumab has shown promise in alleviating these adverse effects without compromising the antitumor efficacy of BsAb therapies [49]. Besides, innovations in BsAb design, like employing Fc-free formats or antibodies with modified Fc domains, are crucial to reduce these risks [50]. Besides, on-target toxicity links directly to the target specificity of BsAbs. While certain tumor-associated antigens (TAAs) demonstrate suitability, others pose risks due to their presence in normal tissues, leading to significant toxicity. For example, BsAbs targeting EpCAM have shown this problem [51]. Moreover, the strong affinity of BsAbs to their targets can lead to on-target CRS. However, unlike tissue toxicity, on-target CRS is typically transient and can be managed with dose modulation and supportive care [47].
Besides, the effectiveness of BsAbs in solid tumors is critically impacted by the TME. A significant challenge is the insufficient T-cell infiltration in immune-desert tumors, which limits BsAb efficacy [52]. Innovative interventions, such as the use of oncolytic reovirus, have been employed to improve T-cell infiltration, transforming 'cold' tumors into more responsive 'inflamed' ones, thereby overcoming resistance to the T cell-engaging BsAb therapy [53]. Additionally, the immunosuppressive nature of the TME, marked by the upregulation of PD-1 and PD-L1 during BsAb therapy, presents another hurdle [54]. The moderate efficacy of BiTEs in solid tumors has led to the exploration of adjunct therapies like checkpoint inhibitors and T-cell costimuli to enhance their antitumor activity. Such combinations have shown promise in preclinical studies by overcoming T-cell exhaustion and amplifying BiTE effectiveness [55, 56]. Additionally, strategies like combining BiTEs with T-cell costimuli like 4-1BB agonists, have been effective in boosting BiTE performance [57]. In sum, combining BsAbs with other immunotherapies can enhance therapeutic efficacy, though results vary depending on BsAb composition, target antigen, and tumor types. Thus, the complex dynamics between BsAbs and the TME in solid tumors require multifaceted and innovative therapeutic strategies to fully harness their potential.
Anti-TGFβ × PD-L1 BsAb
The role of TGFβ in cancer immunology and immunotherapy
The transforming growth factor beta (TGFβ) signaling pathway exhibits a dual nature in cancer biology, serving both tumor-suppressing and tumor-promoting roles, depending on the specific cell and tissue context [58]. In normal cells, TGFβ functions to maintain cellular homeostasis and prevent tumor initiation, primarily by arresting the cell cycle, promoting cellular differentiation, and triggering cell apoptosis [59]. The pathway's response varies across cell types due to differential expression of factors like Smad proteins [60]. Contrastingly, in cancer cells, the regulatory role of TGFβ is often disrupted or altered due to mutations or epigenetic changes, leading to a shift from controlling proliferation to facilitating cancer progression [61]. In the TME, hyperactive TGFβ signaling, typically inhibitory in normal epithelial cells, paradoxically supports tumor growth, invasion, and metastatic behavior [62]. Notably, TGF-β-induced epithelial to mesenchymal transition (EMT) is crucial in cancer development, invasion, and spread [63, 64]. The flexibility and reversibility of EMT in response to TGFβ levels underscore its significance as a potential therapeutic target, especially since it fosters a stem-like phenotype linked to tumor progression and resistance to chemotherapy [65, 66]. A comprehensive understanding of TGFβ's contrasting roles in different cancer types and tissues, along with its impact on the TME, is essential for devising targeted treatments to curb cancer progression.
Notably, accumulating evidences demonstrate that TGFβ stands as a central player in the intricate landscape of cancer immunology and immunotherapy, exerting dual effects on tumorigenesis and immune modulation. Its role in the TME is multifaceted, as it not only contributes to the promotion of tumorigenesis but also establishes an immunosuppressive milieu that shields cancer cells from immune surveillance [44]. The immunosuppressive functions of TGFβ are manifested through its ability to inhibit the activation and function of various immune cells, including NK cells, T cells, and dendritic cells (DCs) [67,68,69]. Moreover, TGFβ enhances the differentiation and expansion of immunosuppressive regulatory T cells (Tregs), further tilting the balance in favor of immune evasion by cancer cells [70]. In the context of cancer immunotherapy, the immunosuppressive nature of TGFβ poses a significant hurdle. Strategies aimed at neutralizing or inhibiting TGFβ signaling have emerged as promising avenues to enhance the efficacy of immunotherapies [71]. Notably, the development of anti-TGFβ × PD-L1 BsAb represents a groundbreaking approach to simultaneously target multiple immunosuppressive pathways within the TME, thereby unleashing the full potential of the immune system against cancer.
It is noteworthy that the dual role of TGFβ in cancer underscores the necessity of understanding its contextual influences for effective patient selection in anti-TGFβ therapies. The pleiotropic activities of TGFβ signaling pose a challenge in developing antagonists for cancer treatment, particularly due to the lack of specific biomarkers and established dosing regimens [60]. To integrate TGFβ blockade agents effectively into frontline cancer therapy, future clinical trials need to focus on bioinformatics and identifying molecular biomarkers for patient stratification and treatment optimization.
M7824 and other bifunctional antibodies
M7824, a novel bifunctional fusion protein, represents a significant stride in PD-L1 × TGFβ dual-blockade therapy (Table 1). This innovative agent combines an anti-PD-L1 domain in the Fab with a TGFβ receptor in the Fc, allowing for simultaneous targeting of both immunosuppressive pathways. M7824 was designed to target PD-L1 molecules on tumor cell, localizing a trap molecule in the TME to capture immunosuppressive TGF-β. Then, M7824 is internalized by cells expressing PD-L1, leading to the removal of M7824-bound TGF-β [42]. In theory, M7824 is expected to exhibit greater specificity for tumor cells compared to a combination of two monoclonal antibodies due to its physical bridging effect. In animal models, M7824 exhibited potent antitumor efficacy, obviously retarding the tumor growth and prolonging survival [42]. Beyond its direct antitumor effects, M7824 induced a substantial reshaping of the TME, including the prevention or reversal of TGFβ-mediated epithelial-mesenchymal transition in cancer cells [72]. This alteration enhances tumor cell susceptibility to immune-mediated attack and chemotherapeutic agents. The fusion protein upregulated the quantities and activities of cytotoxic lymphocytes while concurrently decreasing the proportions of immunosuppressive subsets, including Tregs, myeloid-derived suppressor cells (MDSC), and M2-like macrophages [42]. Additionally, M7824 induced tumor matrix remodeling, contributing to improved immune cell infiltration and reinforcing its potential as a multifaceted immunotherapeutic agent [42]. Moreover, when combined with radiation, chemotherapy, and other immunotherapeutic agents, it enhances overall antitumor activity [73]. In the phase 1 trial, M7824 provided promising responses, particularly in NSCLC with high PD-L1 expression (NCT02517398) (Table 2) [40, 43].
The success of M7824 has catalyzed the exploration and development of additional anti-TGFβ × PD-L1 bifunctional proteins landscape. Among these, SHR-1701, with a structure reminiscent of M7824, combines anti-PD-L1 domain with an N-terminal-truncated TGFβRII [74]. In a phase 1 clinical trial (NCT05179239), SHR-1701 exhibited antitumor activity in recurrent metastatic cervical cancer [75]. Similarly, the bifunctional protein BR102, comprising an anti-PD-L1 antibody and TGFβRII ectodomain, demonstrated antitumor activity in murine tumor models [76]. These emerging antibodies, including SHR-1701 and BR102, contribute to the expanding repertoire of potential anti-TGFβ × PD-L1 blockade therapies, promising novel therapeutic strategies for the complex landscape of cancer immunotherapy.
YM101 and BiTP
YM101, heralded as the world's first publicly reported anti-TGFβ × PD-L1 BsAb, marks a pivotal advancement in the field of cancer immunotherapy. Engineered using the Check-BODY™ technology platform, YM101 represents a testament to the innovative strategies employed to combat the dual challenges posed by PD-L1 and TGF-β [21]. Preclinical investigations revealed YM101's ability to effectively counteract the effects of both TGF-β and PD-1 × PD-L1 signaling. Moreover, in vivo evidence demonstrated that YM101 outperformed anti-TGF-β and anti-PD-L1 monotherapies in terms of antitumor activity. We hypothesize that this improved antitumor effect may be attributed to the enhanced tumor specificity resulting from the distinctive physical bridging effect of YM101. However, it is crucial to acknowledge that our current speculation lacks experiment evidence to substantiate it. In upcoming research, it will be imperative to employ techniques such as isotope labeling to further validate and demonstrate the advantages of YM101, specifically in terms of its potential for increased tumor specificity and the associated enhancement of antitumor effects.
Besides, YM101 played a transformative role in shaping the TME, promoting the formation of inflamed tumors characterized by increased numbers and activities of tumor-infiltrating lymphocytes (TIL) [21]. Additionally, YM101 shifted the balance of macrophage polarization towards the antitumor M1 phenotype, further enhancing its immunotherapeutic potential [21]. Besides, in preclinical studies, the combination of STING agonists and YM101 demonstrated potent and durable antitumor immune protection by targeting three independent and complementary pathways [77]. STING agonists induce DC maturation and activate macrophages, reigniting immunologically cold tumors and enhancing both innate and adaptive immune responses systemically. When combined with YM101, STING agonists synergized to normalize the TME and impede tumor growth in non-inflamed models [78].
Inspired by the encouraging preclinical results, the development of the alternative molecule for clinical trials (BiTP) followed suit. Sharing a similar structure with YM101 and constructed using the Check-BODY™ platform, BiTP demonstrated efficacy in murine triple-negative breast cancer (TNBC) models [79]. Efficacy experiments in humanized TNBC models indicated that BiTP exhibited superior antitumor efficacy compared to corresponding monotherapies. BiTP reduces collagen generation, enhances T-cell penetration, and increases the infiltration of lymphocytes into the tumor [79]. At the present stage, multiple clinical trials of BiTP are ongoing, including CTR20211776 (for solid tumors) and CTR20223410 (for pancreatic cancer). Generally, the development of anti-TGF-β × PD-L1 BsAb, exemplified by YM101, BiTP, and M7824, represents a transformative approach to cancer immunotherapy [61, 80, 81]. These innovative agents, designed to concurrently target multiple immunosuppressive pathways, have shown remarkable efficacy in preclinical and clinical settings. The synergistic effects observed in combination therapies further underscore the potential of these antibodies to overcome resistance mechanisms and broaden their applicability across diverse tumor types.
Anti-CD47 × PD-L1 BsAb
CD47 plays a pivotal role in cancer by delivering a "don't eat me" signal to macrophages when binding to its ligand signal-regulatory protein alpha (SIRPα) on tumor cells [82]. Antibodies disrupting CD47 or its ligand have shown therapeutic effects in preclinical studies and clinical trials [83]. CD47 blockade enhances antigen presentation, phagocytosis, and immune infiltration in various tumor models, supporting the development of CD47 blockade immunotherapy agents [84,85,86,87]. Furthermore, the dual blockade of CD47/SIRPα and PD-1/PD-L1 signaling, which respectively suppress innate and adaptive immune responses, has shown enhanced therapeutic efficacy in various cancer types, providing a promising avenue for cancer treatment that stimulates both arms of the immune system [88]. Based on knobs-into-holes (KIH) platform, Wang et al. developed an anti-CD47 × PD-L1 BsAb 6MW3211, which was designed with a common light chain, exhibiting low affinity to CD47 and high affinity to PD-L1 [89]. This unique affinity profile allows preferential binding to PD-L1 of tumor cells, suppressing the CD47 signaling pathway [89]. 6MW3211 demonstrates potent therapeutic efficacy in diverse mouse models and shows promising pharmacokinetics and safety profiles in vivo [89]. The coexpression of CD47 and PD-L1 on various human tumors, confirmed by multiplex fluorescent immunohistochemistry staining, supports the potential of 6MW3211 for clinical trials targeting PD-L1+ CD47+ cancers [89].
Besides, Chen et al. constructed an affinity-tuned anti-CD47 × PD-L1 BsAb (hBisAb) to improve antibody selectivity and therapeutic efficacy [90]. hBisAb was developed utilizing knobs-in-holes technology and a common light chain architecture for its IgG1 format. This humanized antibody demonstrates moderate affinity for CD47 and a highly potent affinity for PD-L1, as evidenced by kinetic rate constants obtained via surface plasmon resonance and cell-based assays. Specifically designed to prioritize PD-L1 binding, the antibody effectively blocks the PD-1/PD-L1 interaction and also inhibits the CD47/SIRPα axis [90]. This dual-action mechanism not only enhances T cell functionality but also significantly boosts phagocytosis of tumor cells by macrophages, outperforming monotherapies targeting either checkpoint alone [90]. In vitro and in vivo studies reveal that the hBisAb exhibits a remarkable selectivity for tumor cells over red blood cells, addressing a common challenge of CD47-targeted therapies by minimizing unwanted hematologic effects. This selectivity is further underscored by the antibody’s preferential binding to PD-L1-expressing cells in the TME, reducing off-target effects and improving therapeutic safety [90]. The bispecific antibody, particularly in its IgG1 form, has shown superior efficacy in promoting antibody-dependent cellular phagocytosis (ADCP) and DC-mediated T cell activation, leading to significant tumor growth inhibition and improved survival rates in syngeneic murine models. Notably, this approach mitigates the potential toxicity often associated with CD47 targeting, as evidenced by the maintenance of normal red blood cells counts and body weight in treated mice, highlighting the bispecific antibody’s enhanced antitumor efficacy and reduced side effects [90].
Furthermore, there are some other anti-CD47 × PD-L1 BsAbs have been reported. For instance, IBI322, was designed to improve therapeutic selectivity and efficacy by preferentially binding to PD-L1+CD47+ tumor cells, inducing tumor cell phagocytosis while minimizing impact on CD47+PD-L1− cells like red blood cells [91]. Similarly, a dual-targeting fusion protein, IAB, effectively engaged both CD47 and PD-L1, demonstrating potent antitumor activity and playing a vital role in activating innate and adaptive immunity against tumors [92]. These innovative approaches underscore the potential of dual checkpoint blockade, simultaneously targeting CD47 and PD-L1, to improve therapeutic outcomes while mitigating toxicities associated with traditional antibodies.
Anti-VEGF/PD-1 and anti-VEGF/PD-L1 BsAb
VEGF, induced by the hypoxic TME, stimulates endothelial cell proliferation and angiogenesis [93]. Additionally, VEGF exerts immunosuppressive effects, promoting the recruitment of immunosuppressive cells and hindering immune cell infiltration [94]. Combining anti-vascular targeting drugs with ICIs has demonstrated synergistic antitumor effects in various cancers, highlighting the potential of dual therapeutic strategies to address both angiogenesis and immune response in cancer treatment [95]. At the present stage, multiple anti-VEGF × PD-1 and anti-VEGF × PD-L1 BsAbs have been successfully developed for cancer immunotherapy.
Hassanzadeh et al. constructed a bivalent anti-PD-L1 × VEGF nanobody, which demonstrated efficient inhibition of angiogenesis in vitro [96]. Besides, the BsAb HB0025, targeting PD-L1 and VEGF, was developed using mAb-Trap technology. The preclinical studies showed that HB0025 was more effective in suppressing tumor growth compared to anti-PD-L1 antibody or VEGFR1D2 fusion protein alone [97]. Moreover, Xiong et al. developed a fully human bispecific single-chain diabody (BsDb) that targets VEGF165 and PD-1. This BsDb demonstrated high specificity, inhibiting VEGF165-induced activities in human umbilical vein endothelial cells and enhancing T cell proliferation and IFN-γ production [98]. In mouse models, the BsDb exhibited potent antitumor activity by suppressing angiogenesis and activating immune responses, suggesting its potential as a dual-targeting BsAb for cancer therapy [98]. Importantly, the phase 2 clinical trial assessed the efficacy and safety of AK112, a humanized IgG1 anti-VEGF × PD-1 BsAb, in combination with chemotherapy in advanced NSCLC [99]. The study included three cohorts with different treatment histories and genomic alterations. The confirmed objective response rates (ORR) in cohorts 1, 2, and 3 were 53.5%, 68.4%, and 40.0%, respectively [99]. The findings suggest that AK112 plus platinum-doublet presents promising antitumor activity and safety, providing a potential new treatment option for advanced NSCLC patients [99].
Anti-4-1BB × PD-L1 BsAb
4-1BB (CD137) is an inducible costimulatory molecule expressed by activated NK and T cells [100, 101]. 4-1BB signaling, triggered by interaction with its ligand on professional antigen-presenting cells (APCs), activates pathways leading to enhanced cytokine generation, survival, proliferation, and immunological memory [102, 103]. In the TME, 4-1BB serves as a marker for tumor-specific cytotoxic T lymphocytes (CTLs) and is often co-expressed with PD-1 [104]. 4-1BB activation has shown promising antitumor responses in preclinical models, and the combination of 4-1BB agonist antibodies with PD-1/PD-L1 inhibitors synergistically enhances antitumor immunity [105,106,107,108,109]. Currently, the use of 4-1BB agonists combined with anti-PD-1 therapies faces a significant challenge especially systemic toxicity. For example, the clinical development of a therapeutic CD137 agonist antibody was discontinued due to dose-dependent hepatitis caused by the systemic activation of the 4-1BB pathway. Theoretically, the BsAb technique holds promise, as it could potentially activate 4-1BB through PD-L1 engagement, thereby enhancing tumor-specific T cell responses. This approach appears promising because PD-1 and 4-1BB are both co-expressed on tumor-specific CD8 + CTLs [110].
Several anti-4-1BB × PD-L1 BsAbs have been developed to enhance the therapeutic efficacy of ICIs by combining 4-1BB agonists with these inhibitors. MCLA-145 was engineered as an IgG1 molecule with specific modifications to the Fc CH3 domain to encourage heavy chain heterodimerization and to the CH2 domain to prevent Fc receptor binding. In vitro experiments indicated that MCLA-145 could potently activate T cells, strengthens T cell priming, differentiation, and immune memory, and exhibits superior antitumor activity compared to ICI comparators [110]. Importantly, MCLA-145 demonstrates no graft-versus-host disease and minimal adverse effects in non-human primates [110]. Mechanically, MCLA-145 functions by binding to PD-L1 on tumor cells and CD137 on T effector cells, facilitating the creation of an "immunological synapse." In this synapse, T cells can exposure to enhanced TCR signaling as PD-1 inhibition is relieved, and CD137 activation is intensified. Subsequent investigations have confirmed that the activation of CD137 signaling by MCLA-145 is conditional and occurs when neighboring cells express more than 5000 copies of PD-L1. This conditional activation offers potential advantages in safety and effectiveness. It is important to note that even under conditions of maximum saturation, MCLA-145 cannot trigger CD137 signaling in the absence of neighboring cells expressing PD-L1 [110]. Another bispecific antibody, ABL503, selectively activates 4-1BB signaling only in the context of PD-L1, avoiding dose-dependent toxicity observed in patients treated with anti-4-1BB agonistic antibodies [111]. ABL503 exhibits potent antitumor activity and improved safety profiles in preclinical models [111].
PRS-344/S095012 is developed to block the PD-1/PD-L1 pathway and localize 4-1BB co-stimulation to a PD-L1+ TME [112]. This bispecific molecule effectively combines ICI with TME-localized 4-1BB-mediated immunostimulation, demonstrating superior T-cell stimulation and antitumor activity in murine models compared to the combination of monoclonal antibodies [112]. Additionally, HK010, an Fc-mutated IgG4 anti-4-1BB × PD-L1 BsAb, exhibits a strong antitumor effect by simultaneously blocking PD-1/PD-L1 signaling and stimulating 4-1BB signaling [113]. HK010 shows potent antitumor immunity, induces durable antigen-specific immune memory, and is well-tolerated in preclinical models, suggesting a promising option for cancer immunotherapy [114]. Additionally, PM1003, a single-domain antibody towards a unique epitope of 4-1BB, is used in the engineering of multi-specific antibodies, such as anti-PD-L1 × 4-1BB BsAbs, to localize 4-1BB activation within the TME, resulting in potent inhibition of PD-L1 activity and antitumor activity with minimal toxicity in vivo [115].
Notably, in the phase 1 clinical trial (NCT03917381), the potential of DuoBody-4-1BB × PD-L1 (GEN1046), a first-in-class bispecific immunotherapy agent, was investigated in patients with advanced refractory solid tumors [116]. In preclinical models, GEN1046 demonstrated superior effects on T-cell proliferation, cytokine generation, and cytotoxicity function compared to clinically approved anti-PD-1/PD-L1 agents [116]. The ongoing first-in-human study revealed manageable safety, pharmacodynamic immune effects consistent with its mechanism of action, and early clinical activity, with a disease control rate of 65.6% (40/61) observed in patients, including those resistant to prior anti-PD-1/PD-L1 immunotherapy [116]. GEN1046's encouraging preclinical and clinical results suggest its potential to fill a clinical gap in patients with immunotherapy-relapsed or refractory disease, positioning it as a promising candidate for combination therapy with other immunotherapy agents [116]. In summary, these developments collectively provide novel strategies for cancer immunotherapy with enhanced efficacy and safety.
Anti-LAG-3 × PD-L1 BsAb
LAG-3, an identified transmembrane protein on activated T cells and NK cells, delivers inhibitory signals to suppress T cell proliferation [117,118,119]. Monoclonal antibodies blocking LAG-3 and MHC-II interaction are currently being assessed for their potential antitumor effects [120, 121]. However, the coexpression of LAG-3 and PD-1 in tumors implies their involvement in T-cell exhaustion [122]. The combined administration of anti-PD-1/PD-L1 and anti-LAG-3 antibodies displays a synergistic ability to inhibit tumor growth, as evidenced in phase 2/3 trials where the combination of relatlimab and nivolumab yielded significantly prolonged PFS compared to nivolumab alone [123,124,125]. At present, the combination of relatlimab and nivolumab has been approved for advanced melanoma [123, 124]. Besides, combinations like ieramilimab (an anti-LAG-3 antibody) alongside spartalizumab (an anti-PD-1 antibody) have demonstrated sustained positive responses in various patient groups, including those with non-small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, mesothelioma, and triple-negative breast cancer (TNBC). These responses were observed in patients who had not previously received anti-PD-1/L1 treatments and in melanoma and renal cell carcinoma patients who had undergone prior anti-PD1/L1 therapy [126]. The rationale for developing anti-LAG-3/PD-L1 bispecific antibodies arises from the observed coexpression in tumors, indicating a potential role in T cell exhaustion.
IBI323 and ABL501 represent promising BsAbs targeting LAG-3 and PD-L1, aiming to overcome the limited effectiveness observed in anti-PD-1/PD-L1 treatments for advanced tumors. IBI323 not only maintained the blockade activities of its parental antibodies but also introduced a novel cell-bridging function [127]. This innovative mechanism translated into heightened immune stimulatory activity in mixed leukocyte reactions and more robust antitumor responses in humanized mouse models, correlated with an increase in tumor-specific T cells [127]. Similarly, ABL501 was constructed from an anti-LAG-3 IgG4 antibody linked to a PD-L1-targeting scFv through a (G4S)3 linker, featuring a strategic S224P amino acid substitution to enhance stability. It effectively binds to its targets without eliciting Fc-mediated effector functions like ADCC and CDC, focusing its action on checkpoint blockade. In vitro experiments showed that ABL501 efficiently targeted both LAG-3 and PD-L1 pathways, outperforming individual anti-LAG-3 and anti-PD-L1 antibodies in enhancing the activation of effector T cells [128]. ABL501 demonstrated compelling in vivo antitumor efficacy in humanized xenograft models, underscoring its potential clinical significance [128]. The examination of immune profiles in peripheral blood highlights a heightened presence of the LAG-3 + PD-1 + memory CD4 + T cell subset in relapsed cholangiocarcinoma patients who underwent chemotherapy [128]. Notably, this subset predicts increased responsiveness to ABL501, providing valuable support for its ongoing first-in-human trial (NCT05101109) [128]. Mechanically, ABL501 promoted DC maturation and capacity to prime T cells, leading to improved cross-presentation of antigens and more robust CD8 + T cell activation. Additionally, ABL501 directly increased CD8 + T cell cytotoxicity against tumors. Its efficacy hinges on simultaneous engagement of LAG-3 and PD-L1, facilitating effective T cell-tumor cell interactions. By acting as a T cell engager and promoting T cell activation while blocking inhibitory signals, ABL501 orchestrates a potent antitumor immune response [128].
Besides, the anti-LAG-3 × PD-L1 BsAb FS118 exhibits promising preclinical and clinical results, offering a novel approach for cancer immunotherapy [129]. In preclinical investigations, FS118 demonstrated simultaneous binding to LAG-3 and PD-L1 with high affinity, surpassing the antitumor activity of the combination of anti-LAG-3 and anti-PD-L1 antibodies [130]. Mechanistic studies in syngeneic tumor mouse models revealed significant tumor growth suppression with the surrogate mLAG-3 × PD-L1 antibody. Notably, the murine surrogate led to decreased LAG-3 abundance of T cells, while the combination of individual antibodies increased LAG-3 expression [130]. Moreover, binding of the surrogate mLAG-3/PD-L1 antibody resulted in the rapid shedding of mouse LAG-3 into the blood [130]. In clinical studies, a phase 1 trial (NCT03440437) demonstrated the safety and tolerability of FS118 in patients with advanced, anti-PD-1/PD-L1-resistant cancers [131]. FS118 showed a recommended phase 2 dose of 10 mg/kg weekly, sustained pharmacodynamic activity, and an overall disease control rate of 46.5%, particularly notable in patients with acquired resistance to PD-1/PD-L1-targeted therapy [131]. This study supports the continued investigation of FS118 for patients with refractory cancers, highlighting its potential as an effective dual PD-L1 × LAG-3 blockade strategy. Apart from solid tumors, LAG-3-targeting BsAbs also exhibited promising efficacy in hematological malignancies. In the phase 1 clinical trial NCT03219268, the efficacy of anti-LAG-3 × PD-1 BsAb Tebotelimab was explored in patients with solid tumors or hematologic malignancies [132]. Notably, 34% of patients showed tumor reduction, with positive responses in various cancer types, including cases resistant to anti-PD-1 treatment [132].
Anti-PD-1/CTLA-4 BsAb
CTLA-4 and PD-1 are immune checkpoints that inhibit various T cell functions, and their activation leads to T cell functional inhibition through multiple mechanisms [133]. CTLA-4, when induced upon activation, competes with CD28-mediated activation and removes costimulatory ligands from APCs [134]. PD-1, when expressed by T lymphocytes, acts as an inhibitor reducing cytotoxicity and cytokine generation. Both checkpoints are co-opted by tumors for immune evasion. Antibodies blocking CTLA-4 or PD-1 have shown antitumor activity in preclinical and clinical settings, and combination therapy has demonstrated improved responses in various cancers [135]. Besides, the TME analysis reveals a higher ratio of PD-1+CTLA-4+ cells in tumors compared to normal tissues, supporting the rationale for targeting PD-1+ CTLA-4+ cells to selectively block checkpoints in the TME while avoiding influences on normal tissues [136].
The systemic blockade of the PD-1/PD-L1 axis is foundational in cancer immunotherapy, especially considering its clinical significance and well-regarded safety profile. Therefore, a superior combination therapeutic should ensure it retains the effectiveness of PD-1 blockade without diminishing its capacity to interrupt PD-1 interactions with its ligands. However, the systemic CTLA-4 inhibition presents a higher risk of adverse effects. To mitigate these risks, anti-PD-1 × CTLA-4 BsAb provides refining the CTLA-4 inhibitory function to specifically target cells that co-express PD-1 and CTLA-4 within the TME [136]. For instance, MGD019 represents an advanced BsAb engineered to simultaneously target PD-1 and CTLA-4. This BsAb is uniquely designed with a tetravalent structure, utilizing a high-affinity anti-PD-1 monoclonal antibody, alongside an anti-CTLA-4 monoclonal antibody with properties that block ligands in a manner similar to the well-known ipilimumab [136]. The construction of MGD019 on the Dual-Affinity Re-Targeting platform with the 2 × 2 symmetric format, incorporating a hinge-stabilized IgG4 backbone. The unique structure of MGD019 allows for a robust blockade of PD-1 and a conditional inhibition of CTLA-4, tailored to the TME [136]. It mimics the in vitro PD-1 blockade efficacy of its anti-PD-1 precursor while modulating the CTLA-4 blockade to be most effective in cells expressing both PD-1 and CTLA-4. This specificity ensures localized CTLA-4 inhibition in the TME, enhancing safety by avoiding widespread Treg depletion [136]. Notably, the capacity of MGD019 capacity to block the interaction between PD-1 and its ligands with high efficiency, combined with its adaptable CTLA-4 blockade strategy, demonstrates significant antitumor activity with a manageable safety profile in patients with advanced solid tumors [136].
Moreover, the IgG4 Fc region of MGD019 confers a reduced capacity for Fc-mediated ADCC, thereby decreasing the inadvertent elimination of activated T cells and Tregs [136]. The effect of Treg depletion on the therapeutic efficacy and safety of ipilimumab remains under examination, yet the potential of Fc regions in anti-CTLA-4 antibodies to induce such depletion has been linked to both beneficial and detrimental outcomes in preclinical studies [137, 138]. By circumventing the depletion of Tregs while maintaining effective CTLA-4 blockade in the TME, MGD019 is designed to improve patient safety and uphold the beneficial effects of CTLA-4 antagonism. The immunosuppressive role of Tregs predominantly involves CTLA-4-mediated T cell exhaustion. Therefore, the blockade of CTLA-4 by MGD019 in the TME is expected to be sufficiently intense to compensate for the absence of Treg depletion [136]. This strategy, which prevents Treg reduction and ensures strong CTLA-4 inhibition in the TME, seeks to optimize the pivotal functions of these immune checkpoints in cancer immunotherapy, potentially yielding enhanced therapeutic benefits while minimizing adverse effects relative to conventional antibody treatments [136].
Additionally, the anti-PD-1/CTLA-4 BsAb QL1706 showed a manageable safety profile in a phase 1/1b study for advanced solid tumors refractory to standard therapies [139]. Across all patients at the recommended dose, the objective response rate was 16.9%, with a median duration of response of 11.7 months [139]. Notably, immunotherapy-naïve patients, particularly those with NSCLC, nasopharyngeal carcinoma, and cervical cancer, exhibited promising antitumor activities, with response rates of 24.2%, 38.7%, and 28.3%, respectively [139]. QL1706 is currently under evaluation in randomized phase 2/3 trials for further assessment of its efficacy [139]. Furthermore, MEDI5752, a novel monovalent bispecific antibody, enhances PD-1 blockade by selectively inhibiting CTLA4 on PD-1 + activated T cells [140]. It reduces the required dose for IL-2 secretion and rapidly internalizes and degrades PD-1. With a preference for tumor localization, MEDI5752 demonstrates superior in vivo activity compared to anti-PD-1 and anti-CTLA4 antibody combinations [140]. Two patients with advanced solid tumors showed robust partial responses to MEDI5752 treatment. This represents a significant advancement in cancer immunotherapy, offering distinct benefits by selectively targeting CTLA4 on PD-1 + T cells [140].
Cadonilimab is a tetravalent bispecific IgG1 antibody with an innovative Fc-null design, aming to eliminate Fc-mediated effector function for safety and efficacy considerations [141]. It exhibits biological activity comparable to the combination of CTLA-4 and PD-1 antibodies. Remarkably, cadonilimab displays higher binding avidity in a high-density PD-1 and CTLA-4 setting, offering potential advantages in tumor-like environments [141]. In the phase 1b/2 trial NCT03852251, the efficacy of cadonilimab was explored in advanced solid tumors [142]. Cadonilimab demonstrated encouraging efficacy, with objective response rates of 32.3% in cervical cancer, 18.2% in esophageal squamous cell carcinoma, and 16.7% in hepatocellular carcinoma [142]. These findings underscore the potential effectiveness of cadonilimab in achieving positive tumor responses in diverse advanced solid tumor types [142]. At present, Cadonilimab has received approval in China for treating relapsed or metastatic cervical cancer after platinum-based chemotherapy [143].
Other BsAbs targeting PD-1/PD-L1 signaling
Besides the agents mentioned above, other BsAbs have been developed and are undergoing evaluation in clinical trials, such as anti-PD-L1/CD3, anti-PD-1/PD-L1, and anti-TIM-3/PD-L1 BsAbs. For instance, recent advances in the development of anti-PD-L1/CD3 BsAb have addressed key challenges associated with existing T-cell engagers. One notable innovation is the Protease-Activated PSTAGylated BiTE (PAPB), which is designed for solid tumors. PAPB incorporates a shielding polypeptide domain (PSTAG), a protease-activated linker, and a BiTE core with scFvs targeting PD-L1 and CD3. PAPB demonstrates a dose-dependent binding of the BiTE core to PD-L1 and CD3, with the ability to release the core in response to MMP2 in the TME, significantly prolonging its plasma half-life [144]. Furthermore, a novel anti-PD-L1/CD3 nanobody-based BiTE demonstrates cytotoxic activity on melanoma cells correlated with PD-L1 expression levels, highlighting its potential in treating PD-L1-overexpressing melanoma. Collectively, these advancements signify promising steps toward enhancing the safety, duration of action, and efficacy of anti-PD-L1/CD3 antibodies in the realm of solid tumor immunotherapy [145].
TIM-3 serves a critical role in cancer immunology as a negative regulator of immune response [146, 147]. In cancer, TIM-3 expression specifically identifies the most dysfunctional subset of CD8+ T cells, indicating their exhaustion [148]. Studies in preclinical cancer models demonstrate significant efficacy in co-blockading the TIM-3 and PD-1 pathways, both in solid and hematologic malignancies [149]. Ongoing clinical trials, particularly in solid tumors, are exploring the potential of anti-TIM-3 in combination with anti-PD-1, showcasing its promise as a target for cancer immunotherapy [52, 150, 151]. In the phase 1 study NCT03752177, LY3415244, a TIM-3/PD-L1 BsAb, was evaluated for safety and efficacy in patients with advanced solid tumors [152]. While some patients showed promising outcomes, such as a near partial response in a PD-1 refractory NSCLC patient (-29.6%), the trial faced challenges [152]. Notably, 16.7% of patients experienced clinically significant anaphylactic infusion-related reactions. All patients developed treatment-emergent antidrug antibodies (TE-ADA), impacting soluble TIM-3 target engagement and leading to early termination of the study [152]. Despite these challenges, the patient outcomes, particularly in the context of PD-1 refractory cancer, highlight the potential clinical impact of LY3415244, warranting further exploration and consideration in future studies.
Conclusion and perspective
The advent and clinical validation of BsAbs targeting the PD-1/PD-L1 axis alongside other immune regulatory molecules mark a pivotal evolution in the landscape of cancer immunotherapy. This review has delved into the innovative strides made in the realm of BsAbs, particularly focusing on their development, mechanisms of action, and therapeutic potential in managing solid tumors. The exploration of BsAbs such as anti-TGFβ × PD-L1, anti-CD47 × PD-L1, and others, underlines a strategic endeavor to amplify antitumor immunity, overcome immune evasion, and address the limitations inherent in monotherapy approaches. While the therapeutic promise of these agents is underscored by both preclinical and emerging clinical successes, the journey towards their optimal integration into cancer care will require careful research and attention to detail.
Firstly, future research must concentrate on enhancing the specificity, efficacy, and safety profiles of BsAbs. This includes the development of next-generation BsAbs with reduced immunogenicity, improved tumor penetration, and tailored pharmacokinetics characteristics. Advanced molecular engineering techniques can facilitate the design of BsAbs that selectively accumulate within the TME, minimizing systemic exposure and associated toxicities. Besides, the complexity of the TME remains a formidable challenge to the efficacy of immunotherapies. Novel BsAbs that can modulate the suppressive TME, enhance antigen presentation, and promote T cell infiltration and activation within tumors are of particular interest. Strategies combining BsAbs with agents that disrupt physical barriers within the TME or neutralize suppressive cell populations could yield synergistic antitumor effects. Moreover, identifying predictive biomarkers for responsiveness to BsAb therapies is crucial. Comprehensive genomic, proteomic, and immunological profiling of tumors could unveil biomarkers that predict therapeutic response, guide patient selection, and facilitate personalized treatment approaches. This precision medicine approach would optimize therapeutic outcomes and mitigate the risk of adverse effects. Furthermore, the integration of BsAbs with other treatment modalities, including chemotherapy, targeted therapy, radiation, and other immunotherapies, holds great promises. Rational combination strategies based on mechanistic rationales and preclinical evidence can potentiate antitumor efficacy, counteract resistance mechanisms, and broaden the therapeutic window of BsAbs.
The exploration of BsAbs in cancer immunotherapy opens a new frontier in our fight against cancer, promising to enhance the precision, potency, and persistence of immune-mediated antitumor responses. The future of cancer treatment with BsAbs beckons a paradigm where the synergy of targeting multiple immune checkpoints or combining immune modulation with other therapeutic strategies can provide durable, effective, and safer treatment options for patients worldwide. The substantive prospects for BsAbs in cancer care not only highlight a promising therapeutic avenue but also underscore our collective commitment to turning the tide against cancer through immunological means.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- PD-1:
-
Programmed cell death 1
- ITIM:
-
Immune receptor tyrosine-based inhibitory motif
- ITSM:
-
Immune receptor tyrosine-based switch motif
- BsAb:
-
Bispecific antibody
- TAA:
-
Tumor-associated antigen
- BiTE:
-
Bispecific T cell engager
- NSCLC:
-
Non-small cell lung cancer
- TGFβ:
-
Transforming growth factor-β
- DC:
-
Dendritic cell
- Treg:
-
Regulatory T cell
- TME:
-
Tumor microenvironment
- MDSC:
-
Myeloid-derived suppressor cell
- TIL:
-
Tumor-infiltrating lymphocyte
- TNBC:
-
Triple-negative breast cancer
- STING:
-
Stimulator of an interferon gene
- KIH:
-
Knobs-into-hole
- VEGFR1D2:
-
Vascular endothelial growth factor receptor 1 domain 2
- BsDb:
-
Bispecific single-chain diabody
- ORR:
-
Objective response rate
- APC:
-
Antigen-presenting cell
- CTL:
-
Cytotoxic T lymphocyte
References
Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42.
Chen RY, Zhu Y, Shen YY, Xu QY, Tang HY, Cui NX, et al. The role of PD-1 signaling in health and immune-related diseases. Front Immunol. 2023;14:1163633.
He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–9.
Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–8.
Kurachi M. CD8(+) T cell exhaustion. Semin Immunopathol. 2019;41:327–37.
Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018;378:1976–86.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24–33.
Li H, Er Saw P, Song E. Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics. Cell Mol Immunol. 2020;17:451–61.
Liu C, Yang M, Zhang D, Chen M, Zhu D. Clinical cancer immunotherapy: Current progress and prospects. Front Immunol. 2022;13: 961805.
Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14:10.
Chen Y, Hu H, Yuan X, Fan X, Zhang C. Advances in Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma. Front Immunol. 2022;13: 896752.
Chen Y, Zheng X, Wu C. The Role of the Tumor Microenvironment and Treatment Strategies in Colorectal Cancer. Front Immunol. 2021;12: 792691.
Moore EK, Strazza M, Mor A. Combination Approaches to Target PD-1 Signaling in Cancer. Front Immunol. 2022;13: 927265.
Wang X, Guo G, Guan H, Yu Y, Lu J, Yu J. Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. J Exp Clin Cancer Res. 2019;38:87.
Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17:129.
Labrijn AF, Janmaat ML, Reichert JM, Parren P. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18:585–608.
Yi M, Zhang J, Li A, Niu M, Yan Y, Jiao Y, et al. The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1. J Hematol Oncol. 2021;14:27.
Suurs FV, Lub-de Hooge MN, de Vries EGE, de Groot DJA. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol Ther. 2019;201:103–19.
Zhou Y, Penny HL, Kroenke MA, Bautista B, Hainline K, Chea LS, et al. Immunogenicity assessment of bispecific antibody-based immunotherapy in oncology. J Immunother Cancer. 2022;10: e004225.
Li T, Wang X, Niu M, Wang M, Zhou J, Wu K, et al. Bispecific antibody targeting TGF-β and PD-L1 for synergistic cancer immunotherapy. Front Immunol. 2023;14:1196970.
Sedykh SE, Prinz VV, Buneva VN, Nevinsky GA. Bispecific antibodies: design, therapy, perspectives. Drug Des Devel Ther. 2018;12:195–208.
Yu S, Li A, Liu Q, Yuan X, Xu H, Jiao D, et al. Recent advances of bispecific antibodies in solid tumors. J Hematol Oncol. 2017;10:155.
Fan G, Wang Z, Hao M, Li J. Bispecific antibodies and their applications. J Hematol Oncol. 2015;8:130.
Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104.
Goebeler ME, Bargou RC. T cell-engaging therapies - BiTEs and beyond. Nat Rev Clin Oncol. 2020;17:418–34.
Goéré D, Flament C, Rusakiewicz S, Poirier-Colame V, Kepp O, Martins I, et al. Potent immunomodulatory effects of the trifunctional antibody catumaxomab. Cancer Res. 2013;73:4663–73.
Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharmacol Ther. 2012;136:334–42.
Sanford M. Blinatumomab: first global approval. Drugs. 2015;75:321–7.
Kaplan JB, Grischenko M, Giles FJ. Blinatumomab for the treatment of acute lymphoblastic leukemia. Invest New Drugs. 2015;33:1271–9.
Budde LE, Sehn LH, Matasar M, Schuster SJ, Assouline S, Giri P, et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23:1055–65.
Mosunetuzumab KC. First Approval. Drugs. 2022;82:1229–34.
Zhou S, Liu M, Ren F, Meng X, Yu J. The landscape of bispecific T cell engager in cancer treatment. Biomark Res. 2021;9:38.
Scott EM, Duffy MR, Freedman JD, Fisher KD, Seymour LW. Solid Tumor Immunotherapy with T Cell Engager-Armed Oncolytic Viruses. Macromol Biosci. 2018. https://doi.org/10.1002/mabi.201700187.
Huang Q, Cai WQ, Han ZW, Wang MY, Zhou Y, Cheng JT, et al. Bispecific T cell engagers and their synergistic tumor immunotherapy with oncolytic viruses. Am J Cancer Res. 2021;11:2430–55.
Jin S, Sun Y, Liang X, Gu X, Ning J, Xu Y, et al. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct Target Ther. 2022;7:39.
Paz-Ares L, Kim TM, Vicente D, Felip E, Lee DH, Lee KH, et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Second-Line Treatment of Patients With NSCLC: Results From an Expansion Cohort of a Phase 1 Trial. J Thorac Oncol. 2020;15:1210–22.
Wu Y, Yi M, Zhu S, Wang H, Wu K. Recent advances and challenges of bispecific antibodies in solid tumors. Exp Hematol Oncol. 2021;10:56.
Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med. 2018;10:eaan5488.
Strauss J, Heery CR, Schlom J, Madan RA, Cao L, Kang Z, et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin Cancer Res. 2018;24:1287–95.
Lind H, Gameiro SR, Jochems C, Donahue RN, Strauss J, Gulley JM, et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J Immunother Cancer. 2020;8: e000433.
Leclercq-Cohen G, Steinhoff N, Albertí Servera L, Nassiri S, Danilin S, Piccione E, et al. Dissecting the Mechanisms Underlying the Cytokine Release Syndrome (CRS) Mediated by T-Cell Bispecific Antibodies. Clin Cancer Res. 2023;29:4449–63.
Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95.
Baeuerle PA, Wesche H. T-cell-engaging antibodies for the treatment of solid tumors: challenges and opportunities. Curr Opin Oncol. 2022;34:552–8.
Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016:567–72.
Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–7.
Liguori L, Polcaro G, Nigro A, Conti V, Sellitto C, Perri F, et al. Bispecific Antibodies: A Novel Approach for the Treatment of Solid Tumors. Pharmaceutics. 2022;14:2442.
Kebenko M, Goebeler ME, Wolf M, Hasenburg A, Seggewiss-Bernhardt R, Ritter B, et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors. Oncoimmunology. 2018;7: e1450710.
Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21:28.
Groeneveldt C, Kinderman P, van den Wollenberg DJM, van den Oever RL, Middelburg J, Mustafa DAM, et al. Preconditioning of the tumor microenvironment with oncolytic reovirus converts CD3-bispecific antibody treatment into effective immunotherapy. J Immunother Cancer. 2020;8: e001191.
Chang CH, Wang Y, Li R, Rossi DL, Liu D, Rossi EA, et al. Combination Therapy with Bispecific Antibodies and PD-1 Blockade Enhances the Antitumor Potency of T Cells. Cancer Res. 2017;77:5384–94.
Sam J, Colombetti S, Fauti T, Roller A, Biehl M, Fahrni L, et al. Combination of T-Cell Bispecific Antibodies With PD-L1 Checkpoint Inhibition Elicits Superior Anti-Tumor Activity. Front Oncol. 2020;10: 575737.
Osada T, Patel SP, Hammond SA, Osada K, Morse MA, Lyerly HK. CEA/CD3-bispecific T cell-engaging (BiTE) antibody-mediated T lymphocyte cytotoxicity maximized by inhibition of both PD1 and PD-L1. Cancer Immunol Immunother. 2015;64:677–88.
Belmontes B, Sawant DV, Zhong W, Tan H, Kaul A, Aeffner F, et al. Immunotherapy combinations overcome resistance to bispecific T cell engager treatment in T cell-cold solid tumors. Sci Transl Med. 2021;13:eabd1524.
Colak S, Ten Dijke P. Targeting TGF-β Signaling in Cancer. Trends Cancer. 2017;3:56–71.
Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30.
Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-β pathway. J Hematol Oncol. 2021;14:55.
Bai X, Yi M, Jiao Y, Chu Q, Wu K. Blocking TGF-β Signaling To Enhance The Efficacy Of Immune Checkpoint Inhibitor. Onco Targets Ther. 2019;12:9527–38.
Hao Y, Baker D, Ten Dijke P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int J Mol Sci. 2019;20:2767.
Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–84.
Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–72.
Kim BN, Ahn DH, Kang N, Yeo CD, Kim YK, Lee KY, et al. TGF-β induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer. Sci Rep. 2020;10:10597.
Liu S, Zhang C, Wang B, Zhang H, Qin G, Li C, et al. Regulatory T cells promote glioma cell stemness through TGF-β-NF-κB-IL6-STAT3 signaling. Cancer Immunol Immunother. 2021;70:2601–16.
Slattery K, Woods E, Zaiatz-Bittencourt V, Marks S, Chew S, Conroy M, et al. TGFβ drives NK cell metabolic dysfunction in human metastatic breast cancer. J Immunother Cancer. 2021;9: e002044.
Horn LA, Riskin J, Hempel HA, Fousek K, Lind H, Hamilton DH, et al. Simultaneous inhibition of CXCR1/2, TGF-β, and PD-L1 remodels the tumor and its microenvironment to drive antitumor immunity. J Immunother Cancer. 2020;8: e000326.
Canè S, Van Snick J, Uyttenhove C, Pilotte L, Van den Eynde BJ. TGFβ1 neutralization displays therapeutic efficacy through both an immunomodulatory and a non-immune tumor-intrinsic mechanism. J Immunother Cancer. 2021;9: e001798.
Moreau JM, Velegraki M, Bolyard C, Rosenblum MD, Li Z. Transforming growth factor-β1 in regulatory T cell biology. Sci Immunol. 2022;7:eabi4613.
Tauriello DVF, Sancho E, Batlle E. Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer. 2022;22:25–44.
David JM, Dominguez C, McCampbell KK, Gulley JL, Schlom J, Palena C. A novel bifunctional anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology. 2017;6: e1349589.
Knudson KM, Hicks KC, Luo X, Chen JQ, Schlom J, Gameiro SR. M7824, a novel bifunctional anti-PD-L1/TGFβ Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology. 2018;7: e1426519.
Cheng B, Ding K, Chen P, Ji J, Luo T, Guo X, et al. Anti-PD-L1/TGF-βR fusion protein (SHR-1701) overcomes disrupted lymphocyte recovery-induced resistance to PD-1/PD-L1 inhibitors in lung cancer. Cancer Commun (Lond). 2022;42:17–36.
Feng J, Tang D, Wang J, Zhou Q, Peng J, Lou H, et al. SHR-1701, a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, for Recurrent or Metastatic Cervical Cancer: A Clinical Expansion Cohort of a Phase I Study. Clin Cancer Res. 2022;28:5297–305.
Wu ZH, Li N, Gao ZZ, Chen G, Nie L, Zhou YQ, et al. Development of the Novel Bifunctional Fusion Protein BR102 That Simultaneously Targets PD-L1 and TGF-β for Anticancer Immunotherapy. Cancers (Basel). 2022;14:4964.
Yi M, Niu M, Wu Y, Ge H, Jiao D, Zhu S, et al. Combination of oral STING agonist MSA-2 and anti-TGF-β/PD-L1 bispecific antibody YM101: a novel immune cocktail therapy for non-inflamed tumors. J Hematol Oncol. 2022;15:142.
Yi M, Niu M, Zhang J, Li S, Zhu S, Yan Y, et al. Combine and conquer: manganese synergizing anti-TGF-β/PD-L1 bispecific antibody YM101 to overcome immunotherapy resistance in non-inflamed cancers. J Hematol Oncol. 2021;14:146.
Yi M, Wu Y, Niu M, Zhu S, Zhang J, Yan Y, et al. Anti-TGF-β/PD-L1 bispecific antibody promotes T cell infiltration and exhibits enhanced antitumor activity in triple-negative breast cancer. J Immunother Cancer. 2022;10: e005543.
Gulley JL, Schlom J, Barcellos-Hoff MH, Wang XJ, Seoane J, Audhuy F, et al. Dual inhibition of TGF-β and PD-L1: a novel approach to cancer treatment. Mol Oncol. 2022;16:2117–34.
Yi M, Li T, Niu M, Wu Y, Zhao Z, Wu K. TGF-β: A novel predictor and target for anti-PD-1/PD-L1 therapy. Front Immunol. 2022;13:1061394.
Logtenberg MEW, Scheeren FA, Schumacher TN. The CD47-SIRPα Immune Checkpoint. Immunity. 2020;52:742–52.
Zhao H, Song S, Ma J, Yan Z, Xie H, Feng Y, et al. CD47 as a promising therapeutic target in oncology. Front Immunol. 2022;13: 757480.
van Duijn A, Van der Burg SH, Scheeren FA. CD47/SIRPα axis: bridging innate and adaptive immunity. J Immunother Cancer. 2022;10: e004589.
Wang S, Wu Q, Chen T, Su R, Pan C, Qian J, et al. Blocking CD47 promotes antitumour immunity through CD103(+) dendritic cell-NK cell axis in murine hepatocellular carcinoma model. J Hepatol. 2022;77:467–78.
Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90.
Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–15.
Yi M, Li T, Niu M, Mei Q, Zhao B, Chu Q, et al. Exploiting innate immunity for cancer immunotherapy. Mol Cancer. 2023;22:187.
Wang R, Zhang C, Cao Y, Wang J, Jiao S, Zhang J, et al. Blockade of dual immune checkpoint inhibitory signals with a CD47/PD-L1 bispecific antibody for cancer treatment. Theranostics. 2023;13:148–60.
Chen SH, Dominik PK, Stanfield J, Ding S, Yang W, Kurd N, et al. Dual checkpoint blockade of CD47 and PD-L1 using an affinity-tuned bispecific antibody maximizes antitumor immunity. J Immunother Cancer. 2021;9: e003464.
Wang Y, Ni H, Zhou S, He K, Gao Y, Wu W, et al. Tumor-selective blockade of CD47 signaling with a CD47/PD-L1 bispecific antibody for enhanced anti-tumor activity and limited toxicity. Cancer Immunol Immunother. 2021;70:365–76.
Liu B, Guo H, Xu J, Qin T, Guo Q, Gu N, et al. Elimination of tumor by CD47/PD-L1 dual-targeting fusion protein that engages innate and adaptive immune responses. MAbs. 2018;10:315–24.
Yu P, Wang Y, Yuan D, Sun Y, Qin S, Li T. Vascular normalization: reshaping the tumor microenvironment and augmenting antitumor immunity for ovarian cancer. Front Immunol. 2023;14:1276694.
Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18:60.
Niu M, Yi M, Wu Y, Lyu L, He Q, Yang R, et al. Synergistic efficacy of simultaneous anti-TGF-β/VEGF bispecific antibody and PD-1 blockade in cancer therapy. J Hematol Oncol. 2023;16:94.
Hassanzadeh Eskafi A, Oghalaei A, Mahboudi F, Ghaderi H, Behdani M, Shoari A, et al. Investigation of the therapeutic potential of recombinant bispecific bivalent anti-PD-L1/VEGF nanobody in inhibition of angiogenesis. Immunopharmacol Immunotoxicol. 2023;45:197–202.
Cui X, Jia H, Xin H, Zhang L, Chen S, Xia S, et al. A Novel Bispecific Antibody Targeting PD-L1 and VEGF With Combined Anti-Tumor Activities. Front Immunol. 2021;12: 778978.
Xiong C, Mao Y, Wu T, Kang N, Zhao M, Di R, et al. Optimized Expression and Characterization of a Novel Fully Human Bispecific Single-Chain Diabody Targeting Vascular Endothelial Growth Factor165 and Programmed Death-1 in Pichia pastoris and Evaluation of Antitumor Activity In Vivo. Int J Mol Sci. 2018;19:2900.
Zhao Y, Chen G, Chen J, Zhuang L, Du Y, Yu Q, et al. AK112, a novel PD-1/VEGF bispecific antibody, in combination with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC): an open-label, multicenter, phase II trial. EClinicalMedicine. 2023;62: 102106.
Etxeberria I, Glez-Vaz J, Teijeira Á, Melero I. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open. 2020;4: e000733.
Vinay DS, Kwon BS. 4–1BB signaling beyond T cells. Cell Mol Immunol. 2011;8:281–4.
Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4–1BB: mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49–57.
Kim AMJ, Nemeth MR, Lim SO. 4–1BB: A promising target for cancer immunotherapy. Front Oncol. 2022;12: 968360.
Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124:2246–59.
Claus C, Ferrara C, Xu W, Sam J, Uhlenbrock F, et al. Tumor-targeted 4–1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci Transl Med. 2019;11:eaav5989.
Claus C, Ferrara-Koller C, Klein C. The emerging landscape of novel 4–1BB (CD137) agonistic drugs for cancer immunotherapy. MAbs. 2023;15:2167189.
Qi X, Li F, Wu Y, Cheng C, Han P, Wang J, et al. Optimization of 4–1BB antibody for cancer immunotherapy by balancing agonistic strength with FcγR affinity. Nat Commun. 2019;10:2141.
Shindo Y, Yoshimura K, Kuramasu A, Watanabe Y, Ito H, Kondo T, et al. Combination immunotherapy with 4–1BB activation and PD-1 blockade enhances antitumor efficacy in a mouse model of subcutaneous tumor. Anticancer Res. 2015;35:129–36.
Chen S, Lee LF, Fisher TS, Jessen B, Elliott M, Evering W, et al. Combination of 4–1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2015;3:149–60.
Geuijen C, Tacken P, Wang LC, Klooster R, van Loo PF, Zhou J, et al. A human CD137×PD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade. Nat Commun. 2021;12:4445.
Jeong S, Park E, Kim HD, Sung E, Kim H, Jeon J, et al. Novel anti-4-1BB×PD-L1 bispecific antibody augments anti-tumor immunity through tumor-directed T-cell activation and checkpoint blockade. J Immunother Cancer. 2021;9: e002428.
Peper-Gabriel JK, Pavlidou M, Pattarini L, Morales-Kastresana A, Jaquin TJ, Gallou C, et al. The PD-L1/4-1BB Bispecific Antibody-Anticalin Fusion Protein PRS-344/S095012 Elicits Strong T-Cell Stimulation in a Tumor-Localized Manner. Clin Cancer Res. 2022;28:3387–99.
Muik A, Altintas I, Gieseke F, Schoedel KB, Burm SM, Toker A, et al. An Fc-inert PD-L1×4-1BB bispecific antibody mediates potent anti-tumor immunity in mice by combining checkpoint inhibition and conditional 4–1BB co-stimulation. Oncoimmunology. 2022;11:2030135.
Cheng LS, Zhu M, Gao Y, Liu WT, Yin W, Zhou P, et al. An Fc-muted bispecific antibody targeting PD-L1 and 4–1BB induces antitumor immune activity in colorectal cancer without systemic toxicity. Cell Mol Biol Lett. 2023;28:47.
Zhai T, Wang C, Xu Y, Huang W, Yuan Z, Wang T, et al. Generation of a safe and efficacious llama single-domain antibody fragment (vHH) targeting the membrane-proximal region of 4–1BB for engineering therapeutic bispecific antibodies for cancer. J Immunother Cancer. 2021;9: e002131.
Muik A, Garralda E, Altintas I, Gieseke F, Geva R, Ben-Ami E, et al. Preclinical Characterization and Phase I Trial Results of a Bispecific Antibody Targeting PD-L1 and 4–1BB (GEN1046) in Patients with Advanced Refractory Solid Tumors. Cancer Discov. 2022;12:1248–65.
Chocarro L, Blanco E, Zuazo M, Arasanz H, Bocanegra A, Fernández-Rubio L, et al. Understanding LAG-3 Signaling. Int J Mol Sci. 2021;22:5282.
Aggarwal V, Workman CJ, Vignali DAA. LAG-3 as the third checkpoint inhibitor. Nat Immunol. 2023;24:1415–22.
Maruhashi T, Sugiura D, Okazaki IM, Okazaki T. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8: e001014.
Cai L, Li Y, Tan J, Xu L, Li Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J Hematol Oncol. 2023;16:101.
Maruhashi T, Sugiura D, Okazaki IM, Shimizu K, Maeda TK, Ikubo J, et al. Binding of LAG-3 to stable peptide-MHC class II limits T cell function and suppresses autoimmunity and anti-cancer immunity. Immunity. 2022;55:912-24.e8.
Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27.
Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med. 2022;386:24–34.
Amaria RN, Postow M, Burton EM, Tetzlaff MT, Ross MI, Torres-Cabala C, et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature. 2022;611:155–60.
Paik J. Nivolumab Plus Relatlimab: First Approval. Drugs. 2022;82:925–31.
Lin CC, Garralda E, Schöffski P, Hong DS, Siu LL, Martin M, et al. A phase 2, multicenter, open-label study of anti-LAG-3 ieramilimab in combination with anti-PD-1 spartalizumab in patients with advanced solid malignancies. Oncoimmunology. 2024;13:2290787.
Jiang H, Ni H, Zhang P, Guo X, Wu M, Shen H, et al. PD-L1/LAG-3 bispecific antibody enhances tumor-specific immunity. Oncoimmunology. 2021;10:1943180.
Sung E, Ko M, Won JY, Jo Y, Park E, Kim H, et al. LAG-3xPD-L1 bispecific antibody potentiates antitumor responses of T cells through dendritic cell activation. Mol Ther. 2022;30:2800–16.
Kroloff MJ, Holz JB, Stern O, Shepherd CJ, Morrow M, Kayitalire L, et al. Durable response of anaplastic thyroid carcinoma to FS118, a bispecific LAG-3/PD-L1 antibody, after checkpoint inhibitor progression: a case report. J Immunother Cancer. 2022;10: e005225.
Kraman M, Faroudi M, Allen NL, Kmiecik K, Gliddon D, Seal C, et al. FS118, a Bispecific Antibody Targeting LAG-3 and PD-L1, Enhances T-Cell Activation Resulting in Potent Antitumor Activity. Clin Cancer Res. 2020;26:3333–44.
Yap TA, LoRusso PM, Wong DJ, Hu-Lieskovan S, Papadopoulos KP, Holz JB, et al. A Phase 1 First-in-Human Study of FS118, a Tetravalent Bispecific Antibody Targeting LAG-3 and PD-L1 in Patients with Advanced Cancer and PD-L1 Resistance. Clin Cancer Res. 2023;29:888–98.
Luke JJ, Patel MR, Blumenschein GR, Hamilton E, Chmielowski B, Ulahannan SV, et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: a phase 1 trial. Nat Med. 2023;29:2814–24.
Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18:155.
Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65.
Wu K, Yi M, Qin S, Chu Q, Zheng X, Wu K. The efficacy and safety of combination of PD-1 and CTLA-4 inhibitors: a meta-analysis. Exp Hematol Oncol. 2019;8:26.
Berezhnoy A, Sumrow BJ, Stahl K, Shah K, Liu D, Li J, et al. Development and Preliminary Clinical Activity of PD-1-Guided CTLA-4 Blocking Bispecific DART Molecule. Cell Rep Med. 2020;1: 100163.
Pai CS, Simons DM, Lu X, Evans M, Wei J, Wang YH, et al. Tumor-conditional anti-CTLA4 uncouples antitumor efficacy from immunotherapy-related toxicity. J Clin Invest. 2019;129:349–63.
Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42.
Zhao Y, Ma Y, Zang A, Cheng Y, Zhang Y, Wang X, et al. First-in-human phase I/Ib study of QL1706 (PSB205), a bifunctional PD1/CTLA4 dual blocker, in patients with advanced solid tumors. J Hematol Oncol. 2023;16:50.
Dovedi SJ, Elder MJ, Yang C, Sitnikova SI, Irving L, Hansen A, et al. Design and Efficacy of a Monovalent Bispecific PD-1/CTLA4 Antibody That Enhances CTLA4 Blockade on PD-1(+) Activated T Cells. Cancer Discov. 2021;11:1100–17.
Pang X, Huang Z, Zhong T, Zhang P, Wang ZM, Xia M, et al. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. MAbs. 2023;15:2180794.
Gao X, Xu N, Li Z, Shen L, Ji K, Zheng Z, et al. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): a multicentre, open-label, phase 1b/2 trial. Lancet Oncol. 2023;24:1134–46.
Keam SJ. Cadonilimab: First Approval. Drugs. 2022;82:1333–9.
Liu D, Bao L, Zhu H, Yue Y, Tian J, Gao X, et al. Microenvironment-responsive anti-PD-L1 × CD3 bispecific T-cell engager for solid tumor immunotherapy. J Control Release. 2023;354:606–14.
Li B, Wang S, Shan B, Li B, Li F. A PD-L1xCD3 bispecific nanobody as a novel T-cell engager in treating PD-L1 overexpression melanoma. Mol Immunol. 2023;163:20–7.
Acharya N, Sabatos-Peyton C, Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. 2020;8: e000911.
Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res. 2014;2:393–8.
Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86.
Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–51.
Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–10.
Tian T, Li Z. Targeting Tim-3 in Cancer With Resistance to PD-1/PD-L1 Blockade. Front Oncol. 2021;11: 731175.
Hellmann MD, Bivi N, Calderon B, Shimizu T, Delafontaine B, Liu ZT, et al. Safety and Immunogenicity of LY3415244, a Bispecific Antibody Against TIM-3 and PD-L1, in Patients With Advanced Solid Tumors. Clin Cancer Res. 2021;27:2773–81.
Acknowledgements
Not applicable.
Funding
This work was supported by Natural Science Foundation of Zhejiang Province (LQ24H160007), China Postdoctoral Science Foundation (No. 2022M722766 and 2023M743016), and Postdoctoral Fellowship Program of CPSF (No. GZB20230642).
Author information
Authors and Affiliations
Contributions
TL performed the selection of literature, drafted the manuscript and prepared the figures. MN and JZ collected the related references and participated in discussion. MY and KW designed this review and revised the manuscript. All authors contributed to this manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, T., Niu, M., Zhou, J. et al. The enhanced antitumor activity of bispecific antibody targeting PD-1/PD-L1 signaling. Cell Commun Signal 22, 179 (2024). https://doi.org/10.1186/s12964-024-01562-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-024-01562-5