Abstract
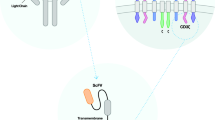
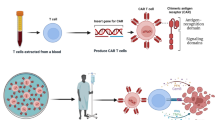
Chimeric antigen receptor (CAR)-T cell therapy is a promising new treatment for cancer that involves genetically modifying a patient’s T-cells to recognize and attack cancer cells. This review provides an overview of the latest discoveries and clinical trials related to CAR-T cell therapy, as well as the concept and applications of the therapy. The review also discusses the limitations and potential side effects of CAR-T cell therapy, including the high cost and the risk of cytokine release syndrome and neurotoxicity. While CAR-T cell therapy has shown promising results in the treatment of hematologic malignancies, ongoing research is needed to improve the efficacy and safety of the therapy and expand its use to solid tumors. With continued research and development, CAR-T cell therapy has the potential to revolutionize cancer treatment and improve outcomes for patients with cancer.

(Source: Biorender)

(Source: Biorender)

Similar content being viewed by others
References
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021. Epub 2021/04/06. https://doi.org/10.1002/ijc.33588. PubMed PMID: 33818764.
Labanieh L, Majzner RG, Mackall CL. Programming CAR-T cells to kill cancer. Nat Biomed Eng. 2018;2(6):377–91. Epub 2019/04/24. https://doi.org/10.1038/s41551-018-0235-9. PubMed PMID: 31011197.
Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–98. Epub 2013/04/04. https://doi.org/10.1158/2159-8290.CD-12-0548. PubMed PMID: 23550147; PubMed Central PMCID: PMCPMC3667586.
Guedan S, Calderon H, Posey AD, Jr., Maus MV. Engineering and design of chimeric antigen receptors. Mol Ther Methods Clin Dev. 2019;12:145–56. Epub 2019/01/23. https://doi.org/10.1016/j.omtm.2018.12.009. PubMed PMID: 30666307; PubMed Central PMCID: PMCPMC6330382.
Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125(26):4017–23. Epub 2015/05/23. https://doi.org/10.1182/blood-2014-12-580068. PubMed PMID: 25999455; PubMed Central PMCID: PMCPMC4481592.
James SE, Greenberg PD, Jensen MC, Lin Y, Wang J, Till BG, et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J Immunol. 2008;180(10):7028–38. Epub 2008/05/06. https://doi.org/10.4049/jimmunol.180.10.7028. PubMed PMID: 18453625; PubMed Central PMCID: PMCPMC2585549.
Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13(18 Pt 1):5426–35. Epub 2007/09/15. https://doi.org/10.1158/1078-0432.Ccr-07-0674. PubMed PMID: 17855649.
Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172(1):104–13. Epub 2003/12/23. https://doi.org/10.4049/jimmunol.172.1.104. PubMed PMID: 14688315.
Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–84. Epub 2004/02/13. https://doi.org/10.1038/sj.leu.2403302. PubMed PMID: 14961035.
Teng MW, Kershaw MH, Moeller M, Smyth MJ, Darcy PK. Immunotherapy of cancer using systemically delivered gene-modified human T lymphocytes. Hum Gene Ther. 2004;15(7):699–708. Epub 2004/07/10. https://doi.org/10.1089/1043034041361235. PubMed PMID: 15242530.
Tang XY, Sun Y, Zhang A, Hu GL, Cao W, Wang DH, et al. Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label phase I trial protocol. BMJ Open. 2016;6(12):e013904. Epub 2017/01/01. https://doi.org/10.1136/bmjopen-2016-013904. PubMed PMID: 28039295; PubMed Central PMCID: PMCPMC5223707.
Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18(2):413–20. Epub 2009/09/24. https://doi.org/10.1038/mt.2009.210. PubMed PMID: 19773745; PubMed Central PMCID: PMCPMC2839303.
Maus MV, June CH. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer Res. 2016;22(8):1875–84. Epub 2016/04/17. https://doi.org/10.1158/1078-0432.CCR-15-1433. PubMed PMID: 27084741; PubMed Central PMCID: PMCPMC4843171.
Yu S, Li A, Liu Q, Li T, Yuan X, Han X, et al. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10(1):78. Epub 2017/03/31. https://doi.org/10.1186/s13045-017-0444-9. PubMed PMID: 28356156; PubMed Central PMCID: PMCPMC5372296.
Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15(8):1145–54. https://doi.org/10.1517/14712598.2015.1046430.
Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang C-H, Saso K, et al. A novel chimeric antigen receptor containing a JAK–STAT signaling domain mediates superior antitumor effects. Nat Med. 2018;24(3):352–9. https://doi.org/10.1038/nm.4478.
Hosseinkhani N, Derakhshani A, Kooshkaki O, Abdoli Shadbad M, Hajiasgharzadeh K, Baghbanzadeh A, et al. Immune checkpoints and CAR-T cells: the pioneers in future cancer therapies? Int J Mol Sci. 2020;21(21). Epub 2020/11/11. https://doi.org/10.3390/ijms21218305. PubMed PMID: 33167514; PubMed Central PMCID: PMCPMC7663909.
Schwella N, Braun A, Ahrens N, Rick O, Salama A. Leukapheresis after high-dose chemotherapy and autologous peripheral blood progenitor cell transplantation: a novel approach to harvest a second autograft. Transfusion. 2003;43(2):259–64. Epub 2003/02/01. https://doi.org/10.1046/j.1537-2995.2003.00306.x. PubMed PMID: 12559023.
McGuirk J, Waller EK, Qayed M, Abhyankar S, Ericson S, Holman P, et al. Building blocks for institutional preparation of CTL019 delivery. Cytotherapy. 2017;19(9):1015–24. Epub 2017/07/30. https://doi.org/10.1016/j.jcyt.2017.06.001. PubMed PMID: 28754600.
Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–64. Epub 2009/04/23. https://doi.org/10.1038/mt.2009.83. PubMed PMID: 19384291; PubMed Central PMCID: PMCPMC2805264.
Pang Y, Hou X, Yang C, Liu Y, Jiang G. Advances on chimeric antigen receptor-modified T-cell therapy for oncotherapy. Mol Cancer. 2018;17(1):91. Epub 2018/05/18. https://doi.org/10.1186/s12943-018-0840-y. PubMed PMID: 29769134; PubMed Central PMCID: PMCPMC5956614.
Riet T, Holzinger A, Dörrie J, Schaft N, Schuler G, Abken H. Nonviral RNA transfection to transiently modify T cells with chimeric antigen receptors for adoptive therapy. Methods Mol Biol. 2013;969:187–201. Epub 2013/01/09. https://doi.org/10.1007/978-1-62703-260-5_12. PubMed PMID: 23296935.
Crossrates M. Novartis receives first ever FDA approval for a CAR-T cell therapy, Kymriah (TM) (CTL019), for children and young adults with B-cell ALL that is refractory or has relapsed at least twice. 2017.
Davila ML, Brentjens RJ. CD19-targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2016;14(10):802–8. Epub 2016/12/09. PubMed PMID: 27930631; PubMed Central PMCID: PMCPMC5536094.
Fala L. Yescarta (axicabtagene ciloleucel) second CAR T-cell therapy approved for patients with certain types of large B-cell lymphoma. 2018.
Munshi NC, Anderson LD, Jr., Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–16. Epub 2021/02/25. https://doi.org/10.1056/NEJMoa2024850. PubMed PMID: 33626253.
Roex G, Timmers M, Wouters K, Campillo-Davo D, Flumens D, Schroyens W, et al. Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. J Hematol Oncol. 2020;13(1):164. https://doi.org/10.1186/s13045-020-01001-1.
Yang J, Zhou W, Li D, Niu T, Wang W. BCMA-targeting chimeric antigen receptor T-cell therapy for multiple myeloma. Cancer Lett. 2023;553:215949. Epub 2022/10/11. https://doi.org/10.1016/j.canlet.2022.215949. PubMed PMID: 36216149.
Markham A. Belantamab mafodotin: first approval. Drugs. 2020;80(15):1607–13.
Baines AC, Ershler R, Kanapuru B, Xu Q, Shen G, Li L, et al. FDA approval summary: belantamab mafodotin for patients with relapsed or refractory multiple myeloma. Clin Cancer Res. 2022;28(21):4629–33.
Chekol Abebe E, Yibeltal Shiferaw M, Tadele Admasu F, Asmamaw Dejenie T. Ciltacabtagene autoleucel: the second anti-BCMA CAR T-cell therapeutic armamentarium of relapsed or refractory multiple myeloma. Front Immunol. 2022;13:991092. Epub 20220902. https://doi.org/10.3389/fimmu.2022.991092. PubMed PMID: 36119032; PubMed Central PMCID: PMCPMC9479060.
Hua G, Scanlan R, Straining R, Carlson DS. Teclistamab-cqyv: the first bispecific T-cell engager antibody for the treatment of patients with relapsed or refractory multiple myeloma. J Adv Pract Oncol. 2023;14(2):163–71. Epub 20230301. https://doi.org/10.6004/jadpro.2023.14.2.7. PubMed PMID: 37009408; PubMed Central PMCID: PMCPMC10062534.
Martin TG, Rossi A, Essell JH, Siegel DSD, Mailankody S, Saini N, et al. A first-in-human phase 1, multicenter, open-label study of CB-011, a next-generation CRISPR-genome edited allogeneic anti-BCMA immune-cloaked CAR-T cell therapy, in patients with relapsed/refractory multiple myeloma (CAMMOUFLAGE trial). J Clini Oncol. 2023;41(16_suppl):TPS8063-TPS. https://doi.org/10.1200/JCO.2023.41.16_suppl.TPS8063.
Cao G, Zhang G, Liu M, Liu J, Wang Q, Zhu L, et al. GPC3-targeted CAR-T cells secreting B7H3-targeted BiTE exhibit potent cytotoxicity activity against hepatocellular carcinoma cell in the in vitro assay. Biochem Biophys Rep. 2022;31:101324. Epub 2022/08/30. https://doi.org/10.1016/j.bbrep.2022.101324. PubMed PMID: 36032401; PubMed Central PMCID: PMCPMC9399963.
Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi J, et al. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin Cancer Res. 2020;26(15):3979–89. Epub 2020/05/07. https://doi.org/10.1158/1078-0432.Ccr-19-3259. PubMed PMID: 32371538.
Xie C, Cecilia Monge BM, Mabry-Hrones D, Coffman KL, Hicks S, Redd B, et al. A phase I study of GPC3 targeted CAR-T cell therapy in advanced GPC3-expressing hepatocellular carcinoma (HCC). J Clin Oncol. 2023;41(4_suppl):TPS624-TPS. https://doi.org/10.1200/JCO.2023.41.4_suppl.TPS624.
Durgin JS, Henderson F, Jr., Nasrallah MP, Mohan S, Wang S, Lacey SF, et al. Case report: prolonged survival following EGFRvIII CAR T cell treatment for recurrent glioblastoma. Front Oncol. 2021;11:669071. Epub 2021/05/25. https://doi.org/10.3389/fonc.2021.669071. PubMed PMID: 34026647; PubMed Central PMCID: PMCPMC8138201.
O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399). Epub 2017/07/21. https://doi.org/10.1126/scitranslmed.aaa0984. PubMed PMID: 28724573; PubMed Central PMCID: PMCPMC5762203.
Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151. Epub 2014/11/08. https://doi.org/10.1126/scitranslmed.3010162. PubMed PMID: 25378643; PubMed Central PMCID: PMCPMC4373413.
Adusumilli PS, Zauderer MG, Rivière I, Solomon SB, Rusch VW, O'Cearbhaill RE, et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 2021;11(11):2748–63. Epub 2021/07/17. https://doi.org/10.1158/2159-8290.Cd-21-0407. PubMed PMID: 34266984; PubMed Central PMCID: PMCPMC8563385.
Castelletti L, Yeo D, van Zandwijk N, Rasko JEJ. Anti-mesothelin CAR T cell therapy for malignant mesothelioma. Biomark Res. 2021;9(1):11. https://doi.org/10.1186/s40364-021-00264-1.
Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov. 2016;6(2):133–46. Epub 2015/10/28. https://doi.org/10.1158/2159-8290.Cd-15-0583. PubMed PMID: 26503962; PubMed Central PMCID: PMCPMC4744527.
Budi HS, Ahmad FN, Achmad H, Ansari MJ, Mikhailova MV, Suksatan W, et al. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor (CAR) for tumor immunotherapy; recent progress. Stem Cell Res Ther. 2022;13(1):40. https://doi.org/10.1186/s13287-022-02719-0.
Xu J, Meng Q, Sun H, Zhang X, Yun J, Li B, et al. HER2-specific chimeric antigen receptor-T cells for targeted therapy of metastatic colorectal cancer. Cell Death Dis. 2021;12(12):1109. Epub 20211127. https://doi.org/10.1038/s41419-021-04100-0. PubMed PMID: 34839348; PubMed Central PMCID: PMCPMC8627513.
Amatya C, Pegues MA, Lam N, Vanasse D, Geldres C, Choi S, et al. Development of CAR T cells expressing a suicide gene plus a chimeric antigen receptor targeting signaling lymphocytic-activation molecule F7. Mol Ther. 2021;29(2):702–17. Epub 20201014. https://doi.org/10.1016/j.ymthe.2020.10.008. PubMed PMID: 33129371; PubMed Central PMCID: PMCPMC7854354.
Mailankody S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, et al. GPRC5D-targeted CAR T cells for myeloma. N Engl J Med. 2022;387(13):1196–206. https://doi.org/10.1056/NEJMoa2209900. (PubMed PMID: 36170501).
Smith EL, Harrington K, Staehr M, Masakayan R, Jones J, Long TJ, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11(485). https://doi.org/10.1126/scitranslmed.aau7746. PubMed PMID: 30918115; PubMed Central PMCID: PMCPMC7508042.
Tang X, Zhou Y, Li W, Tang Q, Chen R, Zhu J, et al. T cells expressing a LMP1-specific chimeric antigen receptor mediate antitumor effects against LMP1-positive nasopharyngeal carcinoma cells in vitro and in vivo. J Biomed Res. 2014;28(6):468–75. Epub 20141201. https://doi.org/10.7555/jbr.28.20140066. PubMed PMID: 25469116; PubMed Central PMCID: PMCPMC4250525.
Münz C. Redirecting T cells against Epstein–Barr virus infection and associated oncogenesis. Cells. 2020;9(6). Epub 20200604. https://doi.org/10.3390/cells9061400. PubMed PMID: 32512847; PubMed Central PMCID: PMCPMC7349826.
Peng H, Nerreter T, Mestermann K, Wachter J, Chang J, Hudecek M, et al. ROR1-targeting switchable CAR-T cells for cancer therapy. Oncogene. 2022;41(34):4104–14. Epub 20220720. https://doi.org/10.1038/s41388-022-02416-5. PubMed PMID: 35859167; PubMed Central PMCID: PMCPMC9398970.
Wallstabe L, Göttlich C, Nelke LC, Kühnemundt J, Schwarz T, Nerreter T, et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight. 2019;4(18). Epub 20190919. https://doi.org/10.1172/jci.insight.126345. PubMed PMID: 31415244; PubMed Central PMCID: PMCPMC6795380.
Shi H, Yu F, Mao Y, Ju Q, Wu Y, Bai W, et al. EphA2 chimeric antigen receptor-modified T cells for the immunotherapy of esophageal squamous cell carcinoma. J Thorac Dis. 2018;10(5):2779–88. https://doi.org/10.21037/jtd.2018.04.91.PubMedPMID:29997940;PubMedCentralPMCID:PMCPMC6006048.
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–55. Epub 20150820. https://doi.org/10.1038/nrc3982. PubMed PMID: 26289314.
Tarp MA, Sørensen AL, Mandel U, Paulsen H, Burchell J, Taylor-Papadimitriou J, et al. Identification of a novel cancer-specific immunodominant glycopeptide epitope in the MUC1 tandem repeat. Glycobiology. 2007;17(2):197–209. Epub 20061018. https://doi.org/10.1093/glycob/cwl061. PubMed PMID: 17050588.
Posey AD, Jr., Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity. 2016;44(6):1444–54. Epub 2016/06/23. https://doi.org/10.1016/j.immuni.2016.05.014. PubMed PMID: 27332733; PubMed Central PMCID: PMCPMC5358667.
Künkele A, Taraseviciute A, Finn LS, Johnson AJ, Berger C, Finney O, et al. Preclinical assessment of CD171-directed CAR T-cell adoptive therapy for childhood neuroblastoma: CE7 epitope target safety and product manufacturing feasibility. Clin Cancer Res. 2017;23(2):466–77. Epub 20160707. https://doi.org/10.1158/1078-0432.Ccr-16-0354. PubMed PMID: 27390347.
Golinelli G, Grisendi G, Prapa M, Bestagno M, Spano C, Rossignoli F, et al. Targeting GD2-positive glioblastoma by chimeric antigen receptor empowered mesenchymal progenitors. Cancer Gene Ther. 2020;27(7–8):558–70. Epub 20181122. https://doi.org/10.1038/s41417-018-0062-x. PubMed PMID: 30464207; PubMed Central PMCID: PMCPMC7445885.
Yvon E, Del Vecchio M, Savoldo B, Hoyos V, Dutour A, Anichini A, et al. Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin Cancer Res. 2009;15(18):5852–60. Epub 20090908. https://doi.org/10.1158/1078-0432.Ccr-08-3163. PubMed PMID: 19737958; PubMed Central PMCID: PMCPMC2745508.
Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86(24):10024–8. Epub 1989/12/01. https://doi.org/10.1073/pnas.86.24.10024. PubMed PMID: 2513569; PubMed Central PMCID: PMCPMC298636.
Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90(2):720–4. Epub 1993/01/15. https://doi.org/10.1073/pnas.90.2.720. PubMed PMID: 8421711; PubMed Central PMCID: PMCPMC45737.
Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20(1):70–5. Epub 2001/12/26. https://doi.org/10.1038/nbt0102-70. PubMed PMID: 11753365.
Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9(3):279–86. Epub 2003/02/13. https://doi.org/10.1038/nm827. PubMed PMID: 12579196.
Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21(4):904–12. Epub 2013/02/21. https://doi.org/10.1038/mt.2013.17. PubMed PMID: 23423337; PubMed Central PMCID: PMCPMC5189272.
Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. Epub 2011/08/13. https://doi.org/10.1056/NEJMoa1103849. PubMed PMID: 21830940; PubMed Central PMCID: PMCPMC3387277.
Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. Epub 2013/03/22. https://doi.org/10.1126/scitranslmed.3005930. PubMed PMID: 23515080; PubMed Central PMCID: PMCPMC3742551.
Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15(8):1145–54. Epub 2015/05/20. https://doi.org/10.1517/14712598.2015.1046430. PubMed PMID: 25985798.
Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543(7643):113–7. Epub 2017/02/23. https://doi.org/10.1038/nature21405. PubMed PMID: 28225754; PubMed Central PMCID: PMCPMC5558614.
Cartron G, Fox CP, Liu FF, Kostic A, Hasskarl J, Li D, et al. Matching-adjusted indirect treatment comparison of chimeric antigen receptor T-cell therapies for third-line or later treatment of relapsed or refractory large B-cell lymphoma: lisocabtagene maraleucel versus tisagenlecleucel. Exp Hematol Oncol. 2022;11(1):1–16.
CRS CRS, NT NT, Lymphohistiocytosis H. US Food and Drug Administration approves Bristol Myers Squibb’s and bluebird bio’s Abecma (idecabtagene vicleucel), the first anti-BCMA CAR T cell therapy for relapsed or refractory multiple myeloma. Abecma is a first-in-class BCMA-directed personalized immune cell therapy delivered as a one-time infusion for triple-class exposed patients with multiple survival. 2021;5:11–2.
Mian A, Hill BT. Brexucabtagene autoleucel for the treatment of relapsed/refractory mantle cell lymphoma. Expert Opin Biol Ther. 2021;21(4):435–41.
St-Pierre F, Gordon LI. Lisocabtagene maraleucel in the treatment of relapsed/refractory large B-cell lymphoma. Future Oncol. 2022;(0).
Cooper ML, Choi J, Staser K, Ritchey JK, Devenport JM, Eckardt K, et al. An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia. 2018;32(9):1970–83. Epub 2018/02/28. https://doi.org/10.1038/s41375-018-0065-5. PubMed PMID: 29483708; PubMed Central PMCID: PMCPMC6102094.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185–99. Epub 2020/01/05. https://doi.org/10.1038/s41573-019-0051-2. PubMed PMID: 31900462.
Morgan MA, Büning H, Sauer M, Schambach A. Use of cell and genome modification technologies to generate improved “off-the-shelf” CAR T and CAR NK Cells. Front Immunol. 2020;11:1965. Epub 2020/09/10. https://doi.org/10.3389/fimmu.2020.01965. PubMed PMID: 32903482; PubMed Central PMCID: PMCPMC7438733.
León-Triana O, Pérez-Martínez A, Ramírez-Orellana M, Pérez-García VM. Dual-target CAR-Ts with on- and off-tumour activity may override immune suppression in solid cancers: a mathematical proof of concept. Cancers (Basel). 2021;13(4). Epub 2021/02/13. https://doi.org/10.3390/cancers13040703. PubMed PMID: 33572301; PubMed Central PMCID: PMCPMC7916125.
Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419–31. https://doi.org/10.1038/s41591-021-01436-0.
Mei H, Li C, Jiang H, Zhao X, Huang Z, Jin D, et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J Hematol Oncol. 2021;14(1):161. https://doi.org/10.1186/s13045-021-01170-7.
Zeng W, Zhang Q, Zhu Y, Ou R, Peng L, Wang B, et al. Engineering novel CD19/CD22 dual-target CAR-T cells for improved anti-tumor activity. Cancer Invest. 2022;40(3):282–92. Epub 2021/11/20. https://doi.org/10.1080/07357907.2021.2005798. PubMed PMID: 34797742.
van der Schans JJ, van de Donk N, Mutis T. Dual targeting to overcome current challenges in multiple myeloma CAR T-cell treatment. Front Oncol. 2020;10:1362. Epub 2020/08/28. https://doi.org/10.3389/fonc.2020.01362. PubMed PMID: 32850436; PubMed Central PMCID: PMCPMC7419675.
Faust JR, Hamill D, Kolb EA, Gopalakrishnapillai A, Barwe SP. Mesothelin: an immunotherapeutic target beyond solid tumors. Cancers (Basel). 2022;14(6). Epub 2022/03/26. https://doi.org/10.3390/cancers14061550. PubMed PMID: 35326701; PubMed Central PMCID: PMCPMC8946840.
Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8.
Donnadieu E, Dupré L, Pinho LG, Cotta-de-Almeida V. Surmounting the obstacles that impede effective CAR T cell trafficking to solid tumors. J Leucocyte Biol. 2020;108(4):1067–79.
Ajina A, Maher J. Prospects for combined use of oncolytic viruses and CAR T-cells. J Immunother Cancer. 2017;5:1–27.
Morgan RA. Human tumor xenografts: the good, the bad, and the ugly. Mol Ther. 2012;20(5):882–4.
Jiang X, Xu J, Liu M, Xing H, Wang Z, Huang L, et al. Adoptive CD8+ T cell therapy against cancer: challenges and opportunities. Cancer Lett. 2019;462:23–32.
Hu J, Sun C, Bernatchez C, Xia X, Hwu P, Dotti G, et al. T-cell homing therapy for reducing regulatory T cells and preserving effector T-cell function in large solid tumors T-cell infiltration therapy for solid tumors. Clin Cancer Res. 2018;24(12):2920–34.
Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–41.
Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334(6062):1573–7.
Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Can Res. 2002;62(5):1462–70.
Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–107.
Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36(5):471–82.
Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20(3):326–36.
Castella M, Caballero-Baños M, Ortiz-Maldonado V, González-Navarro EA, Suñé G, Antoñana-Vidósola A, et al. Point-of-care CAR T-cell production (ARI-0001) using a closed semi-automatic bioreactor: experience from an academic phase I clinical trial. Front Immunol. 2020;11:482. Epub 2020/06/13. https://doi.org/10.3389/fimmu.2020.00482. PubMed PMID: 32528460; PubMed Central PMCID: PMCPMC7259426.
Jackson Z, Roe A, Sharma AA, Lopes F, Talla A, Kleinsorge-Block S, et al. Automated manufacture of autologous CD19 CAR-T cells for treatment of Non-hodgkin lymphoma. Front Immunol. 2020;11:1941. Epub 2020/08/28. https://doi.org/10.3389/fimmu.2020.01941. PubMed PMID: 32849651; PubMed Central PMCID: PMCPMC7427107.
Rohit Reddy S, Llukmani A, Hashim A, Haddad DR, Patel DS, Ahmad F, et al. The role of chimeric antigen receptor-T cell therapy in the treatment of hematological malignancies: advantages, trials, and tribulations, and the road ahead. Cureus. 2021;13(2):e13552. Epub 20210225. https://doi.org/10.7759/cureus.13552. PubMed PMID: 33815972; PubMed Central PMCID: PMCPMC8007123.
Brentjens RJ, Curran KJ. Novel cellular therapies for leukemia: CAR-modified T cells targeted to the CD19 antigen. Hematol Am Soc Hematol Educ Program. 2012;2012:143–51. Epub 2012/12/13. https://doi.org/10.1182/asheducation-2012.1.143. PubMed PMID: 23233573; PubMed Central PMCID: PMCPMC5536093.
Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8(10):1219–26. https://doi.org/10.1158/2159-8290.Cd-18-0442.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69.
Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Investig. 2019;129(8):3464.
Zah E, Lin M-Y, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells bispecific T cells prevent B-cell antigen escape. Cancer Immunol Res. 2016;4(6):498–508.
Green DJ, Pont M, Sather BD, Cowan AJ, Turtle CJ, Till BG, et al. Fully human Bcma targeted chimeric antigen receptor T cells administered in a defined composition demonstrate potency at low doses in advanced stage high risk multiple myeloma. Blood. 2018;132(Supplement 1):1011.
Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–9.
Lemoine J, Ruella M, Houot R. Born to survive: how cancer cells resist CAR T cell therapy. J Hematol Oncol. 2021;14(1):199. https://doi.org/10.1186/s13045-021-01209-9.
Liu Z, Zhou Z, Dang Q, Xu H, Lv J, Li H, et al. Immunosuppression in tumor immune microenvironment and its optimization from CAR-T cell therapy. Theranostics. 2022;12(14):6273–90. Epub 2022/09/29. https://doi.org/10.7150/thno.76854. PubMed PMID: 36168626; PubMed Central PMCID: PMCPMC9475465.
Kailayangiri S, Altvater B, Wiebel M, Jamitzky S, Rossig C. Overcoming heterogeneity of antigen expression for effective CAR T cell targeting of cancers. Cancers (Basel). 2020;12(5). Epub 20200426. https://doi.org/10.3390/cancers12051075. PubMed PMID: 32357417; PubMed Central PMCID: PMCPMC7281243.
Gumber D, Wang LD. Improving CAR-T immunotherapy: overcoming the challenges of T cell exhaustion. EBioMedicine. 2022;77:103941. Epub 20220315. https://doi.org/10.1016/j.ebiom.2022.103941. PubMed PMID: 35301179; PubMed Central PMCID: PMCPMC8927848.
Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16(9):566–81. Epub 2016/08/24. https://doi.org/10.1038/nrc.2016.97. PubMed PMID: 27550819; PubMed Central PMCID: PMCPMC5543811.
Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147–67.
Li D, Li N, Zhang YF, Fu H, Feng M, Schneider D, et al. Persistent polyfunctional chimeric antigen receptor T cells that target glypican 3 eliminate orthotopic hepatocellular carcinomas in mice. Gastroenterology. 2020;158(8):2250–65.e20. Epub 2020/02/16. https://doi.org/10.1053/j.gastro.2020.02.011. PubMed PMID: 32060001; PubMed Central PMCID: PMCPMC7282931.
Xue W, Chen S, Yin H, Tammela T, Papagiannakopoulos T, Joshi NS, et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature. 2014;514(7522):380–4. Epub 2014/08/15. https://doi.org/10.1038/nature13589. PubMed PMID: 25119044; PubMed Central PMCID: PMCPMC4199937.
Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–30. Epub 2016/05/22. https://doi.org/10.1182/blood-2016-04-703751. PubMed PMID: 27207799; PubMed Central PMCID: PMCPMC4929924.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25(4):625–38. Epub 2018/12/29. https://doi.org/10.1016/j.bbmt.2018.12.758. PubMed PMID: 30592986.
Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20(2):119–22. Epub 2014/03/29. https://doi.org/10.1097/ppo.0000000000000035. PubMed PMID: 24667956; PubMed Central PMCID: PMCPMC4119809.
Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J Hematol Oncol. 2017;10(1):53. Epub 2017/02/23. https://doi.org/10.1186/s13045-017-0423-1. PubMed PMID: 28222796; PubMed Central PMCID: PMCPMC5320663.
Choi G, Shin G, Bae S. Price and prejudice? The value of chimeric antigen receptor (CAR) T-cell therapy. Int J Environ Res Public Health. 2022;19(19). Epub 2022/10/15. https://doi.org/10.3390/ijerph191912366. PubMed PMID: 36231661; PubMed Central PMCID: PMCPMC9566791.
Funding
This research received no funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, literature search, writing, review and editing P.D., literature search, writing, A.D. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with animals/human participants performed by any of the authors.
Informed consent
Not applicable.
Research involving human participants and/or animals
No.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dabas, P., Danda, A. Revolutionizing cancer treatment: a comprehensive review of CAR-T cell therapy. Med Oncol 40, 275 (2023). https://doi.org/10.1007/s12032-023-02146-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02146-y