Abstract
Programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) is a negative modulatory signaling pathway for activation of T cell. It is acknowledged that PD-1/PD-L1 axis plays a crucial role in the progression of tumor by altering status of immune surveillance. As one of the most promising immune therapy strategies, PD-1/PD-L1 inhibitor is a breakthrough for the therapy of some refractory tumors. However, response rate of PD-1/PD-L1 inhibitors in overall patients is unsatisfactory, which limits the application in clinical practice. Therefore, biomarkers which could effectively predict the efficacy of PD-1/PD-L1 inhibitors are crucial for patient selection. Biomarkers reflecting tumor immune microenvironment and tumor cell intrinsic features, such as PD-L1 expression, density of tumor infiltrating lymphocyte (TIL), tumor mutational burden, and mismatch-repair (MMR) deficiency, have been noticed to associate with treatment effect of anti-PD-1/anti-PD-L1 therapy. Furthermore, gut microbiota, circulating biomarkers, and patient previous history have been found as valuable predictors as well. Therefore establishing a comprehensive assessment framework involving multiple biomarkers would be meaningful to interrogate tumor immune landscape and select sensitive patients.
Similar content being viewed by others
Background
Novel cancer immunotherapy is the most promising cancer treatment strategy, mainly including chimeric antigen receptor T cell, bispecific antibodies and immune checkpoint inhibitors [1,2,3,4]. Programmed cell death protein 1/programmed cell death-ligand 1 (PD-1/PD-L1) axis is a vital immune checkpoint signaling pathway which could downregulate magnitude of inflammation response and maintain immune homeostasis [5]. Immune receptor tyrosine based inhibitory motif (ITIM) and immune receptor tyrosine-based switch motif (ITSM) are core structures of PD-1, which transduct extracellular signal and recruit Src homology 2 domain containing phosphatases 1/2 (SHP1/2) within the cell [6]. PD-1/PD-L1 axis impairs activation of T cell by inhibiting Ras-Raf-MEK-ERK and PI3K-AKT signaling pathways which are generally believed to promote proliferation and differentiation of T cell [7]. The inhibitory regulation of PD-1/PD-L1 is usually compared to a brake for activation of T cell [8].
In the evolution of immunity, PD-1/PD-L1 axis is an indispensable pathway to maintain immune tolerance and prevent autoimmunity diseases [9,10,11]. However, PD-1/PD-L1 axis influence the balance between tumor immune surveillance and immune resistance as well [12, 13]. Elevated PD-L1 expression on tumor cell or tumor infiltrating lymphocyte (TIL) results in the exhaustion of T cell [14], thus the attenuated tumor-specific immunity promoting tumor progression [15].
Based on the mechanism mentioned above, PD-1/PD-L1 inhibitors block the negative regulatory signal pathways and unleash T cell from exhausted status [16]. Since first PD-1/PD-L1 inhibitor (pembrolizumab) was approved by Food and Drug Administration in 2014, many immune checkpoint inhibitors have been applied in clinical practice [17, 18]. PD-1/PD-L1 inhibitors show potent and durable anti-tumor effects, especially in some refractory tumors [4, 19, 20]. Even though the relatively low response rate limits the application in patients, PD-1/PD-L1 inhibitors attract extensive attention [21,22,23].
In clinical practice, the primary problem for application of PD-1/PD-L1 inhibitors is the unsatisfactory response rate in overall patients. Therefore, patient selection should be implemented prior to PD-1/PD-L1 inhibitors therapy [24, 25]. Identifying predictive biomarkers to distinguish patients most likely to respond to immunotherapy from overall individuals would decrease treatment cost and avoid immune-related adverse events.
Tumor microenvironment related biomarkers
A possible mechanism of tumor immune escape is adaptive immune resistance, indicating the feedback that IFN-γ-induced upregulation of PD-1/PD-L1 axis could downregulate the cytokines and suppress the immune response in tumor microenvironment [26, 27]. Tumor regression induced by PD-1/PD-L1 inhibitors is influenced by some tumor microenvironment related factors such as PD-L1 status and pre-existing tumor infiltrating lymphocyte (TIL) [13, 26].
PD-L1 expression
Relationship between PD-L1 expression and therapeutic response rate
As the most widely adopted predictor, the role of PD-L1 expression has been investigated in many clinical trials (Table 1). Status of PD-L1 expression (positive/negative) is measured by proportion of PD-L1 expressing tumor cell (TC) and/or immune cell (IC). However, the conclusions from multiple trials are not consistent. Generally believed, high PD-L1 expression is related to increased response rate and clinical benefit in anti-PD-1/anti-PD-L1 therapy [28, 29]. In the phase 2 study Keynote-052, patients with urothelial cancer were treated with pembrolizumab, and increased positive predictive value was obtained along with increased PD-L1 expression cutoff value in the range of 1–10% [30]. And the subgroup with PD-L1 expression above 10% showed higher objective response rate than subgroup with PD-L1 expression below 1% (39% vs. 11%) [30]. However, the correlation between elevated PD-L1 expression and higher response rate is overthrown in some trials. In the study Checkmate-032 which involved patients with urothelial cancer, no significant difference in objective response rate (24.0% vs. 26.2%) was observed between PD-L1 expression positive subgroup (≥1%) and negative subgroup (< 1%) [31].
Many hypotheses have been put forward to explain the difference. Firstly, as the immunohistochemistry (IHC) is widely adopted in detection of PD-L1 expression, different cutoff values and scoring systems are used in separate clinical trials [24, 32]. And different antibodies and IHC platforms lead to the incomparability of results among trials as well [33]. Moreover, upregulated PD-L1 expression could be attributed to multiple causes. Intracellular oncogenic variations such as loss of PTEN and exposure to TIL-derived cytokines both contribute to upregulated PD-L1 expression [34]. However, immunity dependent PD-L1 upregulation is more meaningful to reactivate the tumor killing activity of TIL while intracellular oncogenic signaling pathway mediated upregulated PD-L1 has limited predictive value [34]. Lastly, due to intratumoral heterogeneity and dynamic alteration of PD-L1 expression along with treatment and cancer progression, the actual status of PD-L1 would be misinterpreted [35, 36].
The predictive value of PD-L1 expression in combination therapy
In spite of many limitations mentioned above, PD-L1 status is still a core predictor of treatment effect. However, this viewpoint is challenged in the context of combination strategy. A recent clinical trial interrogated the efficacy of combination strategy including atezolizumab, bevacizumab, carboplatin, and paclitaxel (ABCP) in metastatic non-squamous NSCLC patients [37]. Prognosis of patients receiving ABCP was improved significantly compared with treatment consisting of bevacizumab, carboplatin, and paclitaxel (BCP) [37]. Notably, for patients without epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) variations, ABCP group had prolonged RFS (HR = 0.77, p < 0.05, in PD-L1− patients) and OS (HR = 0.78, p = 0.02, in PD-L1− and PD-L1+ patients) regardless of PD-L1 status in comparison with BCP group [37]. Due to enhanced migration of neoantigen specific T cell and attenuated immune suppression caused by anti-angiogenesis and other treatments, it is difficult to predict alteration of immune microenvironment of PD-L1− patient post combination treatment [38, 39]. Therefore, in the context of combination of multiple drugs, the predictive value of PD-L1 expression is vague and deserves further investigation.
The heterogeneity of PD-L1 expression
Heterogeneous distribution of PD-L1+ tumor or stromal cell results in discordance between biopsy specimen and resection tissue [40]. Therefore, when resection tissue is not available, especially for some advanced cancer patients, PD-L1 expression of the whole tumor microenvironment might be displayed inaccurately [40, 41]. In the meanwhile, the probability of false negative event is increased. Notably, multiple cores biopsy showed higher sensitivity for selection of PD-L1+ patients compared with single core biopsy [40]. Besides, expression of PD-L1 variates during cancer evolution and treatment which is another obstacle to profiling immune microenvironment landscape. Kelly RJ et al. found that a significant shift from PD-L1− to PD-L1+ status in 50% advanced esophageal adenocarcinoma patients post chemo-radiation (OR = 6.5, p < 0.01) [42]. Tumor immune environment is subject to the influence of multiple factors, which determines the balance of immune surveillance and tolerance status.
TIL
TIL is a vital component influencing tumor immune microenvironment. Furthermore, TIL density has been confirmed to associate with adaptive upregulation of PD-L1 and clinical benefits [43]. Pre-existing TIL is unleashed by PD-1/PD-L1 inhibitors and then contributes to tumor regression [44, 45]. Recently, a tumor immune microenvironment model which consists of TIL status (presence or absence) and PD-L1 expression status (positive or negative) is established for immunotherapy prediction [46]. Cancer patients are classified into four types in the model, and Type I (PD-L1+TIL+) tumor is most likely to respond to PD-1/PD-L1 blockade therapy [46]. However, Type III (PD-L1+TIL−) tumor is prone to resist to monotherapy of PD-1/PD-L1 inhibitors while the combination of PD-1/PD-L1 inhibitors and adjuvant therapy recruiting T cell into tumor bed would help to reverse the resistance [46]. CD8+ TIL is believed to be a vital player in killing tumor cell directly and maintaining the immune surveillance which could be spoilt by the signaling produced by PD-1/PD-L1 axis [47]. Solomon B et al. found that high density of CD8+ TIL was related with prolonged OS (HR: 0.4, 95%CI: 0.2–0.9, p = 0.017) [47].
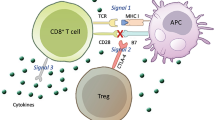
Simultaneously, in another model based on the status of TIL, tumor immune microenvironment is classified into three subtypes: immune inflamed subtype, excluded infiltrate subtype, and immune ignorance subtype [48]. Recently, transforming growth factor β (TGF-β) signaling pathway attracts extensive attention because of its influence on T cell infiltration and distribution in tumor bed [49,50,51]. Mariathasan S et al. conducted a study which enrolled metastatic urothelial cancer patients receiving atezolizumab treatment [49]. In the study, it was noticed that infiltration of T cell into tumor bed might be hampered by activated TGF-β signaling pathway in peritumoral fibroblast (Fig. 1) [49]. And simultaneously, tumor-specific T cell tended to distribute in peritumoral stroma rather than in intratumoral parenchyma [49]. The combined application of TGF-β signaling pathway blockade and PD-1/PD-L1 blockade had the significant advantage in tumor control with conversion of tumor environment from excluded infiltrate subtype to immune inflamed subtype [49,50,51]. Notably, high pan fibroblast TGF-β response signature (TGF-β, TGF-β receptor, etc.) is related with non-response and tumor progression, especially for patient belonging to excluded infiltrate subtype [49].
TIL derived interferon-γ (IFN-γ)
IFN-γ signaling pathway is a double-edged sword in immune surveillance. On the one hand, CD8+ T cell inhibits tumor cell proliferation and enhances immune activity by secreting IFN-γ. On the other hand, T cell-derived IFN-γ upregulates PD-L1 expression on tumor cell as a shield to protect tumor cells from the immune surveillance’s attack [52, 53]. Upregulated PD-L1 driven by IFN-γ is the hallmark of potential tumor killing activation which is corresponded to Type I (PD-L1+TIL+) tumor above-mentioned. IFN-γ expression is generally believed to predict a favorable immune microenvironment to anti-PD-1/PD-L1 therapy [54]. IFNG mRNA expression extracted from formalin-fixed paraffin-embedded tissue specimens is positively related with the effect of anti-PD-1/PD-L1 treatment [55]. However, with PD-1/PD-L1 blockade, constant exposure to IFN-γ leads to survival selective pressure that tumor cells with defect in IFN-γ signaling pathway are most likely to proliferate (Fig. 2) [56]. Loss of downstream signals of IFN-γ is related to adaptive drug resistance during immunotherapy [52]. As a consequence, intact IFN-γ signaling pathway is a necessary but non-sufficient determinant for robust anti-tumor effect.
The role of IFN-γ signaling pathway in adaptive immune resistance and immune surveillance. IFN-γ binds to IFN-γ receptor (IFNGR) on the tumor cell membrane and then activates associated Janus kinase (JAK). Subsequent recruitment and phosphorylation of signal transducers and activators of transcription 1 (STAT1) regulate transcription of Interferon Regulatory Factor-1(IRF-1) in nucleus. IRF-1 promotes PD-L1 expression while interferon-stimulated gene (ISG) transcription induced by phosphorylated STAT1 enhances immune response and inhibits tumor proliferation. Phosphoinositide 3-kinase (PI3K)-AKT pathway promotes activation of STAT1. Constant exposure to IFN-γ by anti-PD-1/PD-L1 results in survival selective pressure. Accumulated IFN-γ signaling pathway mutation or epigenetic alteration abrogates CD8+ T cell mediated tumor cytotoxicity
In fact, apart from IFN-γ, other inflammatory cytokines could induce adaptive immune resistance in multiple cancers. Tumor necrosis factor-α (TNF-α) mediates the de-differentiation of melanoma cell [13]. Moreover, TNF-α, Interleukin-6 (IL-6), and TGF-β are related to epithelial-to-mesenchymal transition (EMT) in multiple cancers such as melanoma and breast cancer [57, 58]. Notably, the cross-talk between TGFβ/TGFβRII pathway and PD-1/PD-L1 axis has been verified to contribute to T cell anergy in transplantation tolerance, but the mechanism should be investigated in tumor immune microenvironment further [59].
Tumor intrinsic feature related biomarkers
Tumor mutational burden
As a biomarker independent of PD-L1 expression, accumulated mutations with increased potentiality of neoantigen results in elevated immunogenicity (Fig. 3) [60, 61]. Correspondingly, activated immune microenvironment is favorable to tumor shrink in the context of anti-PD-1/PD-L1 treatment [62]. Based on Next-Generation Sequencing, it is available to profile nonsynonymous somatic mutations of tumor cell [63]. The level of tumor mutational burden (TMB) is evaluated by mutations per megabase [60]. A pooled analysis involving 27 tumor types/subtypes revealed a significant correlation between TMB and objective response rate (correlation coefficient: 0.74) [64]. Notably, clonal mutations (shared by all tumor cells) and subclonal mutations (expressing on a fraction of tumor cells) affect tumor specific immunity differently [65]. McGranahan N et al. found that homogeneous tumor with high TMB associated with increased clinical benefits and sensitivity to anti-PD-1/PD-L1 therapy [65]. However, tumor with high subclonal mutation rate tends to accompany poor anti-PD-1/PD-L1 effect [60]. Single-site biopsy might overestimate level of clonal mutation due to the interference from subclonal mutation which might explain the poor response of some patients with high TMB [62, 65].
Mechanisms of main biomarkers predicting efficacy of PD-1/PD-L1 inhibitors. Firstly, PD-L1 status reflects adaptive immune resistance which is therapeutic target of PD-1/PD-L1 inhibitors. Mismatch repair deficiency (dMMR) and high microsatellite instability (MSI-H) correlates strongly with high tumor mutational burden (TMB). In the meanwhile, TMB enhances the immunogenicity. Thirdly, tumor infiltrating lymphocyte (TIL) represents potential immune surveillance which could be reactivated by agents. Specific gut microbiota promotes differentiation of T cell, as well as lymphocyte homing and recirculation. Besides, peripheral CD14+CD16−HLA-DRhi monocyte promotes migration of T cell to tumor bed. Lastly, variation of circulating tumor DNA (ctDNA) and PD-L1+ circulating tumor cell presents effect of agent in early stage
Mismatch repair deficiency and microsatellite instability
Mismatch repair (MMR) system participates in rectifying base-base mismatch, insertion, and deletion defect during DNA replication [66]. Members belonging to MMR system including MutL homolog 1 (MLH1), MutS protein homolog 2 (MSH2), MutS homolog 6 (MSH6), and PMS1 homolog 2 (PMS2) contribute to maintaining genomic stability while reduction or depletion of MMR promotes oncogenesis, especially in gastrointestinal cancers [63, 67]. Mismatch repair deficiency (dMMR) leads to the accumulation of mutation as well as production of potential neoantigen (Fig. 3). Furthermore, MMR IHC and microsatellite instability (MSI) analyzed by Polymerase Chain Reaction (PCR) revealed a high concordance between dMMR and MSI [68]. In fact, the primary reason of MSI is epigenetic or genetic variation of MMR [69, 70]. Xiao X et al. found existence of MSI in all ovarian cancer patients with dMMR [71]. MSI-high (MSI-H)/dMMR associates with favorable prognosis of patients receiving anti-PD-1/PD-L1 therapy [72]. Kumar R et al. observed that anti-PD-1 promoted dMMR tumor cell apoptosis by cytotoxicity of CD8+ T cell in vitro in comparison with MMR proficient tumor cell [73]. Le DT et al. conducted a study to explore the influence of MSI-H on anti-PD-L1 therapy, and satisfactory treatment effect was observed (objective radiographic response rate: 53%, complete response rate: 21%) in multiple cancer patients with dMMR [74]. Enhanced treatment effect resulting from MSI-H/dMMR is attributed to increased density of TIL, elevated TMB, upregulated PD-L1 expression, and more potent tumor-specific immune response [72, 75, 76].
Oncogenic driver mutations and other mutations
It has been found that some driver mutations affect PD-L1 expression such as mutation of EGFR, Kirsten rat sarcoma viral oncogene homology (KRAS), and ALK [77]. EGFR activating mutation (mEGFR) upregulates PD-L1 expression and impedes the activation of TIL [78]. Contrary to expectation, patients harboring mEGFR tends to have poorer response in comparison with patients with wild EGFR during anti-PD-1/PD-L1 therapy. PD-L1 expression could be regulated by both extracellular immune factor and intracellular oncogenic driver signal. Given the activated EGFR-mediated PD-L1 expression by PI3K-AKT-STAT3/mTOR signaling pathways as well as simultaneous mEGFR-induced IFN-γ decline, it is hard to estimate whether PD-L1 expression is regulated just depending on EGFR status [78, 79]. Besides, mEGFR is relevant to low TMB and compromised tumor-specific immune response [78]. In contrast to mEGFR, meta-analysis revealed that NSCLC patients harboring KRAS mutation are more likely to belong to PD-L1 positive subtype [80]. And Coelho MA et al. found that hyperactive KRAS enhanced stability of PD-L1 mRNA by MEK-ERK signal pathway [81]. Notably, co-occurring mutation with mutated KRAS affects tumor microenvironment in different ways. Mutated KRAS with co-occurring serine/threonine kinase 11/liver kinase B1 variation associates with upregulated expression of PD-L1 while co-occurring mutation with TP53 accompanies high TMB abundance [81]. Moreover, ALK arrangement in inflammatory myofibroblastic tumor is related to decreased CD8+ TIL as well as downregulated PD-L1 expression [82]. Except for driver mutations, some other somatic mutations modulate tumor-specific immune response as well. Kataegis is a special mutation pattern which is caused by variation of apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3 (APOBEC3) [83]. Boichard A et al. found that Kataegis and APOBEC3 overexpression participated in regulation of PD-L1 expression [83]. Furthermore, polymerase δ1 (POLD1) and polymerase ε (POLE) variations lead to extremely high frequency of somatic mutation which affects tumor immunogenicity [84, 85].
Gut microbiota
Cross-talk between gut microbiota and host immunity influences anti-tumor effect of anti-PD-1/PD-L1 therapy and the predictive value of gut microbiota has been noticed recently (Table 2) [86]. Using mouse xenograft model, Ayelet Sivan et al. observed that fecal microbiome transplantation could restore the sensitivity to anti-PD-L1 treatment and improve anti-tumor activity in non-responding mice [87]. And increased Bifidobacterium abundance accounts for the alteration mentioned above [87]. Besides, Gopalakrishnan V et al. noticed the relationship between high abundance of Faecalibacterium genus and elevated response rate in patients receiving anti-PD-1 treatment [88]. In the meanwhile, dysbacteriosis caused by utilization of antibiotics was proved to influence the efficacy of anti-PD-1/PD-L1 therapy. And the poor response to agents could be reversed by recolonization of Akkermansia muciniphila [89]. Though the exact modulatory mechanism is unclear, many factors are proposed to enhanced tumor control (Fig. 3). Firstly, Bifidobacterium promotes maturation and activation of dendritic cell (DC) which enhances neoantigen presentation process [87]. Secondly, recolonization of Akkermansia muciniphila and Enterococcus hirae associates with appearance of CD4+ central memory T cell (TCM) in tumor bed [89]. And TCM leads to increased CD4/Foxp3+ ratio in tumor bed by enhancing recruitment and chemotactic migration of T cell [89]. Thirdly, bacteria could be sensed by host immunity and then influences the differentiation of lymphocytes such as Th1 and pTh17 in second immune organ. The alteration of microbiota composition might change the tumor immune microenvironment by the homing and recirculation of lymphocytes [89, 90]. Furthermore, bacterial metabolites such as short chain fatty acid (SCFA) participates in energy metabolism of immune cell which might affects the function of immunity [91]. Finally, potential molecular mimicry between gut microbiota and tumor might participates in tumor-specific immune response [92]. Therefore, analyzing gut microbiota composition would be favorable to predict treatment effect of anti-PD-1/PD-L1 therapy.
Biomarkers in peripheral blood
Compared with biopsy sample from tumor tissue, peripheral blood sample is more available and less heterogeneous. Due to negligible invasion, it is an ideal access to monitor shift of biomarkers in peripheral blood for optimized therapy strategy (Fig. 3) [93].
Peripheral immune cell
Using mass cytometry and bioinformatics analysis, Krieg C et al. observed that high abundance of peripheral CD14+CD16−HLA-DRhi monocyte at baseline associated with higher response rate in anti-PD-1/PD-L1 therapy. And the increased markers on membrane such as intercellular cell adhesion molecule-1 (ICAM-1) and human leukocyte antigen-antigen D related (HLA-DR) indicate enhanced migration and activation of monocyte. Besides, responding patients tended to have decreased T cell in peripheral blood in comparison with non-responding patients. Supposedly, CD14+CD16−HLA-DRhi monocyte promotes the infiltration of T cell from peripheral blood into tumor bed which results in enhanced T cell-mediated tumor killing activity [94]. Besides, Kamphorst AO et al. noticed that early expansion of peripheral PD-1+Ki-67+CD8+ T cells after anti-PD-1 treatment was related to better treatment effect. And peripheral PD-1+Ki-67+CD8+ T cell was detected to express more activation-associated markers such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and inducible T cell costimulator (ICOS) [95]. Furthermore, Fujisawa Y et al. found that neutrophil/lymphocyte ratio and lactate dehydrogenase (LDH) level associated with response to nivolumab in melanoma patients. Elevated neutrophil/lymphocyte ratio (> 2.2) predicted poor treatment effect (OR = 4.16, p = 0.0026) while increased peripheral LDH was related with poor response tendency without statistical significance (OR = 2.53, p = 0.081) [96]. Contrary to neutrophil, increased relative eosinophil count (≥1.5%) could be used as a favorable predictor in melanoma patients receiving pembrolizumab [97].
Circulating tumor DNA and PD-L1high circulating tumor cell
Radiological assessment is widely applied to evaluate the treatment effect of anti-PD-1/PD-L1. However, interference of pseudo-progression and non-real time reflection of tumor burden might affect the selection of subsequent treatment strategy [98]. It was observed that circulating tumor DNA (ctDNA) in responding patient decreased quickly in 5 days after first nivolumab administration. The phenomenon is meaningful that the shift in ctDNA is prior to second administration and radiological change [99]. Compared with detectable abundance at baseline, undetectable ctDNA after therapy beginning indicates robust anti-tumor effect which is valuable for early patient selection [98, 100]. Similarly, decreased PD-L1+ circulating tumor cell after treatment beginning is related to robust anti-tumor response. However, patients with high abundance of PD-L1+ circulating tumor cell at baseline tend to be sensitive to anti-PD-L1 therapy [101].
Soluble PD-L1
Splice variants of PD-L1 which lack transmembrane or intracellular domain lead to secretion of soluble PD-L1 (sPD-L1) [102]. Similar to membrane-binding PD-L1, sPD-L1 hampers the activation and proliferation of T cell as well [103]. It is generally acknowledged that increased level of sPD-L1 before treatment associates with poor prognosis which is attributed to high tumor burden, elevated alternative splicing, and exhausted immune response [102, 104]. Zhou J et al. found that high sPD-L1 at baseline was related with increased risk of tumor progression. However, rapidly increased sPD-L1 level after immune checkpoint inhibitors treatment indicated potent tumor-specific immune response and high partial response rate (around 70%) [102].
Peripheral cytokine and other parameters
Peripheral cytokines reflect status of tumor immune microenvironment and response to anti-PD-1/PD-L1 treatment [93]. Prolactin (PRL) participates in maturation and activation of immunity while high PRL inhibits immune response by IL-10 [105]. Adaptive hyperprolactinemia associates with poor response during nivolumab treatment and patients with stable concentration of PRL exhibit significant higher response rate (p = 0.004) [105]. Moreover, a phase 2 study revealed that pretreatment high level of IFN-γ, IL-6, and IL-10 in peripheral blood were relevant to increased objective response rate in melanoma patients receiving nivolumab [106]. Besides, tumor-derived vascular endothelial growth factor (VEGF) promotes tumor progression by angiogenesis and immunosuppression in tumor microenvironment [107]. Anti-angiogenesis therapy not only inhibits neo-vascular formation, but also upregulates the quantity of TIL significantly [108]. Patients receiving anti-PD-L1 combined with anti-VEGF therapy exhibited higher response rate than monotherapy [107, 109]. Cytokines participate in immune response directly, and the predictive value of cytokine in peripheral blood needs to explore further.
Patient previous history, pathological feature, and other predictors
Chronic obstructive pulmonary disease (COPD) participates in oncogenesis and COPD-associated chronic inflammation influences immune environment of lung cancer patient in the meanwhile [34]. Biton J et al. interrogated treatment response of lung cancer patients receiving nivolumab. Lung cancer patients with co-existing COPD tended to harbor higher inhibitory markers such as PD-1 and TIM-3, which indicated more severe exhaustion of TIL in comparison with patients without COPD [34, 110]. NSCLC patients with co-existing COPD had favorable prognosis during nivolumab treatment and increased correlation between PD-L1 expression and response rate [34]. Notably, cigarette exposure contributes to oncogenesis of lung cancer as well as occurrence of COPD [111]. Because cigarette exposure leads to increased TMB which might cause enhanced the sensitivity to immunotherapy, it is necessary to rule out the interference from cigarette exposure [112]. By analyzing TMB, KRAS, and TP53 variations in COPD+ patients, no significant enrichment of smoking signature was observed in COPD+ patients [34]. Therefore, COPD is speculated as a potential predictor for anti-PD-1/PD-L1 treatment. Besides, immune microenvironment alters among tumors with different pathological features. In three subtypes of lung adenocarcinoma, the level of TMB and immune cell signature change significantly [113]. Tumor belonging to proximal inflammatory subtype tends to have higher TMB, TP53 variation, and immune cell signature, while tumor belonging to terminal respiratory unit subtype is most likely to harbor low TMB without TP53 mutation [113]. And the predictive value of pathological feature needs to be verified in large sample size. Intriguingly, a recent pilot study revealed the correlation between family history of cancer and treatment effect of anti-PD-1/PD-L1 therapy [114]. Multiple cancers patients with family history of cancer had significantly improved objective response rate (p = 0.0024) and favorable outcome [114].
Conclusion
PD-L1 expression is generally believed as a surrogate of pre-existing immune specific immune activity and can be upregulated by IFN-γ in tumor microenvironment [115]. However, other factors simultaneously influence PD-L1 expression such as intracellular oncogenic signaling pathway apart from adaptive immune resistance. Therefore, total PD-L1 including IFN-γ-derived and IFN-γ-independent PD-L1 is not accurate to reflect tumor immune surveillance status [115]. Combination of PD-L1 expression, TIL, TMB, genetic and epigenetic variation of IFN-γ provides a comprehensive prospective on tumor immune landscape. Moreover, circulating biomarkers and gut microbiota play a vital role in dynamic monitoring of tumor immune status due to minimum invasion. With the increased understanding of tumor immune escape, establishing a wide-ranging framework which consists of multiple biomarkers is quite necessary for patient selection and precision medicine.
Abbreviations
- ALK :
-
Anaplastic lymphoma kinase
- APOBEC3:
-
Apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3
- COPD:
-
Chronic obstructive pulmonary disease
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- DC:
-
Dendritic cell
- dMMR:
-
Mismatch repair deficiency
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
epithelial-to-mesenchymal transition
- HR:
-
hazard ratio
- IC:
-
Immune cell
- ICAM-1:
-
Intercellular cell adhesion molecule-1
- ICOS:
-
Inducible T cell costimulator
- ITIM:
-
Immune receptor tyrosine based inhibitory motif
- ITSM:
-
Immune receptor tyrosine-based switch motif
- KRAS :
-
Kirsten rat sarcoma viral oncogene homology
- LDH:
-
Lactate dehydrogenase
- mEGFR :
-
EGFR activating mutation
- MLH1:
-
MutL homolog 1
- MMR:
-
Mismatch repair
- MSH2:
-
MutS protein homolog 2
- MSH6:
-
MutS homolog 6
- NSCLC:
-
Non-small-cell-lung cancer
- OS:
-
Overcall survival
- PD-1/PD-L1:
-
Programmed cell death protein 1/programmed cell death 1 ligand 1
- PMS2:
-
PMS1 homolog 2
- POLD1 :
-
Polymerase δ1
- POLE :
-
polymerase ε
- PRL:
-
Prolactin;
- SCFA:
-
Short chain fatty acid
- SHP1/2:
-
Src homology 2 domain containing phosphatases 1/2
- sPD-L1:
-
soluble PD-L1
- TC:
-
Tumor cell
- TCM :
-
Central memory T cell
- TGF-β:
-
Transforming growth factor β
- TMB:
-
tumor mutational burden
- TNF-α:
-
Tumor necrosis factor-α
- VEGF:
-
Vascular endothelial growth factor
References
Ye B, Stary CM, Li X, Gao Q, Kang C, Xiong X. Engineering chimeric antigen receptor-T cells for cancer treatment. Mol Cancer. 2018;17:32.
Yu S, Li A, Liu Q, Li T, Yuan X, Han X, et al. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10:78.
Yu S, Liu Q, Han X, Qin S, Zhao W, Li A, et al. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp Hematol Oncol. 2017;6:31.
Crist M, Balar A. Atezolizumab in invasive and metastatic urothelial carcinoma. Expert Rev Clin Pharmacol. 2017;10:1295–301.
Kythreotou A, Siddique A, Mauri FA, Bower M, Pinato DJ. PD-L1. J Clin Pathol. 2018;71:189–94.
Boussiotis VA, Chatterjee P, Li L. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J. 2014;20:265–71.
Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5 ra46
LaFleur MW, Muroyama Y, Drake CG, Sharpe AH. Inhibitors of the PD-1 pathway in tumor therapy. J Immunol. 2018;200:375–83.
Dougan M. Checkpoint blockade toxicity and immune homeostasis in the gastrointestinal tract. Front Immunol. 2017;8:1547.
Kuol N, Stojanovska L, Nurgali K, Apostolopoulos V. PD-1/PD-L1 in disease. Immunotherapy. 2018;10:149–60.
Juchem KW, Sacirbegovic F, Zhang C, Sharpe AH, Russell K, McNiff JM, et al. PD-L1 prevents the development of autoimmune heart disease in graft-versus-host disease. J Immunol. 2018;200:834–46.
Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553:91–5.
Ribas A. Adaptive immune resistance: how Cancer protects from immune attack. Cancer Discov. 2015;5:915–9.
Witt DA, Donson AM, Amani V, Moreira DC, Sanford B, Hoffman LM, et al. Specific expression of PD-L1 in RELA-fusion supratentorial ependymoma: implications for PD-1-targeted therapy. Pediatr Blood Cancer. 2018;65:e26960.
Zheng B, Ren T, Huang Y, Sun K, Wang S, Bao X, et al. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. J Hematol Oncol. 2018;11:16.
Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 blockade: have we found the key to unleash the antitumor immune response? Front Immunol. 2017;8:1597.
Karlsson AK, Saleh SN. Checkpoint inhibitors for malignant melanoma: a systematic review and meta-analysis. Clin Cosmet Investig Dermatol. 2017;10:325–39.
Liu B, Song Y, Liu D. Recent development in clinical applications of PD-1 and PD-L1 antibodies for cancer immunotherapy. J Hematol Oncol. 2017;10:174.
Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for Cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561.
Jindal V, Gupta S. Expected paradigm shift in brain metastases therapy-immune checkpoint inhibitors. Mol Neurobiol. 2018; https://doi.org/10.1007/s12035-018-0905-3.
Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9–16.
Polk A, Svane IM, Andersson M, Nielsen D. Checkpoint inhibitors in breast cancer - current status. Cancer Treat Rev. 2017;63:122–34.
Sheng Z, Zhu X, Sun Y, Zhang Y. The efficacy of anti-PD-1/PD-L1 therapy and its comparison with EGFR-TKIs for advanced non-small-cell lung cancer. Oncotarget. 2017;8:57826–35.
Ancevski Hunter K, Socinski MA, Villaruz LC. PD-L1 testing in guiding patient selection for PD-1/PD-L1 inhibitor therapy in lung Cancer. Mol Diagn Ther. 2018;22:1–10.
Janjigian YY, Sanchez-Vega F, Jonsson P, Chatila WK, Hechtman JF, Ku GY, et al. Genetic predictors of response to systemic therapy in Esophagogastric Cancer. Cancer Discov. 2018;8:49–58.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71.
Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol. 2018;11:31.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung Cancer. N Engl J Med. 2016;375:1823–33.
Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129:3419–27.
Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–92.
Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17:1590–8.
Liu D, Wang S, Bindeman W. Clinical applications of PD-L1 bioassays for cancer immunotherapy. J Hematol Oncol. 2017;10:110.
Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 immunohistochemistry assays for lung Cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12:208–22.
Biton J, Ouakrim H, Dechartres A, Alifano M, Mansuet-Lupo A, Si H, et al. Impaired tumor-infiltrating T cells in patients with COPD impacts lung Cancer response to PD-1 blockade. Am J Respir Crit Care Med. 2018; https://doi.org/10.1164/rccm.201706-1110OC.
Li D, Chen R, Wang YW, Fornace AJ Jr, Li HH. Prior irradiation results in elevated programmed cell death protein 1 (PD-1) in T cells. Int J Radiat Biol. 2017;94:488–94.
Vilain RE, Menzies AM, Wilmott JS, Kakavand H, Madore J, Guminski A, et al. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clin Cancer Res. 2017;23:5024–33.
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301.
Bocca P, Di Carlo E, Caruana I, Emionite L, Cilli M, De Angelis B, et al. Bevacizumab-mediated tumor vasculature remodelling improves tumor infiltration and antitumor efficacy of GD2-CAR T cells in a human neuroblastoma preclinical model. Oncoimmunology. 2017;7:e1378843.
McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:749–57.
Munari E, Zamboni G, Lunardi G, Marchionni L, Marconi M, Sommaggio M, et al. PD-L1 expression heterogeneity in non-small cell lung Cancer: defining criteria for harmonization between biopsy specimens and whole sections. J Thorac Oncol. 2018; https://doi.org/10.1016/j.jtho.2018.04.017.
Gradecki SE, Grange JS, Stelow EB. Concordance of PD-L1 expression between Core biopsy and resection specimens of non-small cell lung Cancer. Am J Surg Pathol. 2018; https://doi.org/10.1097/PAS.0000000000001085.
Kelly RJ, Zaidi AH, Smith MA, Omstead AN, Kosovec JE, Matsui D, et al. The dynamic and transient immune microenvironment in locally advanced esophageal adenocarcinoma post Chemoradiation. Ann Surg. 2017; https://doi.org/10.1097/SLA.0000000000002410.
Xing X, Guo J, Wen X, Ding G, Li B, Dong B, et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology. 2018;7:e1356144.
Yagi T, Baba Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, et al. PD-L1 expression, tumor-infiltrating lymphocytes, and clinical outcome in patients with surgically resected esophageal Cancer. Ann Surg. 2017; https://doi.org/10.1097/SLA.0000000000002616.
Tomioka N, Azuma M, Ikarashi M, Yamamoto M, Sato M, Watanabe KI, et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC). Breast Cancer. 2018;25:34–42.
Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–45.
Solomon B, Young RJ, Bressel M, Urban D, Hendry S, Thai A, et al. Prognostic significance of PD-L1(+) and CD8(+) immune cells in HPV(+) oropharyngeal squamous cell carcinoma. Cancer Immunol Res. 2018; https://doi.org/10.1158/2326-6066.CIR-17-0299.
Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for Cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22:1865–74.
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–8.
Ravi R, Noonan KA, Pham V, Bedi R, Zhavoronkov A, Ozerov IV, et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFbeta enhance the efficacy of cancer immunotherapy. Nat Commun. 2018;9:741.
Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11:39.
Gao Y, Yang J, Cai Y, Fu S, Zhang N, Fu X, et al. IFN-gamma-mediated inhibition of lung cancer correlates with PD-L1 expression and is regulated by PI3K-AKT signaling. Int J Cancer. 2018; https://doi.org/10.1002/ijc.31357.
Tremblay-LeMay R, Rastgoo N, Chang H. Modulating PD-L1 expression in multiple myeloma: an alternative strategy to target the PD-1/PD-L1 pathway. J Hematol Oncol. 2018;11:46.
Teng F, Meng X, Kong L, Yu J. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: a systematic review. Cancer Lett. 2018;414:166–73.
Karachaliou N, Gonzalez-Cao M, Crespo G, Drozdowskyj A, Aldeguer E, Gimenez-Capitan A, et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther Adv Med Oncol. 2018;10 1758834017749748
Abril-Rodriguez G, Ribas A. SnapShot: Immune Checkpoint Inhibitors. Cancer Cell. 2017;31:848–e1.
Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490:412–6.
Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–95.
Baas M, Besancon A, Goncalves T, Valette F, Yagita H, Sawitzki B, et al. TGFbeta-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. elife. 2016;5:e08133.
Hodges TR, Ott M, Xiu J, Gatalica Z, Swensen J, Zhou S, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro-Oncology. 2017;19:1047–57.
Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung Cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–41.
Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–608.
Yuza K, Nagahashi M, Watanabe S, Takabe K, Wakai T. Hypermutation and microsatellite instability in gastrointestinal cancers. Oncotarget. 2017;8:112103–15.
Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377:2500–1.
McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9.
Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18:85–98.
Kim ST, Klempner SJ, Park SH, Park JO, Park YS, Lim HY, et al. Correlating programmed death ligand 1 (PD-L1) expression, mismatch repair deficiency, and outcomes across tumor types: implications for immunotherapy. Oncotarget. 2017;8:77415–23.
Yamashita H, Nakayama K, Ishikawa M, Nakamura K, Ishibashi T, Sanuki K, et al. Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget. 2018;9:5652–64.
Geiersbach KB, Samowitz WS. Microsatellite instability and colorectal cancer. Arch Pathol Lab Med. 2011;135:1269–77.
Zeinalian M, Hashemzadeh-Chaleshtori M, Salehi R, Emami MH. Clinical aspects of microsatellite instability testing in colorectal Cancer. Adv Biomed Res. 2018;7:28.
Xiao X, Dong D, He W, Song L, Wang Q, Yue J, et al. Mismatch repair deficiency is associated with MSI phenotype, increased tumor-infiltrating lymphocytes and PD-L1 expression in immune cells in ovarian cancer. Gynecol Oncol. 2018;149:146–54.
Jin Z, Yoon HH. The promise of PD-1 inhibitors in gastro-esophageal cancers: microsatellite instability vs. PD-L1. J Gastrointest Oncol. 2016;7:771–88.
Kumar R, Yu F, Zhen YH, Li B, Wang J, Yang Y, et al. PD-1 blockade restores impaired function of ex vivo expanded CD8(+) T cells and enhances apoptosis in mismatch repair deficient EpCAM(+)PD-L1(+) cancer cells. Onco Targets Ther. 2017;10:3453–65.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
Mills AM, Dill EA, Moskaluk CA, Dziegielewski J, Bullock TN, Dillon PM. The relationship between mismatch repair deficiency and PD-L1 expression in breast carcinoma. Am J Surg Pathol. 2018;42:183–91.
El Jabbour T, Ross JS, Sheehan CE, Affolter KE, Geiersbach KB, Boguniewicz A, et al. PD-L1 protein expression in tumour cells and immune cells in mismatch repair protein-deficient and -proficient colorectal cancer: the foundation study using the SP142 antibody and whole section immunohistochemistry. J Clin Pathol. 2018;71:46–51.
Jiang L, Su X, Zhang T, Yin X, Zhang M, Fu H, et al. PD-L1 expression and its relationship with oncogenic drivers in non-small cell lung cancer (NSCLC). Oncotarget. 2017;8:26845–57.
Li X, Lian Z, Wang S, Xing L, Yu J. Interactions between EGFR and PD-1/PD-L1 pathway: implications for treatment of NSCLC. Cancer Lett. 2018;418:1–9.
Petrelli F, Maltese M, Tomasello G, Conti B, Borgonovo K, Cabiddu M, et al. Clinical and molecular predictors of PD-L1 expression in non-small-cell lung Cancer: systematic review and meta-analysis. Clin Lung Cancer. 2018;19:315–22.
Lan B, Ma C, Zhang C, Chai S, Wang P, Ding L, et al. Association between PD-L1 expression and driver gene status in non-small-cell lung cancer: a meta-analysis. Oncotarget. 2018;9:7684–99.
Coelho MA, de Carne TS, Rana S, Zecchin D, Moore C, Molina-Arcas M, et al. Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 mRNA. Immunity. 2017;47:1083–99. e6
Cha YJ, Shim HS. PD-L1 expression and CD8+ tumor-infiltrating lymphocytes are associated with ALK rearrangement and clinicopathological features in inflammatory myofibroblastic tumors. Oncotarget. 2017;8:89465–74.
Boichard A, Tsigelny IF, Kurzrock R. High expression of PD-1 ligands is associated with kataegis mutational signature and APOBEC3 alterations. Oncoimmunology. 2017;6:e1284719.
Gadducci A, Guerrieri ME. Immune checkpoint inhibitors in gynecological cancers: update of literature and perspectives of clinical research. Anticancer Res. 2017;37:5955–65.
Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung Cancer: a review. JAMA Oncol. 2016;2:1217–22.
Yi M, Yu S, Qin S, Liu Q, Xu H, Zhao W, et al. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J Hematol Oncol. 2018;11:47.
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–9.
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7.
Daillere R, Vetizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45:931–43.
Blacher E, Levy M, Tatirovsky E, Elinav E. Microbiome-modulated metabolites at the Interface of host immunity. J Immunol. 2017;198:572–80.
Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–84.
Mitsuhashi A, Okuma Y. Perspective on immune oncology with liquid biopsy, peripheral blood mononuclear cells, and microbiome with non-invasive biomarkers in cancer patients. Clin Transl Oncol. 2018; https://doi.org/10.1007/s12094-017-1827-7.
Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24:144–53.
Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114:4993–8.
Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, et al. Baseline neutrophil to lymphocyte ratio combined with serum LDH level associated with outcome of nivolumab immunotherapy in a Japanese advanced melanoma population. Br J Dermatol. 2018; https://doi.org/10.1111/bjd.16427.
Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al. Baseline biomarkers for outcome of melanoma patients treated with Pembrolizumab. Clin Cancer Res. 2016;22:5487–96.
Cabel L, Riva F, Servois V, Livartowski A, Daniel C, Rampanou A, et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol. 2017;28:1996–2001.
Iijima Y, Hirotsu Y, Amemiya K, Higashi S, Miyashita Y, Omata M. Rapid decrease of circulating tumor DNA predicted the treatment effect of nivolumab in a lung cancer patient within only 5 days. Respir Med Case Rep. 2017;22:31–3.
Lipson EJ, Velculescu VE, Pritchard TS, Sausen M, Pardoll DM, Topalian SL, et al. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J Immunother Cancer. 2014;2:42.
Yue C, Jiang Y, Li P, Wang Y, Xue J, Li N, et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. OncoImmunology. 2018; e1438111
Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017;5:480–92.
Kruger S, Legenstein ML, Rosgen V, Haas M, Modest DP, Westphalen CB, et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. Oncoimmunology. 2017;6:e1310358.
Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66:877–90.
Caponnetto S, Iannantuono GM, Barchiesi G, Magri V, Gelibter A, Cortesi E. Prolactin as a potential early predictive factor in metastatic non-small cell lung Cancer patients treated with Nivolumab. Oncology. 2017;93:62–6.
Yamazaki N, Kiyohara Y, Uhara H, Iizuka H, Uehara J, Otsuka F, et al. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci. 2017;108:1022–31.
Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624.
Dirkx AE, Oude Egbrink MG, Castermans K, van der Schaft DW, Thijssen VL, Dings RP, et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006;20:621–30.
Atkins MB, Plimack ER, Puzanov I, Fishman MN, McDermott DF, Cho DC, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018;19:405–15.
McKendry RT, Spalluto CM, Burke H, Nicholas B, Cellura D, Al-Shamkhani A, et al. Dysregulation of antiviral function of CD8(+) T cells in the chronic obstructive pulmonary disease lung. Role of the PD-1-PD-L1 Axis. Am J Respir Crit Care Med. 2016;193:642–51.
Kameyama N, Chubachi S, Hegab AE, Yasuda H, Kagawa S, Tsutsumi A, et al. Intermittent exposure to cigarette smoke increases lung tumors and the severity of emphysema more than continuous exposure. Am J Respir Cell Mol Biol. 2018; https://doi.org/10.1165/rcmb.2017-0375OC.
Gibbons DL, Byers LA, Kurie JM. Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 2014;12:3–13.
Faruki H, Mayhew GM, Serody JS, Hayes DN, Perou CM, Lai-Goldman M. Lung adenocarcinoma and squamous cell carcinoma gene expression subtypes demonstrate significant differences in tumor immune landscape. J Thorac Oncol. 2017;12:943–53.
Cortellini A, Bersanelli M, Buti S, Gambale E, Atzori F, Zoratto F, et al. Family history of cancer as surrogate predictor for immunotherapy with anti-PD1/PD-L1 agents: preliminary report of the FAMI-L1 study. Immunotherapy. 2018;10:643–55.
Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–52.
Yamazaki N, Takenouchi T, Fujimoto M, Ihn H, Uchi H, Inozume T, et al. Phase 1b study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE-041). Cancer Chemother Pharmacol. 2017;79:651–60.
Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for platinum- and Cetuximab-refractory head and neck Cancer: results from a single-arm. Phase II Study J Clin Oncol. 2017;35:1542–9.
Hui R, Garon EB, Goldman JW, Leighl NB, Hellmann MD, Patnaik A, et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: a phase 1 trial. Ann Oncol. 2017;28:874–81.
Daud AI, Wolchok JD, Robert C, Hwu WJ, Weber JS, Ribas A, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody Pembrolizumab in melanoma. J Clin Oncol. 2016;34:4102–9.
Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung Cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35:3924–33.
Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–22.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13.
Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–65.
Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–7.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65.
Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76.
McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34:833–42.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7.
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46.
Powles T, O'Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of Durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3:e172411.
Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter. Phase Ib Study J Clin Oncol. 2017;35:2117–24.
Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–8.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81572608, 81172422), Wuhan Science and Technology Bureau (No. 2017060201010170), and the National High Technology Research and Development Program of China (No. 2015AA020301).
Author information
Authors and Affiliations
Contributions
MY and DJ performed the selection of literature, drafted the manuscript, and prepared the Figs. HX, QL and WZ collected the related references and participated in discussion. KW and XH designed this review and revised the manuscript. All authors contributed to this manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yi, M., Jiao, D., Xu, H. et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 17, 129 (2018). https://doi.org/10.1186/s12943-018-0864-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-018-0864-3