Abstract
Background
Drought stress in juvenile stages of crop development and premature leaf senescence induced by drought stress have an impact on biomass production and yield formation of barley (Hordeum vulgare L.). Therefore, in order to get information of regulatory processes involved in the adaptation to drought stress and leaf senescence expression analyses of candidate genes were conducted on a set of 156 barley genotypes in early developmental stages, and expression quantitative trait loci (eQTL) were identified by a genome wide association study.
Results
Significant effects of genotype and treatment were detected for leaf colour measured at BBCH 25 as an indicator of leaf senescence and for the expression level of the genes analysed. Furthermore, significant correlations were detected within the group of genes involved in drought stress (r = 0.84) and those acting in leaf senescence (r = 0.64), as well as between leaf senescence genes and the leaf colour (r = 0.34). Based on these expression data and 3,212 polymorphic single nucleotide polymorphisms (SNP) with a minor allele frequency >5 % derived from the Illumina 9 k iSelect SNP Chip, eight cis eQTL and seven trans eQTL were found. Out of these an eQTL located on chromosome 3H at 142.1 cM is of special interest harbouring two drought stress genes (GAD3 and P5CS2) and one leaf senescence gene (Contig7437), as well as an eQTL on chromosome 5H at 44.5 cM in which two genes (TRIUR3 and AVP1) were identified to be associated to drought stress tolerance in a previous study.
Conclusion
With respect to the expression of genes involved in drought stress and early leaf senescence, genotypic differences exist in barley. Major eQTL for the expression of these genes are located on barley chromosome 3H and 5H. Respective markers may be used in future barley breeding programmes for improving tolerance to drought stress and leaf senescence.
Similar content being viewed by others
Background
In order to analyse genetic networks and stress response, real time polymerase chain reaction (PCR) is an important tool [1]. For several years high-throughput instruments e.g. the BioMark System from Fluidigm have enabled large scale quantitative PCR studies [2]. Because of this and the possibility to analyse a large number of genotypes easily on expression chips [2] a range of genome wide association studies (GWAS) using expression data were conducted in the last years [3–5]. Expression quantitative trait loci (eQTL) were detected first in medicinal studies in humans and later also in plants [6–10]. In plants most eQTL studies were performed for complex pathways and aimed at a better understanding of the molecular networks [11]. Whereas in biotic stress the resistance is often controlled by a single gene, responses to abiotic stresses such as drought stress are controlled by many genes [12–14] and so these processes are particularly suitable for high throughput expression analyses and genetical genomics approaches [15]. Even in early developmental stages drought stress and drought stress induced premature leaf senescence have major influences on yield formation [16]. Therefore, it is of prime importance to understand regulatory processes of drought stress [17] and leaf senescence [18].
In plants drought stress is initiated by water deficit in soil resulting in osmotic and oxidative stress and cellular damage [19]. This leads to defined drought stress responses for instance regarding the maintenance of turgor by an increase of osmoprotective molecules as soluble sugars [20–22], as well as measurable lower water content and decreased growth in the stressed plants compared to a control [23, 24]. Stress perception is assigned by special receptors, such as abscisic acid (ABA) receptors, hexokinases, or ion channel linked receptors [25]. The stress signal is then transducted for example via serine-threonine kinases, serin-threonine phosphatases, calcium dependent protein kinases, or phospholipases [25]. Finally, the gene expression is regulated by effector genes coding for late embryo abundant (LEA) proteins, dehydrin, or reactive oxygen species (ROS) and transcription factors, such as MYB, WRKY, NAC, AP2/ERF, DREB2, or bZIP to activate stress responsive mechanisms, re-establish homeostasis and protect and repair damaged proteins and membranes [13, 19, 25, 26]. Besides the above mentioned genes, drought stress associated metabolites such as osmoprotectants, polyamines and proteins involved in carbon metabolism and apoptosis are part of drought stress tolerance [12, 27]. Disturbing the regulatory processes in drought stress response results in irreversible changes of cellular homeostasis and the destruction of functional and structural proteins and membranes, leading to cell death [19] and decreased yield formation [28]. A huge transcriptome analysis for drought stress associated genes was done for example in barley [29] and wheat [30] showing differential response of genes involved in drought stress tolerance.
Initiated by external signals e.g. various stresses such as drought, as well as by internal factors for example phytohormones leaf senescence often occurs as a natural degradation process at the final stage of plant development [31]. Drought stress induced leaf senescence proceeds in three steps. Perception of drought stress is the initiation phase in which senescence signals are transferred via senescence associated genes (SAG) [32]. These are regulatory genes which often encode transcription factors regulating gene expression by binding to distinct cis-elements of target genes [33]. In the following reorganisation phase resources are transported from source (e.g. roots, leaves) to sink (e.g. fruits, seed) organs being important for yield formation [34]. With this translocation chlorophyll, proteins, lipids and other macromolecules are degraded and the content of antioxidants, ABA and ROS increases induced by a change in gene expression [35, 36]. Differentially expressed genes and their regulation during leaf senescence were identified by transcriptome analysis using microarrays in Arabidopsis thaliana [37, 38]. While the genes for photosynthesis and chloroplast development are down-regulated, the genes for the degradation of macromolecules and recycling of resources are up-regulated [39]. For example, expressed genes for chlorophyll degradation are PA42, Lhcb4 and psbA [40] and genes for N mobilization and transport are transcription factors WRKY [41] and NAC [42] as well as glutamine synthetase [38]. Genes differentially expressed can be grouped to those accelerating leaf senescence and genes delaying leaf senescence [43]. The latter possibly resulting in a “stay green” effect and improved drought tolerance [34, 44]. The reorganisation phase is the crucial step for reversibility, after which senescence is irreversible and leads to the final step where leaves and cells often die [45].
In barley (Hordeum vulgare L.), a crop plant of worldwide importance, most mechanisms for leaf senescence are still not well understood [18, 34]. The response to drought in juvenile stages is less well documented, as only few studies are focused on early developmental stages [20, 24, 46, 47] whereas a lot of studies were conducted for drought stress in the generative stage [48]. Nevertheless, barley is to some extent a model organism for research at a genome wide level. The barley gene space has been published [49] and with this information gene positions can be compared to these data. Comparing the position of the analysed genes in the Morex genome with positions of the detected eQTL, resulted in the co-localization of eQTL and genes involved in drought stress [11, 50]. Therefore, the present study aimed at the identification of eQTL in barley for genes involved in drought stress in the juvenile phase and early leaf senescence (Table 1) based on a genome wide association study.
Results
Leaf senescence
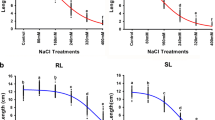
Leaf colour (SPAD, soil plant analysis development) measured at 20 days after drought stress induction (BBCH 25, according to Stauss [51]) being indicative for leaf senescence revealed significant differences between treatments and genotypes but no significant interaction of genotype and treatment was observed at this stage (Fig. 1 and Table 2) giving hint to physiological changes and changes in gene expression.
Relative expression of candidate genes
At the same developmental stage (BBCH 25) expression analyses were conducted for the whole set of 156 genotypes analysing 14 genes (Table 1). The relative expression (-∆∆Ct) ranges from −8.5 to 14.9 (Fig. 2, Additional file 1). In most genotypes all five drought stress related genes (A1, Dhn1, GAD3, NADP_ME and P5CS2) showed a higher expression under stress treatment relative to the control whereas for genes involved in leaf senescence opposite effects were detected for all genes (GSII, hv_36467, LHC1b20 and pHvNF-Y5α) except Contig7437. The genes out of the GWAS [20], i.e. AVP1 and TRIUR3 which are drought stress related genes, were up-regulated, whereas SAPK9 and ETFQO showed a lower expression relative to the control. In total, eight genes were up and six genes were down-regulated relative to the control but not all genotypes responded in the same way.
The mean quality score for all amplifications was 0.954. Because ∆Ct and ∆∆Ct values were not normally distributed (data not shown) further statistical analysis was done with logarithmic values (log2). Analysis of variance (ANOVA) revealed significant (p <0.001) effects for genotype and treatment for the 14 genes except Contig7437 (Table 2).
Highest significant correlations for differences in gene expression were identified within groups, i.e. within the group of drought stress genes, leaf senescence genes and genes out of GWAS (Table 3). The highest correlation was observed for the group of drought stress genes between relative expression of GAD3 and P5CS2 (r = 0.84), for the group of leaf senescence genes for GSII and pHvNF-Y5a (r = 0.64), and for the genes out of GWAS between AVP1 and TRIUR3 (r = 0.54). For no gene the differential expression was significantly correlated to the expression differences of all other genes, but ETFQO was correlated to all except Dhn1, and GAD3 and Contig7437 were correlated to all except GSII and AVP1, and SAPK9 and NADP_ME, respectively. Significant correlations were also detected between the relative SPAD values for change in leaf colour and all leaf senescence genes except hv_36467 with the highest coefficients of correlation for GSII (r = 0.24) and pHvNF-Y5a (r = 0.34). Moreover, significant correlations were observed for relative SPAD values to two genes out of GWAS (r = 0.16 for AVP1 and r = 0.15 for TRIUR3).
Genome wide association study
Significant (p <0.001) marker gene expression associations were detected on all barley chromosomes except 4H with the highest number on chromosome 5H (8 single nucleotide polymorphisms, SNP) (Table 4). The largest transcriptional variance was explained by the marker SCRI_RS_181376 associated to the expression of ETFQO (R2 = 11.55 %) and the highest likelihood of odds (LOD) was observed for the marker SCRI_RS_161614 associated to the expression of TRIUR3 (LOD = 3.82) on barley chromosome 5H. Five SNP were significantly associated to the relative expression of the genes for drought stress, six to those for leaf senescence and seven to the genes out of the previous GWAS. Within the group of drought stress genes, expression differences of three genes (A1, GAD3 and P5CS2) and within the group of leaf senescence genes expression differences of four genes (Contig7437, GSII, hv_36467 and pHvNF-Y5α) were associated to markers. Out of these, three were located on chromosome 3H at 142.1 cM. This eQTL was detected for the relative expression of two drought stress genes (GAD3 and P5CS2) and one leaf senescence gene (Contig7437) which were also highly and significantly correlated (Table 3). Furthermore, an eQTL was observed for the relative expression of A1 on chromosome 5H at 149.9 cM associated to two markers. Associations for the relative expression of three genes (AVP1, ETFQO and TRIUR3) out of the four GWAS genes were detected on barley chromosomes 3H and 5H. For the expression of TRIUR3 three markers were found on 5H at 44.5 cM, and the expression of AVP1 was associated to a marker on chromosome 5H at 62.5 cM.
The five SNP significantly associated to the relative expression of drought stress genes and the seven markers associated to genes out of GWAS all marked cis eQTL, while two trans eQTL were detected for P5CS2 and AVP1 (Table 5). In contrast, for the six markers significantly associated to leaf senescence genes only one cis eQTL was observed for pHvNF-Y5α. In summary, seven trans eQTL were detected and eight cis eQTL for which the Morex contigs showed a high identity to the gene analysed. Furthermore, cis eQTL explained a higher transcriptional variance (R2) than those in trans (Table 4 and Table 5).
Discussion
Drought stress and leaf senescence genes
As shown by the significantly decreased SPAD values at 27 days after sowing (das, BBCH 25), drought stress had an accelerating influence on natural leaf senescence in barley (Fig. 1 and Table 2). Furthermore, the drought stress answer in this juvenile stage was observed by differential expression of 14 genes induced by drought stress or leaf senescence (Table 1, Fig. 2).
A1 is a gene which is induced by ABA or abiotic stresses like drought, cold and heat [19, 52, 53]. In the present study expression under drought stress was higher than in the well watered treatment (Fig. 2). This was also shown by several studies first in barley [53] and other species including transgenics [54–57]. Dehydrins (Dhn) are well known to be expressed under dehydration stress [58]. For instance Dhn1 is described to be up-regulated under drought stress in barley [59, 60] which was also found in this study (Fig. 2). The glutamate decarboxylase gene (GAD3) is regulated by calcium and the protein encoded by this gene catalyzes the reaction of glutamate to γ-aminobutyric acid (GABA) [61, 62]. GABA may be involved in drought stress [63] by up-regulation of genes encoding a GABA receptor [29] which was also shown in the present study (Fig. 2). The NADP-dependent malic enzyme-like (NADP_ME) is involved in lignin biosynthesis, and regulates cytosolic pH through balancing the synthesis and degradation of malate [64]. As described in a drought stress study on barley, this effect is used for control of stomatal closure during the day under water-deficit conditions [29]. Comparable to the present study (Fig. 2) the gene for NADP_ME turned out to be higher expressed under drought stress [29]. The delta 1-pyrroline-5-carboxylate synthase 2 gene (P5CS2) is included in proline synthesis [65]. Content of proline is still controversially discussed as an indicator for drought tolerance [66], but it was shown in a previous study that the proline content increased under drought stress [20]. For approving its role, this gene was selected and showed up-regulation under drought stress (Fig. 2). Up-regulation under drought stress was also observed in tobacco [67] and transgenic rice [68].
The Contig7437 is a senescence associated gene (SAG) which is up-regulated under drought stress, as also shown by Guo et al. [29] in barley for drought stress during the reproductive stage. Other analysed SAGs are hv_36467 and pHvNF-Y5α, which were down-regulated in most genotypes under drought stress in our study (Fig. 2) whereas in literature reverse effects are described. The gene hv_36467 is a SAG12 like gene which is a senescence associated cystein protease and turned out to be up-regulated during natural leaf senescence in barley [69] and during dark induced senescence in tobacco [70]. In Arabidopsis thaliana the gene NFYA5 similar to pHvNF-Y5α was analysed by microarrays showing that the expression of this gene was induced by drought stress and ABA treatments [71], as well as under nitrogen stress [72]. Our data indicate a specific regulation of these two genes under different conditions. The protein encoded by the glutamine synthetase 2 (GSII) gene was found in photosynthetic tissues where its main role is the re-assimilation of photorespiratory ammonia [73, 74]. During senescence, the activity of GSII decreased representing down-regulation of associated genes in rice [73], barley and wheat [75] which was confirmed in the present study (Fig. 2). With chlorophyll degradation during leaf senescence the light harvesting complexes (LHC) of PSI and PSII remain stable, but synthesis rates of apoproteins of LHC decrease early in senescence [76]. In the present study LHC1b20 was down-regulated for most genotypes during drought stress induced leaf senescence in juvenile barley (Fig. 2) which was also shown in rice [77] and barley [78, 79] for natural leaf senescence in the generative stage.
In this study, all five selected drought stress genes were up-regulated under drought stress (Fig. 2) according to literature which demonstrates a clear drought stress answer and a good experimental setup for detecting and analysing drought stress response. In contrast, four out of the five selected genes for leaf senescence were down-regulated (Fig. 2) because a few of these genes are involved in photosynthesis and chloroplast development. Results for three of these genes (Contig7437, GSII and LHC1b20) were in accordance with results known from literature, while this was not the case for two of them (hv_36467 and pHvNF-Y5α). However, for all of these genes the adverse effect was detected for some genotypes (Fig. 2). Results revealed that drought stress in early developmental stages of barley leads to premature induced leaf senescence as already observed by physiological parameters [20] and by expression analysis of drought stress and leaf senescence related genes in this study.
Expression differences in three genes (GAD3, P5CS2 and Contig7437) were significantly associated to barley chromosome 3H at 142.1 cM (Table 4). At this position also quantitative trait loci (QTL) were found for drought stress [20, 80] as well as for leaf senescence [81]. These facts and the high correlation of these genes (Table 3) make this eQTL very interesting for marker assisted breeding in barley.
Genes out of GWAS
To verify the QTL identified for drought stress and drought stress induced leaf senescence by Wehner et al. [20] an expression profile and eQTL analysis was conducted with genes coding for proteins identified within respective QTL. The genes ETFQO, SAPK9, TRIUR3 and AVP1 were differentially expressed (Fig. 2).
The protein encoded by the electron transfer flavoprotein-ubiquinone oxidoreductase gene (ETFQO) is located in the mitochondria where it accepts electrons from ETF, transfers them to ubiquinone and acts downstream in the degradation of chlorophyll during leaf senescence [82, 83]. Expression studies showed that ETFQO is up-regulated under darkness induced leaf senescence [83, 84] whereas in this study on drought stress induced leaf senescence no clear direction was observed (Fig. 2). A gene coding for a serine/threonine-protein kinase (SAPK9) was analysed which can be activated by hyperosmotic stress and ABA in rice [85]. In the present study SAPK9 was down-regulated in most genotypes (Fig. 2). Furthermore, the abscisic acid-inducible protein kinase gene (TRIUR3) which is also involved in dehydration stress response [86] was differentially expressed. Until now, no relative expression analysis has been conducted for this gene, but a huge amount of ABA inducible genes are up-regulated under drought stress in rice [87]. In the present study TRIUR3 was also up-regulated under drought stress (Fig. 2). The nucleotide pyrophosphatase/phosphodiesterase gene (AVP1) is a gene which is up-regulated under drought stress [88] which was confirmed in the current study (Fig. 2). Expression of this gene was also observed in transgenics showing a higher drought stress tolerance [89–92].
Three of these genes (SAPK9, TRIUR3 and AVP1) were located within the QTL on barley chromosome 5H at 45 cM [20]. Furthermore, expression differences of two of them (TRIUR3 and AVP1) were again associated to markers on chromosome 5H around 45 cM (Table 4) and this position was also validated in the Morex genome (Table 5). A high and significant correlation between the relative expression data of both genes as well as to the relative SPAD values (Table 3) promotes this finding. At the same position on chromosome 5H two markers which turned out to be significantly associated to SPAD and biomass yield under drought stress treatment were identified [20]. So, these results [20] and those of this study give hint that the two SNP markers, i.e. BOPA1_9766-787 and SCRI_RS_102075 may be used in marker based selection procedures in barley breeding programmes aiming at the improvement of drought stress tolerance.
For the understanding of complex mechanisms, such as the process of drought stress tolerance and drought stress induced leaf senescence as a basis for future breeding activities it is of prime importance to understand how and when regulatory genes are activated and where they are located in the barley genome. Results of this study contribute to elucidate the regulation of drought stress induced leaf senescence during early developmental stages in barley. The present genetical genomics approach helps to localize and understand transcriptional regulation and gene interaction, both from cis-acting elements and trans-acting factors (Table 5). When analysing the expression regulation of the barley genome, cis eQTL were found for the genes A1, GAD3, pHvNF-Y5α, ETFQO and TRIUR3. Markers which were significantly associated to cis eQTL explained up to 11.55 % of the transcriptional variance (Table 4 and Table 5). Therefore, most of the strongest eQTL acted in cis which was also observed in previous eQTL studies [8, 93, 94].
Factors that act in trans regulating the expression levels of the genes of interest were mainly found for the group of leaf senescence genes. Some of these genes are described as SAGs (Contig7437, hv_36467 and pHvNF-Y5α), because up to now little is known about their function. Results of the present study give hint that these SAGs are regulated in trans.
Conclusion
With respect to the expression of genes involved in drought stress response and early leaf senescence genotypic differences exist in barley. Major eQTL for the expression of these genes are located on barley chromosome 3H and 5H. The eQTL on chromosome 5H coincides with the QTL for drought stress induced leaf senescence identified in a previous GWAS [43]. Respective markers, i.e. BOPA1_9766-787 and SCRI_RS_102075 may be used in future barley breeding programmes for improving tolerance to drought stress and early leaf senescence, respectively.
Methods
Plant material and phenotypic characterisation
Phenotyping, genotyping and QTL analysis were conducted as described in Wehner et al. [20] on a set of 156 winter barley genotypes consisting of 113 German winter barley cultivars (49 two-rowed and 64 six-rowed, [95]) and 43 accessions of the spanish barley core collection (SBCC) [96]. The same set of genotypes as well as the same experimental design was used for expression- and eQTL analysis in the present study. In brief, trials were conducted in greenhouses of the Julius Kühn-Institut in Groß Lüsewitz, Germany and drought stress was applied in a split plot design with three replications per genotype and treatment (control, drought stress). In each pot four plants were sown and all leaves were tied up, except the primary leaf per plant. Drought stress was induced by a termination of watering at the primary leaf stage (BBCH 10, according to Stauss [51]) seven days after sowing (das). From this time drought stress developed slowly till 20 das when the final drought stress level was reached. The drought stress variant was kept at 20 % of the maximal soil water capacity and the control variant at 70 % by weighing the pots resulting in a relative water content (36 das) ranging between 88.8 % and 91.5 % in the control variant and 80.9 % and 86.1 % in the drought stress treatment. The experimental setup and growth conditions for these pot experiment are described in detail as design B in Wehner et al. [20].
At 26 das (BBCH 25) leaf material for RNA extraction was sampled by harvesting one primary leaf per pot taking the middle part for further analyses. Mixed samples out of the three leaf pieces (circa 100 mg) per genotype and treatment (312 samples) each were immediately frozen in liquid nitrogen and stored at −80 °C. Furthermore, to get information on the influence of drought stress on leaf senescence leaf colour (SPAD, Konica Minolta Chlorophyll Meter SPAD-502 Plus, Osaka Japan) was measured 27 das on three primary leaves per pot at five positions each.
RNA isolation and cDNA synthesis
The frozen primary leaves were homogenized with a tube pestle (Biozym) in liquid nitrogen. Total RNA from the primary leaves was isolated with the InviTrap Spin Plant RNA Mini Kit (STRATEC Molecular), using lysis solution RP and following the manufacturer’s instructions. After incubation for 15 min at room temperature, an additional incubation for 3 min at 55 °C was conducted to get a higher RNA yield. Total RNA yield was measured by Qubit fluorometric quantification (Life technologies) and concentration was adjusted to 50 ng. RNA was used for cDNA synthesis with the QuantiTect Reverse Transcription Kit (Qiagen) following the manufacturer’s instructions. cDNA was stored at −20 °C.
Expression analysis using quantitative real-time PCR (qPCR)
A high throughput system (BioMark) was used for expression analysis in which four Fluidigm chips (96.96) were analysed for the 312 samples. Default space on these chips allows to analyse 48 genes in two technical replications. Out of these 48 analysed genes (23 genes involved in drought stress, 12 leaf senescence genes, 11 genes coding for proteins out of a previous GWAS [20] and two reference genes), 14 differentially expressed genes revealing clear differences between genotypes and showing a low number of missing values were selected for the present study. Five of these genes were involved in leaf senescence, five in drought stress response and four genes coding for proteins related to leaf senescence or drought stress out of the previous genome wide association study [20] were chosen. In addition, as a reference gene GAPDH was included (Table 1). To identify the gene for those proteins identified in the GWAS studies by Wehner et al. [20] the significant associated marker sequences were compared to the plant nucleotide collection by Blastn (Basic Local Alignment Search Tool, ncbi [www.ncbi.nlm.nih.gov] accessed June 2014) and the gene with the best hit was chosen for primer design.
Primers (Eurofins HPSF purified) were constructed using the primer designing tool of NCBI ([www.ncbi.nlm.nih.gov/tools/primer-blast] accessed June 2014) with a length of 20 bp, annealing temperature of 59 °C and product size of 100–200 bp (Table 1).
qPCR was performed using the high throughput platform BioMark HD System and the 96.96 Dynamic Array IFC (Fluidigm) following the manufacturer’s instructions. 5 μl Fluidigm sample premix consisted of 1.25 μl pre-amplified cDNA, 0.25 μl of 20x DNA binding dye sample loading reagent (Fluidigm), 2.5 μl of SsoFast EvaGreen Supermix with low ROX (BioRad) and 1 μl of RNase/DNase-free water. Each 5 μl assay premix consisted of 2 μl of 100 μM primers, 2.5 μl assay loading reagent (Fluidigm) and 0.5 μl RNase/DNase-free water. Thermal conditions for qPCR were: 95 °C for 60 s, 30 cycles of 96 °C for 5 s, 60 °C for 20 s plus melting curve analysis. Data were processed using BioMark Real-Time PCR Analysis Software 3.0.2 (Fluidigm). The quality threshold was set at the default setting of 0.65 and linear baseline correction and automatic cycle threshold method were used.
Data analysis
The analysis software (Fluidigm Real- Time PCR Analysis Software) gave cycle threshold (Ct) values and calculated ∆Ct values, as well as a quality score for each amplification. Out of these ∆Ct values calculated out of the Ct value of the gene of interest minus the Ct value of the housekeeping gene (GAPDH) for each genotype, treatment and replication, the relative expression (∆∆Ct) was calculated out of the ∆Ct values for stress treatment minus the ∆Ct values for control treatment for each genotype and replication [97]. ∆∆Ct values without correction of PCR efficiency were used for calculation, because genes were tested and selected by their efficiency in preliminary experiments. A mean PCR efficiency (Quality Score of Fluidigm) was calculated for all amplifications.
Shapiro-Wilk test for normal distribution and analysis of variance (ANOVA) using a linear model were carried out using R 2.15.1 [98] to test effects of genotype (using ∆∆Ct values) and treatment (using ∆Ct values). Furthermore, coefficients of correlation (Spearman) were calculated in R between relative expression of the genes and the relative SPAD values [20, 99]. Moreover, for the SPAD values an ANOVA mixed linear model (MLM) was calculated (replication as random) in R to test effects of genotype, treatment and interaction of genotype and treatment. For relative expression as well as for the SPAD values box whisker plots were calculated in R.
Expression quantitative trait loci (eQTL) analysis
For the 14 selected genes a genome wide association study (GWAS) for eQTL detection was conducted on the 156 genotypes applying a mixed linear model (MLM) using TASSEL 3.0 [100]. For this purpose a genetic map with 3,212 polymorphic SNP markers with minor allele frequencies larger than 5 % [101], a population structure calculated with STRUCTURE 2.3.4 [102] based on 51 simple sequence repeat (SSR) markers covering the whole genome, a kinship calculated with SPAGeDi 1.3d [103] based on 51 SSRs and the relative expression data (means for replications) were used. For comparability the methods were the same as used for GWAS in Wehner et al. [20]. All results with p values <0.001 (likelihood of odds, LOD = 3) were considered as significant marker gene expression associations.
To compare genomic positions of the eQTL with those of the analysed genes, sequences of the genes were compared against high confidential genes (CDS sequences) of the barley Morex genome by Blastn (Basic Local Alignment Search Tool, IPK Barley Blast server [http://webblast.ipk-gatersleben.de/barley/viroblast.php] accessed May 2015) and the Morex contig with the highest identity on the associated linkage group (chromosome) was chosen. With this information eQTL were divided in cis and trans eQTL. cis eQTL coincide with the location of the underlying gene (position <10 cM), whereas trans eQTL are located in other regions of the genome [11].
Abbreviations
- ∆∆Ct:
-
relative expression
- ABA:
-
abscisic acid
- Blast:
-
Basic Local Alignment Search Tool
- Ct:
-
cycle threshold
- das:
-
days after sowing
- e.g:
-
for example
- eQTL:
-
expression quantitative trait locus/loci
- GWAS:
-
genome wide association study
- i.e:
-
id est
- LEA:
-
late embryogenesis abundant protein
- LOD:
-
likelihood of odds
- MLM:
-
mixed linear model
- PCR:
-
polymerase chain reaction
- qPCR:
-
quantitative real-time polymerase chain reaction
- QTL:
-
quantitative trait locus/loci
- ROS:
-
reactive oxygen species
- SAG:
-
senescence associated genes
- SBCC:
-
Spanish Barley Core Collection
- SNP:
-
single nucleotide polymorphism
- SPAD:
-
soil plant analysis development; measurement of chlorophyll content by colour
- SSR:
-
single sequence repeat
References
Korenková V, Scott J, Novosadová V, Jindřichová M, Langerová L, Švec D, et al. Pre-amplification in the context of high-throughput qPCR gene expression experiment. BMC Mol Biol. 2015;16(1):5.
Spurgeon SL, Jones RC, Ramakrishnan R. High throughput gene expression measurement with real time PCR in a microfluidic dynamic array. PLoS One. 2008;3(2), e1662.
Gilad Y, Rifkin SA, Pritchard JK. Revealing the architecture of gene regulation: the promise of eQTL studies. Trends Genet. 2008;24(8):408–15.
Hansen BG, Halkier BA, Kliebenstein DJ. Identifying the molecular basis of QTLs: eQTLs add a new dimension. Trends Plant Sci. 2008;13(2):72–7.
Westra H-J, Franke L. From genome to function by studying eQTLs. Biochim Biophys Acta. 2014;1842(10):1896–902.
Gibson G, Weir B. The quantitative genetics of transcription. Trends Genet. 2005;21(11):616–23.
Potokina E, Prasad M, Malysheva L, Röder M, Graner A. Expression genetics and haplotype analysis reveal cis regulation of serine carboxypeptidase I (Cxp1), a candidate gene for malting quality in barley (Hordeum vulgare L.). Funct Integr Genomics. 2006;6(1):25–35.
West MA, Kim K, Kliebenstein DJ, van Leeuwen H, Michelmore RW, Doerge R, et al. Global eQTL mapping reveals the complex genetic architecture of transcript-level variation in Arabidopsis. Genetics. 2007;175(3):1441–50.
Drost DR, Puranik S, Novaes E, Novaes CR, Dervinis C, Gailing O, et al. Genetical genomics of Populus leaf shape variation. BMC Plant Biol. 2015;15(1):166.
Liu P, Wang CM, Li L, Sun F, Yue GH. Mapping QTLs for oil traits and eQTLs for oleosin genes in jatropha. BMC Plant Biol. 2011;11(1):132.
Druka A, Potokina E, Luo Z, Jiang N, Chen X, Kearsey M, et al. Expression quantitative trait loci analysis in plants. Plant Biotechnol J. 2010;8(1):10–27.
Vinocur B, Altman A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol. 2005;16(2):123–32.
Beck EH, Fettig S, Knake C, Hartig K, Bhattarai T. Specific and unspecific responses of plants to cold and drought stress. J Biosci. 2007;32(3):501–10.
Anjum SA, Xie X-y, Wang L-c, Saleem MF, Man C, Lei W. Morphological, physiological and biochemical responses of plants to drought stress. African J Agri Res. 2011;6(9):2026–32.
Sreenivasulu N, Sopory S, Kishor PK. Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene. 2007;388(1):1–13.
El Hafid R, Smith DH, Karrou M, Samir K. Physiological responses of spring durum wheat cultivars to early-season drought in a Mediterranean environment. Ann Bot-London. 1998;81(2):363–70.
Cattivelli L, Rizza F, Badeck F-W, Mazzucotelli E, Mastrangelo AM, Francia E, et al. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crop Res. 2008;105(1-2):1–14.
Buchanan-Wollaston V, Earl HJ, Harrison E, Mathas E, Navabpour S, Page T, et al. The molecular analysis of leaf senescence – a genomics approach. Plant Biotechnol J. 2003;1:3–22.
Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218(1):1–14.
Wehner G, Balko C, Enders M, Humbeck K, Ordon F. Identification of genomic regions involved in tolerance to drought stress and drought stress induced leaf senescence in juvenile barley. BMC Plant Biol. 2015;15(1):125.
Blum A. Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci. 1989;29:230–3.
Teulat B, Borries C, This D. New QTLs identified for plant water status, water-soluble carbohydrate and osmotic adjustment in a barley population grown in a growth-chamber under two water regimes. Theor Appl Genet. 2001;103(1):161–70.
Jamieson PD, Martin RJ, Francis GS, Wilson DR. Drought effects on biomass production and radiation-use efficiency in barley. Field Crop Res. 1995;43:77–86.
Honsdorf N, March TJ, Hecht A, Eglinton J, Pillen K. Evaluation of juvenile drought stress tolerance and genotyping by sequencing with wild barley introgression lines. Mol Breeding. 2014;34(3):1475–95.
Bhargava S, Sawant K. Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breeding. 2013;132(1):21–32.
Ahuja I, de Vos RC, Bones AM, Hall RD. Plant molecular stress responses face climate change. Trends Plant Sci. 2010;15(12):664–74.
Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J Exp Bot. 2007;58(2):221–7.
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: effects, mechanisms and management. Agron Sust Dev. 2009;29(1):185–212.
Guo P, Baum M, Grando S, Ceccarelli S, Bai G, Li R, et al. Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. J Exp Bot. 2009;60(12):3531–44.
Aprile A, Mastrangelo AM, De Leonardis AM, Galiba G, Roncaglia E, Ferrari F, et al. Transcriptional profiling in response to terminal drought stress reveals differential responses along the wheat genome. BMC Genomics. 2009;10(1):279.
Lim PO, Kim HJ, Nam HG. Leaf senescence. Ann Rev Plant Biol. 2007;58:115–36.
Sarwat M, Naqvi AR, Ahmad P, Ashraf M, Akram NA. Phytohormones and microRNAs as sensors and regulators of leaf senescence: assigning macro roles to small molecules. Biotechnol Adv. 2013;31(8):1153–71.
Balazadeh S, Riaño-Pachón D, Mueller-oeber B. Transcription factors regulating leaf senescence in Arabidopsis thaliana. Plant biology. 2008;10(s1):63–75.
Gregersen PL, Culetic A, Boschian L, Krupinska K. Plant senescence and crop productivity. Plant Mol Biol. 2013;82(6):603–22.
Munne-Bosch S, Alegre L. Die and let live: leaf senescence contributes to plant survival under drought stress. Funct Plant Biol. 2004;31:203–16.
Penfold CA, Buchanan-Wollaston V. Modelling transcriptional networks in leaf senescence. J Exp Bot. 2014;65(14):3859–73.
Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C, et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell. 2011;23(3):873–94.
Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005;42(4):567–85.
Humbeck K. Epigenetic and small RNA regulation of senescence. Plant Mol Biol. 2013;82(6):529–37.
Miersch I, Heise J, Zelmer I, Humbeck K. Differential degradation of the photosynthetic apparatus during leaf senescence in barley (Hordeum vulgare L.). Plant Biol. 2000;2:618–23.
Ay N, Irmler K, Fischer A, Uhlemann R, Reuter G, Humbeck K. Epigenetic programming via histone methylation at WRKY53 controls leaf senescence in Arabidopsis thaliana. Plant J. 2009;58(2):333–46.
Guo Y, Gan S. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006;46(4):601–12.
Li Z, Peng J, Wen X, Guo H. Gene network analysis and functional studies of senescence-associated genes reveal novel regulators of Arabidopsis leaf senescence. J Integr Plant Biol. 2012;54(8):526–39.
Borrell AK, Hammer GL, Henzell RG. Does maintaining green leaf area in sorghum improve yield under drought? II. Dry matter production and yield. Crop Sci. 2000;40(4):1037–48.
Fischer AM. The Complex Regulation of Senescence. Crit Rev Plant Sci. 2012;31(2):124–47.
Ay N, Clauss K, Barth O, Humbeck K. Identification and characterization of novel senescence‐associated genes from barley (Hordeum vulgare) primary leaves. Plant Biol. 2008;10(1):121–35.
Kleber-Janke T, Krupinska K. Isolation of cDNA clones for genes showing enhanced expression in barley leaves during dark-induced senescence as well as during senescence under field conditions. Planta. 1997;203(3):332–40.
Tyagi K, Park MR, Lee HJ, Lee CA, Rehman S, Steffenson B, et al. Fertile crescent region as source of drought tolerance at early stage of plant growth of wild barley (Hordeum vulgare L. ssp. spontaneum). Pak J Bot. 2011;43(1):475–86.
Consortium IBGS, Mayer KF, Waugh R, Brown JW, Schulman A, Langridge P, et al. A physical, genetic and functional sequence assembly of the barley genome. Nature. 2012;491(7426):711–6.
Kliebenstein D. Quantitative genomics: analyzing intraspecific variation using global gene expression polymorphisms or eQTLs. Plant Biol. 2009;60(1):93.
Stauss R. Compendium of Growth Stage Identification Keys for Mono-and Dicotyledonous Plants: Extended BBCH Scale, Autumn 1994: Ciba; 1994.
Marttila S, Tenhola T, Mikkonen A. A barley (Hordeum vulgare L.) LEA3 protein, HVA1, is abundant in protein storage vacuoles. Planta. 1996;199(4):602–11.
Straub PF, Shen Q, Ho T-hD. Structure and promoter analysis of an ABA-and stress-regulated barley gene, HVA1. Plant Mol Biol. 1994;26(2):617–30.
Checker VG, Chhibbar AK, Khurana P. Stress-inducible expression of barley Hva1 gene in transgenic mulberry displays enhanced tolerance against drought, salinity and cold stress. Transgenic Res. 2012;21(5):939–57.
Rohila JS, Jain RK, Wu R. Genetic improvement of Basmati rice for salt and drought tolerance by regulated expression of a barley Hva1 cDNA. Plant Sci. 2002;163(3):525–32.
Bray EA. Plant responses to water deficit. Trends Plant Sci. 1997;2(2):48–54.
Fu D, Huang B, Xiao Y, Muthukrishnan S, Liang GH. Overexpression of barley hva1 gene in creeping bentgrass for improving drought tolerance. Plant Cell Rep. 2007;26(4):467–77.
Marzin S, Mihaly R, Pauk J, Schweizer P. A transient assay system for the assessment of cell-autonomous gene function in dehydration-stressed barley. J Exp Bot. 2008;59(12):3359–69.
Suprunova T, Krugman T, Fahima T, Chen G, Shams I, Korol A, et al. Differential expression of dehydrin genes in wild barley, Hordeum spontaneum, associated with resistance to water deficit. Plant Cell Environ. 2004;27(10):1297–308.
Tommasini L, Svensson JT, Rodriguez EM, Wahid A, Malatrasi M, Kato K, et al. Dehydrin gene expression provides an indicator of low temperature and drought stress: transcriptome-based analysis of barley (Hordeum vulgare L.). Funct Integr Genomics. 2008;8(4):387–405.
Serraj R, Shelp BJ, Sinclair TR. Accumulation of γ‐aminobutyric acid in nodulated soybean in response to drought stress. Physiol Plant. 1998;102(1):79–86.
Ueno H. Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal. 2000;10(1):67–79.
Rhodes D, Handa S, Bressan RA. Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol. 1986;82(4):890–903.
Wheeler MCG, Tronconi MA, Drincovich MF, Andreo CS, Flügge U-I, Maurino VG. A comprehensive analysis of the NADP-malic enzyme gene family of Arabidopsis. Plant Physiol. 2005;139(1):39–51.
Szabados L, Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15(2):89–97.
Kishor P, Kavi B, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014;37(2):300–11.
Dobrá J, Vanková R, Havlová M, Burman AJ, Libus J, Štorchová H. Tobacco leaves and roots differ in the expression of proline metabolism-related genes in the course of drought stress and subsequent recovery. J Plant Physiol. 2011;168(13):1588–97.
Quan R, Hu S, Zhang Z, Zhang H, Zhang Z, Huang R. Overexpression of an ERF transcription factor TSRF1 improves rice drought tolerance. Plant Biotechnol J. 2010;8(4):476–88.
Hollmann J, Gregersen PL, Krupinska K. Identification of predominant genes involved in regulation and execution of senescence-associated nitrogen remobilization in flag leaves of field grown barley. J Exp Bot. 2014;65(14):3963–73.
Carrión CA, Costa ML, Martínez DE, Mohr C, Humbeck K, Guiamet JJ. In vivo inhibition of cysteine proteases provides evidence for the involvement of ‘senescence-associated vacuoles’ in chloroplast protein degradation during dark-induced senescence of tobacco leaves. J Exp Bot. 2013;64(16):4967–80.
Li W-X, Oono Y, Zhu J, He X-J, Wu J-M, Iida K, et al. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell. 2008;20(8):2238–51.
Zhao M, Ding H, Zhu JK, Zhang F, Li WX. Involvement of miR169 in the nitrogen‐starvation responses in Arabidopsis. New Phytol. 2011;190(4):906–15.
Kamachi K, Yamaya T, Hayakawa T, Mae T, Ojima K. Changes in cytosolic glutamine synthetase polypeptide and its mRNA in a leaf blade of rice plants during natural senescence. Plant Physiol. 1992;98(4):1323–9.
Buchanan-Wollaston V. The molecular biology of leaf senescence. J Exp Bot. 1997;48(307):181–99.
Gregersen P, Holm P, Krupinska K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol. 2008;10(1):37–49.
Humbeck K, Quast S, Krupinska K. Functional and molecular changes in the photosynthetic apparatus during senescence of flag leaves from field-grown barley plants. Plant Cell Environ. 1996;19(3):337–44.
Wang Y, Zhang J, Yu J, Jiang X, Sun L, Wu M, et al. Photosynthetic changes of flag leaves during senescence stage in super high-yield hybrid rice LYPJ grown in field condition. Plant Physiol Biochem. 2014;82:194–201.
Krupinska K, Mulisch M, Hollmann J, Tokarz K, Zschiesche W, Kage H, et al. An alternative strategy of dismantling of the chloroplasts during leaf senescence observed in a high-yield variety of barley. Physiol Plant. 2012;144(2):189–200.
Humbeck K, Krupinska K. The abundance of minor chlorophyll a/b-binding proteins CP29 and LHCI of barley (Hordeum vulgare L.) during leaf senescence is controlled by light. J Exp Bot. 2003;54(381):375–83.
Varshney RK, Paulo MJ, Grando S, van Eeuwijk FA, Keizer LCP, Guo P, et al. Genome wide association analyses for drought tolerance related traits in barley (Hordeum vulgare L.). Field Crop Res. 2012;126:171–80.
Li W-T, Liu C, Liu Y-X, Pu Z-E, Dai S-F, Wang J-R, et al. Meta-analysis of QTL associated with tolerance to abiotic stresses in barley. Euphytica. 2013;189(1):31–49.
Araújo WL, Ishizaki K, Nunes-Nesi A, Larson TR, Tohge T, Krahnert I, et al. Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell. 2010;22(5):1549–63.
Ishizaki K, Larson TR, Schauer N, Fernie AR, Graham IA, Leaver CJ. The critical role of Arabidopsis electron-transfer flavoprotein: ubiquinone oxidoreductase during dark-induced starvation. Plant Cell. 2005;17(9):2587–600.
Engqvist MK, Kuhn A, Wienstroer J, Weber K, Jansen EE, Jakobs C, et al. Plant D-2-hydroxyglutarate dehydrogenase participates in the catabolism of lysine especially during senescence. J Biol Chem. 2011;286(13):11382–90.
Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T. Differential activation of the rice sucrose nonfermenting1–related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell. 2004;16(5):1163–77.
Anderberg RJ, Walker-Simmons M. Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinases. P Natl Acad Sci USA. 1992;89(21):10183–7.
Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, et al. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 2003;133(4):1755–67.
Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, et al. Drought-and salt-tolerant plants result from overexpression of the AVP1 H + -pump. P Natl Acad Sci USA. 2001;98(20):11444–9.
Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, et al. Up-regulation of a H + -pyrophosphatase (H + -PPase) as a strategy to engineer drought-resistant crop plants. P Natl Acad Sci USA. 2005;102(52):18830–5.
Pasapula V, Shen G, Kuppu S, Paez-Valencia J, Mendoza M, Hou P, et al. Expression of an Arabidopsis vacuolar H + -pyrophosphatase gene (AVP1) in cotton improves drought-and salt tolerance and increases fibre yield in the field conditions. Plant Biotechnol J. 2011;9(1):88–99.
Bao A-K, Wang S-M, Wu G-Q, Xi J-J, Zhang J-L, Wang C-M. Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci. 2009;176(2):232–40.
Kumar T, Khan MR, Abbas Z, Ali GM. Genetic improvement of sugarcane for drought and salinity stress tolerance using Arabidopsis vacuolar pyrophosphatase (AVP1) gene. Mol Biotechnol. 2014;56(3):199–209.
Holloway B, Li B. Expression QTLs: applications for crop improvement. Mol Breeding. 2010;26(3):381–91.
Holloway B, Luck S, Beatty M, Rafalski J-A, Li B. Genome-wide expression quantitative trait loci (eQTL) analysis in maize. BMC Genomics. 2011;12(1):336.
Rode J, Ahlemeyer J, Friedt W, Ordon F. Identification of marker-trait associations in the German winter barley breeding gene pool (Hordeum vulgare L.). Mol Breeding. 2012;30(2):831–43.
Igartua E, Gracia MP, Lasa JM, Medina B, Molina-Cano JL, Montoya JL, et al. The Spanish barley core collection. Genet Resour Crop Ev. 1998;45:475–81.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–8.
RCore. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org; 2012.
Fischer RA, Maurer R. Drought Resistance in Spring Wheat Cultivars. I Grain Yield Responses. Austral J Agr Res. 1978;29:897–912.
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23(19):2633–5.
Comadran J, Kilian B, Russell J, Ramsay L, Stein N, Ganal M, et al. Natural variation in a homolog of Antirrhinum CENTRORADIALIS contributed to spring growth habit and environmental adaptation in cultivated barley. Nature Genet. 2012;44(12):1388–92.
Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–59.
Hardy OJ, Vekemans X. SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes. 2002;2(4):618–20.
Acknowledgements
The authors thank Dr. Brigitte Ruge-Wehling for the lab facilities for RNA isolation, Dr. Ernesto Igartua CSIC, Spain for providing seeds of the SBCC, the Interdisciplinary Center for Crop Plant Research (IZN) of the Martin-Luther-University of Halle-Wittenberg for funding this project and Prof. Dr. Klaus Pillen for close collaboration.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GW conducted all experiments, including expression, statistical and bioinformatics analyses and mainly wrote the manuscript. EZ provided the Fluidigm BioMark System and supervised the gene expression experiments. CB, KH and FO designed the research, supervised the experimental design and participated in writing the manuscript. All authors approved the final manuscript.
Additional file
Additional file 1:
Relative expression of the 14 genes with mean quality scores for each amplification. aSBCC: spanish barley core collection. bGWAS: genome wide association study. (XLSX 111 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wehner, G., Balko, C., Humbeck, K. et al. Expression profiling of genes involved in drought stress and leaf senescence in juvenile barley. BMC Plant Biol 16, 3 (2016). https://doi.org/10.1186/s12870-015-0701-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-015-0701-4