Abstract
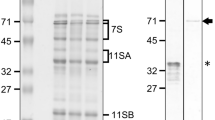
The HVA1 protein belongs to the LEA3 group, which is expressed during the late stage of seed maturation. It is also induced by exogenous abscisic acid (ABA) and a variety of environmental stresses in germinating barley (Hordeum vulgare L.). In the present work, the potential role of HVA1 was investigated by studying its tissue distribution and subcellular localization in mature and stressed seeds by immuno-microscopic methods. In the mature seed, HVA1 protein was detected in all tissues except the non-living starchy endosperm. During germination the amount of HVA1 protein decreased but did not totally disappear. Incubation with 100 μM ABA, cold treatment or drought stress dramatically increased HVA1 expression in the germinated seed. In this work, the distribution of a LEA3 group protein was studied in a cereal seed for the first time by immuno-electron microscopy. In the scutellum and aleurone layer, HVA1 was localized both in the cytoplasm and protein storage vacuoles (PSVs). HVA1 protein was found to be threefold more abundant in PSVs than in the cytoplasm of an unstressed seed tissue. The ratio increased with ABA or stress treatments to at least ninefold. The role of HVA1 in PSVs remains unclear: a previously suggested possibility is ion sequestration to prevent precipitation during stress. On the other hand, HVA1 protein could also be degraded in PSVs. HVA1 protein does not have the signal peptide typical of proteins which are glycosylated and targeted into the vacuole via the Golgi complex. Because HVA1 is not glycosylated, it may use an alternative, ER-independent vacuolar pathway, also found in yeast cells.
Similar content being viewed by others
Abbreviations
- ABA:
-
abscisic acid
- LEA:
-
late embryogenesis abundant
- PSV:
-
protein storage vacuole
References
Anderson BE, Ward JM, Schroeder JI (1994) Evidence for an extracellular reception site for an abscisic acid in Commelina guard cells. Plant Physiol 104: 1177–1183
Ashgar R, Fenton RD, DeMason DA, Close TJ (1994) Nuclear and cytoplasmic localization of maize embryo and aleurone dehydrin. Protoplasma 177: 87–94
Baker JC, Steele C, Dure III L (1988) Sequence and characterization of 6 Lea proteins and their genes from cotton. Plant Mol Biol 11: 277–291
Belanger CF, Brodl MB, Ho T-HD (1986) Heat shock causes destabilization of specific mRNAs and destruction of endoplasmic reticulum in barley aleurone cells. Proc Natl Acad Sci USA 83: 1354–1358
Bray EA (1993) Molecular responses to water deficit. Plant Physiol 103: 1035–1440
Burgess SR, Shewry PR (1986) Identification of homologous globulins from embryos of wheat, barley, rye and oats. J Exp Bot 37: 1863–1871
Bush DS (1993) Regulation of cytosolic calcium in plants. Plant Physiol 103: 7–13
Bush DS, Biswas AK, Jones RL (1993) Hormonal regulation of Ca2+ transport in the endomembrane system of the barley aleurone. Planta 189: 507–515
Chiang H-L, Schekman R (1991) Regulated import and degradation of a cytosolic protein in yeast vacuole. Nature 350: 313–318
Chrispeels MJ (1991) Sorting of proteins in the secretory system. Annu Rev Plant Physiol Plant Mol Biol 42: 21–53
Close TJ, Fenton RD, Moonan F (1993) A view of plant dehydrins using antibodies specific to the carboxy terminal peptide. Plant Mol Biol 23: 279–286
Curry J, Craig FM, Walker-Simmons MK (1991) Sequence analysis of a cDNA encoding a group 3 LEA mRNA inducible by ABA or dehydration stress in wheat. Plant Mol Biol 16: 1073–1076
Dice JF (1990) Peptide sequences that target cytosolic proteins for lysosomal proteolysis. TIBS 15: 305–309
Dure III L (1993a) A repeating 11-mer amino acid motif and plant desiccation. Plant J 3: 363–369
Dure III L (1993b) Structural motifs in Lea proteins. In: Close TJ, Bray EA (eds) Plant responses to cellular dehydration during environmental stress. The American Society of Plant Physiologists, pp 91–103
Dure III L, Crouch M, Harada J, Ho T-HD, Mundy J, Quatrano TT, Sung ZR (1989) Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol 12: 475–486
Fincher GB (1989) Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu Rev Plant Physiol Plant Mol Biol 40: 305–346
Franz G, Hatzopoulos P, Jones TJ, Krauss M, Sung ZR (1989) Molecular and genetic analysis of an embryonic gene, DC 8, from Daucus carota L. Mol Gen Genet 218: 143–151
Gilroy S, Jones RL (1992) Gibberellic acid and abscisic acid coordinately regulate cytoplasmic calcium and secretory activity in barley aleurone protoplasts. Proc Natl Acad Sci USA 89: 3591–3595
Gilroy S, Jones RL (1994) Perception of gibberellic and abscisic acid at the external face of the plasma membrane of barley (Hordeum vulgare L.) aleurone protoplasts. Plant Physiol 104: 1185–1192
Goday A, Jensen AB, Culiánez-Macià FA, Albà MM, Figueras M, Serratosa J, Torrent M, Pagés M (1994) The maize abscisic acid-responsive protein rab17 is located in the nucleus and interacts with nuclear localization signals. Plant Cell 6: 351–360
Griffiths G (1993) Quantitation in immunocytochemistry. In: Griffiths G. Fine structure immunocytochemistry. Springer, Berlin, pp 413–415
Harada JJ, DeLisle AJ, Baden CS, Crouch ML (1989) Unusual sequence of abscisic acid-inducible mRNA which accumulates late in Brassica napus seed development. Plant Mol Biol 12: 198–212
Hong B, Uknes SJ, Ho T-HD (1988) Cloning and characterization of a cDNA encoding a mRNA rapidly induced by ABA in barely aleurone layers. Plant Mol Biol 11: 495–506
Hong B, Barg R, Ho T-HD (1992) Developmental and organ-specific expression of an ABA- and stress-induced protein in barley. Plant Mol Biol 18: 663–674
Jacobsen JV, Knox RB, Pyliotis NA (1971) The structure and composition of aleurone grains in the barley aleurone layer. Planta 101: 189–209
Kervinen J, Sarkkinen P, Kalkkinen N, Mikola L, Saarma M (1993) Hydrolytic specificity of the barley grain aspartic proteinase. Phytochemistry 32: 799–803
Koornneef M, Hanhart CJ, Hihorst HWM, Karssen CM (1989) In vivo inhibition of seed development and reserve protein accumulation in recombinants of abscisic acid biosynthesis and responsiveness mutants in Arabidopsis thaliana. Plant Physiol 90: 463–469
Levanony H, Rubin R, Altschuler Y, Galili G (1992) Evidence for a novel route of wheat storage protein vacuoles. J Cell Biol 119: 1117–1128
Lin LS, Ho TH-D (1986) Mode of action of abscisic acid in barley aleurone layers. Induction of new proteins by abscisic acid. Plant Physiol 82: 289–297
Marttila S, Jones BL, Mikkonen A (1995) Differential localization of two acid proteinases in germinating barley (Hordeum vulgare) seed. Physiol Plant 93: 317–327
Mikkonen A, Begbie R, Grant G, Pusztai A (1986) Intracellular localisation of some peptidases and α-mannosidase in cotyledons of resting kidney beans, Phaseolus vulgaris. Physiol Plant 68: 75–80
Mikola L, Mikola J (1980) Mobilization of proline in the starchy endosperm of germinating barley grain. Planta 149: 149–154
Monroe JD, Salminen MD, Preiss J (1991) Nucleotide sequence of a cDNA clone encoding β-amylase from Arabidopsis thaliana. Plant Physiol 97: 1599–1601
Muesch A, Hartmann E, Rohde K, Rubartelli A, Sitia R, Rapoport TA (1990) A novel pathway for secretory proteins? TIBS 15: 86–88
Nakamura K, Matsuoka K (1993) Protein targeting to the vacuole in plant cells. Plant Physiol 101: 1–5
Rahman S, Shewry PR, Miflin BJ (1982) Differential protein accumulation during barley grain development. J Exp Bot 33: 717–728
Rasmussen U, Munck L, Ullrich SE (1990) Immunogold localization of chymotrypsin inhibitor-2, a lysine-rich protein in developing barley endosperm. Planta 180: 272–277
Ried JL, Walker-Simmons MK (1993) Group 3 late embryogenesis abundant proteins in desiccation-tolerant seedlings of wheat (Triticum aestivum L.). Plant Physiol 102: 125–131
Roberts JK, DeSimone NA, Lingle WL, Dure III L (1993) Cellular concentrations and uniformity of cell-type accumulation of two Lea proteins in cotton embryos. Plant Cell 5: 769–780
Sambrook J, Fritsch EF, Maniatis T (1989) Use of chromogenic substrates with enzyme-coupled antibodies. In: Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press, New York, pp 18.74–75
Schneider K, Wells B, Schmelzer E, Salamini F, Bartels D (1993) Desiccation leads to the rapid accumulation of both cytosolic and chloroplastic proteins in the resurrection plant Craterostigma plantagineum. Hochst. Planta 189: 120–131
Seffens WS, Almoguera C, Wilde HD, Von der Haar RA, Thomas TL (1990) Molecular analysis of a phylogenetically conserved carrot gene: developmental and environmental regulation. Dev Genet 11: 65–76
Shewry PR, Miles MJ, Tatham AS (1994) The prolamin storage proteins of wheat and related cereals. Progr Biophys Mol Biol 61: 37–59
Slot JW, Geuze HJ (1985) A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol 38: 87–93
Straub PF, Shen Q, Ho T-HD (1994) Structure and promoter analysis of an ABA- and stress-regulated barley gene, HVA1. Plant Mol Biol 26: 617–630
Thomann EB, Sollinger J, White C, Rivin CJ (1992) Accumulation of group 3 late embryogenesis abundant proteins in Zea mays embryos. Plant Physiol 99: 607–614
Tranberger TJ, Franceschi VR, Hildebrand DF, Grimes HD (1991) The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell 3: 973–987
Varshavsky A (1992) The N-end rule. Cell 69: 725–735
Yoshihisa T, Anraku Y (1990) A novel pathway of import of αmannosidase, a marker enzyme of vacuolar membrane, in Saccharomyces cerevisiae. J Biol Chem 265: 22418–22425
Yupsanis T, Burgess SR, Jackson PJ, Shewry PR (1990) Characterisation of the major protein component from aleurone cells of barley (Hordeum vulgare L.). J Exp Bot 41: 385–392
Zeevaart JAD, Creelman RA (1988) Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 39: 439–473
Author information
Authors and Affiliations
Corresponding author
Additional information
We are grateful to Prof. David Ho (Washington Univ., St. Louis, Mo., USA) for the HVA1 antibody. In the University of Jyväskylä, Finland, the following persons are acknowledged: Dr Eeva-Liisa Punnonen for pA-gold conjugates, Mrs Raija Vassinen for technical assistance, Mr Paavo Niutanen for assistance in photography, Phil.lic. Jyrki Pusenius for help with statistical methods, and MSc. Ilkka Porali and MSc. Paula Sarkkinen for useful advice. Prof. David Ho, Dr. Qingxi Shen (Washington Univ., St. Louis) and Prof. Antero Salminen (Univ. Jyväskylä, Finland) are gratefully acknowledged for critical reading of the manuscript.
Rights and permissions
About this article
Cite this article
Marttila, S., Tenhola, T. & Mikkonen, A. A barley (Hordeum vulgare L.) LEA3 protein, HVA1, is abundant in protein storage vacuoles. Planta 199, 602–611 (1996). https://doi.org/10.1007/BF00195193
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00195193