Abstract
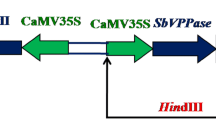
Sugarcane plant is a glycophyte, hence its growth and sucrose contents are severely affected by drought and salinity stresses. Bioengineering approaches offer a plausible and rapid solution to mitigate these losses. Therefore for genetic improvement of sugarcane against these stresses, the present study was conceived to transform Arabidopsis Vacuolar Pyrophosphatase (AVP1) gene—confers tolerance against drought and salinity—into sugarcane through Agrobacterium. For this purpose, highly regenerable apical buds of sugarcane variety CP77-400 were used as explants. EHA105 strain of Agrobacterium harboring pGreen0029 vector containing AVP1 gene driven under 35SCaMV promoter was employed for transformation. The key factors studied include application of acetosyringone, cefotaxime, kanamycin, and co-cultivation period for successful transformation. Maximum regeneration frequency of 77.5 % was achieved on MS media containing 1 mg/l BAP, 1 mg/l Kn, 1 mg/l GA3, 0.25 mg/l NAA, 50 μM acetosyringone, 500 mg/l cefotaxime, and 150 mg/l kanamycin on 3 days of co-cultivation. The results revealed that apical buds are distinctive viable tissues for sugarcane transformation and regeneration to produce a large number of CP77-400 transgenic plants in shorter period of time without intervening mosaics and chimeras. The AVP1 transcripts expression in transgenic lines at various levels was detected by RT-PCR. Longer and profuse root system was observed in transgenic plants in comparison with control plants. Concomitantly, only transgenic plants were able to withstand higher NaCl salt stress as well as scarcity of water thus, showing tolerance against salinity and drought stresses.





Similar content being viewed by others
Abbreviations
- BAP:
-
Benzyl amino purine
- MS:
-
Murashige and Skoog
- SIM:
-
Shoot induction medium
- RIM:
-
Root induction medium
- LB:
-
Luria broth
- NAA:
-
Naphthalene acetic acid
- Kn:
-
Kinetin
- GA3:
-
Gibberellic acid
References
Agrwal, P. K., Agarwal, P., Reddy, M. K., & Sopory, S. K. (2006). Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Reports, 25, 1263–1274.
FAO (Food, Agriculture Organization of the United Nations). (2004). FAO production year book. Rome: FAO.
Burke, E. J., Brown, S. J., & Christidis, N. (2006). Modeling the recent evolution of global drought and projections for the twenty first century with the Hadley centre climate model. Journal of Hydrometeorology, 7, 1113–1125.
Asif, M. A., Zafar, Y., Iqbal, J., Iqbal, M. M., Rashid, U., Ali, G. M., et al. (2011). Enhanced Expression of AtNHX1, in Transgenic Groundnut (Arachis hypogaea L.) Improves Salt and Drought Tolerance. Molecular Biotechnology, 49, 250–256.
Inman-Bamber, N. G., & Smith, D. M. (2005). Water relations in sugarcane and response to water deficits. Field Crops Research, 89, 185–202.
Zhang, S. Z., Yang, B. P., Feng, C. L., Chen, R. K., Luo, J. P., Cai, W. W., et al. (2006). Expression of the Grifola frondosa trehalose synthase gene and improvement of drought-tolerance in sugarcane (Saccharum officinarum L.). Journal of Integrative Plant Biology, 48, 453–459.
Azevedo, R. A., Carvalho, R. F., Cia, M. C., & Gratao, P. L. (2011). Sugarcane under pressure: An overview of biochemical and physiological studies of abiotic stress. Tropical Plant Biology, 4, 42–51.
Plaut, Z., Meinzer, F., & Federman, E. (2000). Leaf development, transpiration and ion uptake and distribution in sugarcane cultivars grown under salinity. Plant and Soil, 218, 59–69.
Akhtar, S., Wahid, A., & Rasul, E. (2003). Emergence, growth and nutrient composition of sugarcane sprouts under NaCl salinity. Biologia Plantarum, 46, 113–116.
Rozeff, N. (1995). Sugarcane and salinity—A review paper. Sugarcane, 5, 8–19.
Munns, R., & Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651–681.
Inman-Bamber, N. G., Bonnett, G. D., Spillman, M. F., Hewitt, M. L., & Jackson, J. (2008). Increasing sucrose accumulation in sugarcane by manipulating leaf extension and photosynthesis with irrigation. Australian Journal of Agricultural Research, 59, 13–26.
Wilkinson, S., & Davies, W. J. (2010). Drought, ozone, ABA and ethylene: new insights from cell to plant community. Plant Cell and Environment, 33, 510–525.
Suprasanna, P., Patade, V. Y., Desai, N. S., Devarumath, R. M., Kawar, P. G., Pagariya, M. C., et al. (2011). Biotechnological Developments in Sugarcane Improvement: An Overview. Sugar Technology, 13, 322–335.
Butterfield, K., Irvine, E., Valdez, G. M., & Mirkov, E. (2002). Inheritance and segregation of virus and herbicide resistance transgenes in sugarcane. Theoretical and Applied Genetics., 104, 797–803.
Gaxiola, R. A., Li, J., Undurraga, S., Dang, L. M., Allen, G. J., Alper, S. L., et al. (2001). Drought- and salt-tolerant plants result from overexpression of the AVP1 H+pump. Proceedings of the National Academy of Sciences of the United States of America, 98, 11444–11449.
Gaxiola, R. A., Fink, G. R., & Hirschi, K. D. (2002). Genetic manipulation of vacuolar proton pumps and transporters. Plant Physiology, 129, 967–973.
Park, S., Li, J., Pittman, J. K., Berkowitz, G. A., Yang, H., Undurraga, S., et al. (2005). Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proceedings of the National Academy of Sciences of the United States of America, 102, 18830–18835.
Cheng, M., Lowe, B. A., Spencer, T. M., Ye, X. D., & Armstrong, C. L. (2004). Factors influencing Agrobacterium-mediated transformation of monocotyledonous species. In Vitro Cellular and Development Biology—Plant, 40, 31–45.
Shrawat, A. K., & Lorz, H. (2006). Agrobacterium-mediated transformation of cereals: A promising approach crossing barriers. Plant Biotechnology Journal, 4, 575–603.
Li, R. Y., & Qu, R. D. (2011). High throughput Agrobacterium-mediated switchgrass transformation. Biomass and Bioenergy, 35, 1046–1054.
Murashige, T., & Skoog, K. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497.
Uzma Khan, M. R., Muhammad, A., Hussain, I., Shah, S. H., Kumar, T., Inam, S., et al. (2012). Rapid in vitro Multiplication of Sugarcane Elite Genotypes and Detection of Sugarcane Mosaic Virus through Two Steps RT-PCR. International Journal of Agriculture and Biology, 14, 870–878.
Rogers, S. O., & Bendich, A. J. (1988). Extraction of DNA from plant tissues. In S. B. Gelvin & R. A. Schilperoort (Eds.), Plant Molecular Biology Manual (pp. A1–A10). Boston: Kluwer Academic Publishers.
Khan, M. R., Khan, I., & Ali, G. M. (2012). MPF2-like MADS-box genes affecting SOC1 and MAF1 expression are implicated in flowering time control. Molecular Biotechnology, 12, 9540–9549.
Herrera-Estrella, L. (1999). Transgenic plants for tropical regions: Some considerations about their development and their transfer to the small farmer. Proceedings of the National Academy of Sciences of the United States of America, 96, 5978–5981.
Serrano, R., & Gaxiola, R. (1994). Microbial models and salt stress tolerance in plants. Critical Reviews in Plant Sciences, 13, 121–138.
Thomson, J. A. (2002). Research needs to improve agricultural productivity and food quality, with emphasis on biotechnology. Journal of Nutrition, 132, 3441S–3442S.
Chaves, M. M., & Oliveira, M. M. (2004). Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. Journal of Experimental Botany, 55, 2365–2384.
Penna, S. (2003). Building stress tolerance through over-producing trehalose in transgenic plants. Trends in Plant Science, 8, 355–357.
Vinocur, B., & Altman, A. (2005). Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Current Opinion in Biotechnology, 16, 123–132.
Zhang, J. Z., Creelman, R. A., & Zhu, J.-K. (2004). From Laboratory to Field. Using Information from Arabidopsis to Engineer Salt, Cold, and Drought Tolerance in Crops. Plant Physiology, 135, 615–621.
Joyce, P., Kuwahata, M., Turner, N., & Lakshmanan, P. (2010). Selection system and cocultivation medium are important determinants of Agrobacterium-mediated transformation of sugarcane. Plant Cell Reports, 29, 173–183.
Gupta, V., Raghuvanshi, S., Gupta, A., Saini, N., Gaur, A., Khan, M. S., et al. (2010). The water-deficit stressand red-rot-related genes in sugarcane. Functional & Integrative Genomics, 10, 207–214.
Trifonova, A., Madsen, S., & Olesen, A. (2001). Agrobacterium-mediated transgene delivery and integration into barley under arrange of In vitro culture conditions. Plant Sciences, 161, 871–880.
Dong, J. Z., Yang, M. Z., Jia, S. R., & Chua, N. H. (1991). Transformation of melon (Cucumismelo L.) and expression from the cauliflower mosaic virus 35S promoter in transgenic melon plants. Nature Biotechnology, 9, 858–863.
Li, X. Q., Liu, C. N., Ritchie, S. W., Peng, J., Gelvin, S. B., & Hodges, T. K. (1992). Factors influencing Agrobacterium-mediated transient expression of Gus Ainrice. Plant Molecular Biology, 20, 1037–1048.
Chaves, M. M., Pereira, J. S., Maroco, J., Rodrigues, M. L., Ricardo, C. P., Osório, M. L., et al. (2002). How plants cope with water stress in the field. Photosynthesis and growth. Annals of Botany, 89, 907–916.
Costa, E., Silva, F., Shvaleva, A., Maroco, J. P., Almeida, M. H., Chaves, M. M., et al. (2004). Responses to water stress in two Eucalyptus globulus clones differing in drought tolerance. Tree Physiology, 24, 1165–1172.
Ober, E. S., & Sharp, R. E. (2003). Electrophysiological responses of maize roots to low water potentials: Relationship to growth and ABA accumulation. Journal of Experimental Botany, 54, 813–824.
Pinheiro, H. A., Damatta, F. M., Chaves, A. R., Loureiro, M. E., & Ducatti, C. (2005). Drought tolerance is associated with rooting depth and stomatal control of water use in clones of Coffea canephora. Annals of Botany, 96, 101–108.
Sharp, R. E., & LeNoble, M. E. (2002). ABA, ethylene and the control of shoot and root growth under water stress. Journal of Experimental Botany, 53, 33–37.
Sharp, R. E., Poroyko, V., Hejlek, L. G., Spollen, W. G., Springer, G. K., Bohnert, H. J., et al. (2004). Root growth maintenance during water deficits: physiology to functional genomics. Journal of Experimental Botany, 55, 2343–2351.
Tschaplinski, T. J., Tuskan, G. A., Gebre, G. M., & Todd, D. E. (1998). Drought resistance of two hybrid Populus clones grown in a large-scale plantation. Tree Physiology, 18, 653–658.
Park, S., Jisheng, L., Jon, K. P., Gerald, A. B., Haibing, Y., Soledad, U., et al. (2005). Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proceedings of the National Academy of Sciences of the United States of America, 102, 18830–18835.
Hirschi, K. D. (1999). Expression of Arabidopsis CAX1 in tobacco: Altered calcium homeostasis and increased stress sensitivity. Plant Cell, 11, 2113–2122.
Buchman, A. R., & Berg, P. (1988). Comparison of intron-dependent and intron-independent gene expression. Molecular and Cellular Biology, 8, 4395–4405.
Chung, S., & Perry, R. (1989). Importance of introns for expression of mouse ribosomal protein gene rpL32. Molecular and Cellular Biology, 9, 2075–2082.
Okkema, P. G., Harrison, S. W., Plunger, V., Aryana, A., & Fire, A. (1993). Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics, 135, 385–404.
Ibrahim, M., Khan, S. A., Zafar, Y., Mansoor, S., Yusuf, A., & Mukhtar, Z. (2009). Expression of a full length Arabidopsis vacuolar H+pyrophosphatase (AVP1) gene in tobacco (Nicotiana tabaccum) to increase tolerance to drought and salt stress. Journal of Phytology, 1, 433–444.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Tanweer Kumar and Uzma have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Kumar, T., Uzma, Khan, M.R. et al. Genetic Improvement of Sugarcane for Drought and Salinity Stress Tolerance Using Arabidopsis Vacuolar Pyrophosphatase (AVP1) Gene. Mol Biotechnol 56, 199–209 (2014). https://doi.org/10.1007/s12033-013-9695-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-013-9695-z