Abstract
Metallic glasses, displaying extraordinary physical and chemical properties, have garnered robust research enthusiasm. Inspired by the exceptional wetting biological surfaces, superhydrophobic surfaces have attracted considerable attention. Superhydrophobic surfaces with both excellent mechanical and chemical stability could be prepared using metallic glasses and have developed considerably over the last few years. In this review, diverse fundamental aspects of wettability are discussed in detail. The methods for preparing superhydrophobic metallic glass surfaces are briefly summarized and compared. The corrosion resistance, self-cleaning, oil/water separation and other potential promising applications of the superhydrophobic surfaces are demonstrated. In the last section, the current limitations in preparative methods for superhydrophobic metallic glass surfaces and future trends in preparation and application are also discussed. It can be used to guide the surface modification of metallic glasses as well as more engineering applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The wettability of solid surfaces plays an essential role in our daily life as well as in many industrial processes. In 1907, a super-anti-wetting surface fabricated via coating soot with a water contact angle (WCA) of virtually 180° was first reported, which is the beginning of researches into surfaces with super wettability [1]. Changing the wettability of solid surfaces attracted increasing attention for both fundamental research and practical applications, especially for superhydrophobic surfaces until Barthlott and Neinhuis reported the “lotus effect” mechanism in 1997 [2]. The low surface energy of wax and the rough micro-nano structure jointly construct the superhydrophobicity (the surface with contact angle (CA) > 150° and sliding angle (SA) < 10°) of the lotus leaf, which brings about increasing research on mimicking natural biology [3,4,5].
Superhydrophobic surfaces inspired by nature are designed and widely applied in the fields of self-cleaning, anti-corrosion, waterproofing, biomedical instrument, marine antifouling, etc. [6]. Examples include bionic low drag and self-cleaning surfaces inspired by the rice leaf and butterfly effect [7, 8]. While a large amount of promising biomimetic materials has been fabricated on polymers, glass, carbon nanotubes, silicon nanowires, and other substrates, the superhydrophobic surfaces obtained on such substrates are invariably low in hardness, easily eroded, and easily destroyed [9,10,11,12]. To overcome this problem, using a metal or metal oxide as a substrate is a better choice, as they are generally not involved with wear and corrosion issues. Recently, the fabrication of superhydrophobic surface on metallic materials such as Al, Cu, stainless steel has received much attention [13,14,15,16]. As shown in Fig. 1, metallic glass was born in the period when metal as a structural material had the lowest influence in the material field [17], and it has deservingly won the favor of the majority of scientific researchers. The study found that constructing superhydrophobic surfaces on metallic glass has superior properties compared to pure metal or stainless steel.
Metallic glasses (MGs) are considered to be potential functional and structural materials used in aerospace devices, biomedical devices, precision devices, and other fields [18,19,20,21]. They have remained one of the most popular and cutting-edge researches since their development in 1960 [22]. Metallic glass is a glassy alloy solid formed by rapid superalloy melt supercooling to glass state transition, and the structure is suddenly "frozen". Different from traditional crystalline alloys, the atomic arrangement of metallic glass does not have symmetrical order on the long range scale, but only shows some order on the short and medium range scale [23]. Compared with conventional crystalline alloys, metallic glasses exhibit superior physical, chemical and mechanical properties, such as ultra-high strength, unique elongation and flexibility, outstanding corrosion resistance, and wear resistance, etc. due to their advantage of having no crystal defects such as grain boundaries and dislocations [24]. In addition, metallic glasses exhibit unique long-range disorder and near-range order in atomic-scale structural disorder. Due to the fact that the amorphous thermal conductivity mechanism relies on the collision of molecules, but there are no molecules in metallic glasses. Therefore, the thermal conductivity of metallic glasses is usually lower. Besides, laser etching of metallic glass surfaces can easily establish micro and nano structures [25]. In recent years, the production of superhydrophobic surfaces on metallic glasses has proven to be feasible, including Zr-based [26], Pd-based [27, 28] and Ce-based [29], etc., which has been extensively investigated.
In this paper, diverse fundamental aspects of wettability are discussed in detail. The methods for preparing superhydrophobic metallic glass surfaces are briefly summarized and compared. The corrosion resistance, self-cleaning, oil/water separation and other potential promising applications of the superhydrophobic surfaces are demonstrated. Besides, the current limitations in preparative methods for superhydrophobic metallic glass surfaces and future trends in preparation and application are also discussed. It can be used to guide the surface modification of metallic glasses as well as more engineering applications.
2 Wettability theory
From a bionic point of view, the lotus leaf with water repellency was used as a research object to reveal the hydrophobic mechanism. Barthlott et al. and Jiang et al. successively reported the key factors to lotus leaves’ hydrophobicity [2]. Nanostructured aligned carbon nanotubes and lotus-like aligned carbon nanotubes were prepared by Jiang et al. They demonstrated that the high CA was caused by the nanostructure and the low sliding angle was caused by the nano- and micro-structure. Furthermore, they found that the trajectory of water droplets on these surfaces was affected by the arrangement of the microarray structures [7].
In the following decade, a mass of biological superhydrophobic surfaces has been researched, such as water strider legs [30], mosquito compound eyes [31], etc. In the systematic study of superhydrophobic surfaces in nature, researchers have summarized two decisive factors for the construction of superhydrophobic surfaces: rough surface structures and low surface energy. For example, wax and micro-nano structures make up the superhydrophobic surface of lotus leaves. In this process, high adhesion of their surfaces was found on some organisms, such as rose petals [32], gecko feet [33].
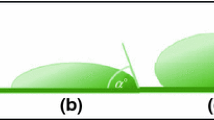
Generally, solid surfaces with CA < 90° are considered hydrophilic, correspondingly solid surfaces with CA > 90° are considered hydrophobic. In 1805, Thomas Young first reported the thermodynamic equilibrium between CA and solid surface tension, which indicates that the CA distinction between hydrophilic and hydrophobic is limited to 90° [34]. Young’s equation is shown in formula 1.
so
where \(\theta\) represent the solid, liquid and vapor three-phase balance CA of the materials, and \({\gamma }_{SV}\) is the solid/vapor interfacial tension, \({\gamma }_{SL}\) is the solid/liquid interfacial tension, \({\gamma }_{LV}\) is the liquid/vapor interfacial tension. However, Berg et al. claimed that 65° might be the boundary between hydrophilicity and hydrophobicity during the study of mixed Langmuir–Blodgett films [35]. Yoon et al. put forward another point of view: they proved that the hydrophobicity and hydrophilicity of solid materials were distinguished by a CA of 65° rather than 90° through experiments using a surface force meter supported by ancillary technology [36]. Long-range attractive forces appeared when a CA greater than 65° existed between the two interfaces. On the contrary, repulsive forces could be detected between interfaces with a CA less than 65°. Similar to this research, Guo et al. regarded an angle of 62.7° as a new division of material hydrophilicity and hydrophobicity after inspecting the apparent and inherent CA of bountiful polymer materials [37]. To solve the problem of Young’s equation being only applicable to ideal materials, two different wetting models suitable for rough surfaces, including the Wenzel model and the Cassie-Baxter model are established. The formulas of the two models are shown in formula 3 and formula 4, respectively [38, 39]:
The droplet fills the groove on the surface entirely in the Wenzel model where \({\theta }_{W}\) represent the apparent CA of rough surfaces, and \(\gamma\) is the solid surface roughness factor, that is, the ratio of the actual surface area of the rough surface to the visible surface area. The Cassie-Baxter model develops the Wenzel model, which assumes that a solid surface with a rough structure and a microscopic heterogeneity is a composite surface (requiring a uniform distribution of components per unit area). It is suitable for the situation where the air is trapped between the rough surfaces to form the solid–liquid-gas three-phase composite contact interface. In the Cassie-Baxter model, \({\theta }_{r}\) represent the apparent CA of rough surfaces, and \({f}_{s}\) is the frictional contact area. Therefore, the superhydrophobic surface can be realized by adjusting the microstructure of the material surface to reduce the ratio of the solid–liquid interface. The scope of applications and schematic diagrams of the above three models are shown in Table 1.
Considering the two decisive factors that affect the hydrophobicity of solid surfaces: surface chemistry and surface roughness, the construction of superhydrophobic surfaces generally have the following methods: 1) nano- and microstructures are constructed on the surface of low-surface-energy materials; 2) one-step construction of solid surface micro-nano structures and low surface energy modification [41, 42]. Many technological approaches, including physical, chemical, and the combination of physical and chemical strategies, have been developed to construct superhydrophobic surfaces so far, such as Etching [43, 44], Sol − Gel [45], Vapor Deposition [46], Electrochemical [47], Plasma Treatment [48], Spin-Coating [49], spraying [50] etc.. For example, Wu et al. presented a novel one-step sol–gel electrochemistry route. In a mixed sol–gel solution containing tetraethoxysilane and dodecyltrimethoxysilane, they prepared superhydrophobic composite films impregnated with an inorganic silica component by single-step electrodeposition [44].
3 The surface free energy of MGS
Surface free energy is the embodiment of intermolecular forces on the surface of an object. At present, measuring the CA is considered the best method to determine the surface energy of a solid, and the Owens -Wendt equation is shown in formula 5 [49]:
where \({\gamma }_{L}\) is the surface energy of the liquid, \({\gamma }_{S}\) is the surface energy of solid, superscript letters D and P denote dispersion force and polar forces, respectively. Through the formula, the CA of two different liquids can be used to calculate SPE of the solid [52]. The research group of Li et al. measured the WCA of Pd-, Mg-, Zr-, Cu-, Fe- and Ni-based bulk metallic glass (BMG), as depicted in Fig. 2 [19]. The WCA ranks from the largest of Pd-based BMG to the smallest of Cu-BMG, which corresponds to the gradual increase of surface energy. The surface energy of BMG for different systems varies greatly, and the addition of other elements into the same system also affects the static hydrophobic angle. Chang et al. gauged the means CA of Zr-based TFMGs at 97.7 ± 0.3°, which indicates its intrinsic hydrophobicity [53]. At present, there are many measurements on the intrinsic hydrophobic angle of Zr-based bulk metallic glasses (BMGs) in different systems, and the results show that the element composition and content are both important factors. Zhang et al. prepared Fe-based metallic glass coating using High-Velocity Oxygen Fuel (HVOF) technology with different feedstock powder sizes. The coating fabricated with the pony-size powders exhibited a hydrophilic property. In contrast, the coating made from the coarse powders exhibited a hydrophobic character with a WCA of 100 ± 2°. The coatings represented distinctly different wettability with the same ingredient and preparation technology. And this provides an essential basis for selecting BMGs to construct a superhydrophobic surface [54].
The essential CAs of water on BMG surfaces [19]
4 Preparation methods for superhydrophobic MG surfaces
As mentioned above, two general principles for the manufacture of superhydrophobic surfaces are the construction of micro-nano structures and low surface energy modification. For metallic glass, superhydrophobic surfaces are commonly constructed by constructing micro-nano structures and then conducting chemical modification. The methods generally include thermoplastic forming technology, etching, spraying, magnetron sputtering, electrochemical machining, etc.
4.1 Thermoplastic forming technology (TPF)
The thermoplastic forming technology is the most common method in preparation of superhydrophobic surfaces on BMGs. Unlike traditional crystalline alloys, metallic glasses have the unique super-plasticity within the supercooled liquid region, that is, they begin to soften when heated to a specific temperature and allows for precise processing, which is known as thermoplastic forming [55, 56]. And this technology has a series of advantages, such as high efficiency, high precision, simple process, and low cost [57,58,59].
Xia et al. prepared superhydrophobic surfaces with excellent stability on Pd-based metallic glass using only the hot-embossing process [27]. As expected, the CA of the patterned surface is much larger than that of a smooth surface and shows an increasing trend with growing pitches. This phenomenon is due to the influence of surface energy gradient. As shown in Fig. 3, the model is consistent with the Cassie-Baxter state. More air is trapped between the droplet and the closed honeycomb structure, which acts as a buffer layer to buffer the diffusion of water and slow down the penetration. And this explains the reason why the patterned surface shows excellent stability.
The SEM micrographs of the hot embossed honeycomb structures with various pitches, (a) 35.5 μm, (b) 75.5 μm, and (c) 115.5 μm. d The 3D profile of the honeycomb pattern with pitch of 75.5 μm. e The line scanning profile of two cells, indiating the average height of 86 lm of the pattern [27]
Similarly, Li et al. successfully prepared a superhydrophobic surface on Zr35Ti30Be26.75Cu8.25 MG [60], which demonstrated high adhesive force. It can be seen from the micro-nano structure model of the Zr-based MG surface in Fig. 4 that a large amount of air is trapped between the droplet and the micro-nano structures, which explains the mechanism of surface superhydrophobicity. And when the surface was turned upside down, nanoscale protuberance resembling gecko feet achieves strong adhesion to the droplets. The mechanism is the change of trapped air pressure before and after inversion. With the trapped air inverted, a negative pressure is generated so that droplets are adsorbed on the surface. Different from the work Li et al. reported, He et al. fabricated a multifunctional BMG with a WCA lager than 155° and a WSA less than 5° through the thermoplastic forming-based process followed by the SiO2/soot deposition [61].
a Schematics of a water droplet on the constructed Zr-based MG surfaces; (b, c) Schematic diagram of the change in the force of the liquid- vapor interface when the water drop is pulled upward [60]
To study the effect of surface structures on hydrophobicity, Hasan et al. create patterns on the surface of Pd-based BMGs at different scales on the surface of Pd-based BMGs using the continuous hot-embossing technique [62]. The result showed that Pd-based BMGs exhibited hydrophilicity under the smooth surface, and the single-scale structures such as micro holes, micro rods, and nanorods conform to the Wenzel model: the increased roughness allows for greater hydrophilicity (Fig. 5). Arora et al. investigated the wettability of various nanotextured metallic glass surfaces, which demonstrated that the variation in surface roughness and roughness could contribute to the difference in CA [63]. Recently, Hasan et al. disclosed the repercussions of surface structure and chemical component on the wetting of BMGs [64]. The result showed that inherently hydrophilic Pd-based MG surface could transform into hydrophobicity through the construction of single-scale microstructures without oxidation. Due to the adsorption of carbon, the wettability of Pd-based MG showed high sensitivity to time (Fig. 6); this situation can be observed in both mechanical demolding and chemical demolding samples.
Influence about surface structure on the wettability of Pt-MG [62]
The wetting kinetics of flat and nano-patterned Pd-MG. (Inset) An SEM image of a nano-patterned Pd-MG [64]
4.2 Chemical and physical etching
4.2.1 Chemical etching
Chemical etching, as a process of subtractive manufacturing through the chemical reaction between the chemical etching solution and the etched surface, is widely used in metals, glass, plastics, and other materials [65]. In 2009, Zhao et al. firstly fabricated a superhydrophobic surface on CaLi-based BMG [66]. Due to the fact that BMGs can be dissolved by water, water etching was used to increase the surface roughness. Similarly, Li et al. used water etching at different times and then modified with a 1.0% ethanol solution of FAS on Ca-based BMG to improve its hydrophobicity and corrosion resistance [67] (Fig. 7). Different from water etching, Liu et al. reported that HCl etching could construct micro-nano scale hierarchical surface structures on Ce-based MG [68]. Though dipping in 0.1 M HCl aqueous solution and FAS chemically modification for 12 h and then heating at 70℃ for 2 h, a superhydrophobic surface with the CA of 157° was successfully manufactured.
The SEM micrographs of Ca-based BMG samples after different surface treatment: a 15 min water etching; b 30 min water etching; c 15 min water etching + FAS; d 30 min water etching + FAS; e Fe coating; f Fe + FAS coating [67]
4.2.2 Laser etching
Laser etching, as a traditional material removal process, has become a principal research direction for BMG micromachining. The principle is that when the high-energy laser beam is concentrated on the surface of the workpiece, the material will be instantly heated, melted, and evaporated due to the interaction of the high-power laser pulse and the surface of the material [25]. Various microstructures, such as dimple-like microstructures and linear microgrooves et. could be fabricated on the MG surface via laser etching through adjusting the operating parameters such as laser processing textures spacing and laser scanning speed [69]. Fornell et al. observed that different intensities of surface laser treatment could regulate the wettability of Cu47.5Zr47.5Al5 MG surface [70]. Similarly, Jiao et al. reported that nanosecond (ns) laser processing texturing with different patterns could change the wettability of Zr-based BMG, where surface roughness played a significant role [71]. Recently, Huang et al. prepared hierarchical structures on the MG surface through laser etching in nitrogen gas [72]. Figure 8 showed the WCA of as-cast in comparison with laser patterned MG surfaces with different pulse overlap rates between two adjacent scanning liners. For r = 47%, the deep and wide micro-grooves trapped a great deal of air. In this case, the WCA of patterned MG surface increased to 144°.
a, b, c CA of MG surface different treatment; d, e Local contact statuses between the water droplet and the laser patterned surfaces corresponding to r = 82% and 47%, respectively [72]
Although laser etching has been widely used in structuring superhydrophobic surfaces on various substrates such as aluminum alloy, stainless steel, etc. [73], it is comparatively new for the construction of MG micro-nano surface. Moreover, the mechanism of the interaction between the MG and the laser is not yet precisely understood. More experimental and theoretical research is needed to avoid the occurrence of oxidation and crystallization during the laser etching process.
4.3 Electrochemical machining (ECM)
Electrochemical machining, as a method for processing metal materials using electrochemical reactions (or electrochemical corrosion), is not limited by the hardness and toughness of materials compared with mechanical machining. The principle of this technique is to use the chemical reaction of gain and loss of electrons on the surface of the cathode and anode to process the metal. Different surface structures can be obtained by controlling the reaction current and reaction time [74]. This method can be used to prepare a superhydrophobic surface on various metallic matrices such as Al, stainless steel, alloy coatings, and other organic coatings. Widely different structures using electrochemical processing have been reported in literature, such as nano-porous network and nano-dendritic structure (shown in Fig. 9), which was due to the change in the applied electric potential range [63]. In practice, electrochemical machining has been used in MG with superhydrophobic surfaces. For example, Pd43Ni10Cu27P20 MG has been electrochemically processed by an acidic medium to change its wettability. Pd-rich MG surfaces with different nanostructures exhibit disparate wettability. The CA was measured to be 112° for the nano-dendritic structure.
SEM images for palladium-rich metallic glass surfaces with (a) a nanorod structure, (b) a nanoporous morphology and (c) nanodendrites [63]
Recently, the development of a superficial hydrophobic film on Mg-based MG substrate using cyclic voltammetry (CV) treatments has been studied where nanoplate and nanosphere morphologies were prepared, followed by modification with stearic acid (SA) [75]. The sample surfaces exhibited a WCA of 131°. Furthermore, Yan et al. investigated the effects of four technological parameters (solution concentration, sweep rate, cycle number, and reaction temperature) in the CV process on the morphology and size of nano-products. This method also improved the corrosion resistance of MG.
4.4 Physical vapor deposition (PVD)
As a method in PVD, magnetron sputtering has the advantages of simple equipment, convenient control, large coating area, and strong adhesion [76]. Sputtering coating is a technology that bombards the target surface with charged particles in a vacuum to deposit the particles on the substrate, which can be used to fabricate thin-film metallic glass (TFMG). When manufacturing superhydrophobic surfaces, this technology can form highly controlled regular nanostructures. On account of the inherent hydrophobicity of TFMG in certain systems, a hydrophobic surface can be formed by coating a layer of TFMG on the surface of the substrate. In this method, the polyacrylonitrile membrane with Zr-based TFMG was created. The TFMG coating, which had a high WCA of 136°, possessed the ability to enable the separation of oil and water [77]. Meanwhile, it improved the chemical and thermal stability of the membrane. Li et al. used a different method to enhance wettability by coating a Fe film on Ca-based BMG [67]. The WCA of the BMG surface increased to 133.6° after coating with the Fe film and chemically modified with FAS, and the corrosion resistance was also improved.
4.5 Thermal spray method
Due to the limitation of the critical size (several centimeter level) in the field of BMGs, BMGs with large sizes are difficult to be prepared. To obtain noticeably sized amorphous specimens, metallic glass coatings have been successfully and widely fabricated on various substrates. The thermal spray technique is widely used for the preparation of high-performance metallic glass coatings [78]. It usually consists of two techniques, as discussed here. One is spraying powder material, including Plasma Spray (PS), High-Velocity Air Fuel Spray (HVAF), High-Velocity Oxygen Fuel Spray (HVOF), Detonation Spray (DS), etc.; the other is spraying powder core wire including Arc Wire Spray (AWS) [79]. Moreover, the high efficiency and low cost are also the advantages of manufacturing metallic glass coating. An early example by Zhang et al. presents the development of highly hydrophobic metallic glass coatings on top of mild steel (AISI 1045) using the HVOF technique, plainly demonstrating the industrial potential of thermally sprayed hydrophobic metallic glass coating [80]. The metallic glass coating also exhibited super-high hardness, excellent corrosion resistance, and clear self-cleaning effect. Zhang et al. fabricated Al-based amorphous/nanocrystalline coating on aluminum alloy 2024 using the HVAF spray technique [81]. The coating exhibited a low porosity (0.42%) and a high amorphous content (80.3%). Through the sealing treated with various sealants, the coating demonstrated different hydrophobic properties. The result showed stearic acid exhibiting the most significant improvement in the coating surface hydrophobicity due to its excellent impermeability and hydrophobicity. The WAC increased from 70° of the as-sprayed coating to 130° after the stearic acid sealing treatment. Meanwhile, the corrosion resistance of the coating was improved.
Plasma Spray (PS) exhibits higher efficiency compared with the conventional HVOF process. Qiao et al. prepared a super-hard superhydrophobic Fe-based metallic glass coating with a WCA of 154° ± 2° and a WSA of 4° ± 1° on the Q235 steel substrate (a carbon structural steel) [82]. The manufacturing process of the superhydrophobic coating used plasma spraying with different spray powers to construct appropriate surface features. FAS17 was then used for surface chemical modification to obtain lower surface energy. The coating with multi-level surface features was fabricated by a spray power of 30 kW. Micron-sized, non-flattening particles, splatter particles, and nano-sized wool-like oxides formed the hierarchical morphology of the coating surface. Recently, Al-based metallic glass coatings with high glass formation ability and prominent performance were prepared using the arc spraying process [83]. The coating exhibited excellent hydrophobicity with a WCA of 146° without modification, indicating that it has the potential to become a superhydrophobic coating. Compared with supersonic flame spraying, the amorphous coating prepared by cold spraying technology has higher amorphous content, denser coating, lower porosity and better thermal stability [84]. It can effectively avoid problems such as oxidation, phase change and thermal cracking caused by high temperature during preparation.
4.6 Other methods
Apart from the previous preparation methods, some other techniques, including hydrothermal method, diamond turning and heat treatment have also been utilized to alter the wettability of bulk metallic glasses [85, 86]. For example, Cui et al. fabricated micro-nano scale hierarchical fracture surfaces of Zr-based MG [58]. Three-point bending method followed by annealing treatment with different times were used to adjust surface wettability. Similarly, Wang et al. prepared a hydrophobic Fe-W MG surface by heat treatment and further chemical modification [85]. Table 2 summarizes the surface preparation method of MG with superhydrophobic surfaces.
5 Promising application
5.1 Corrosion resistance
Corrosion is a widespread problem in daily life, and large economic losses are caused by corrosion each year. To avoid corrosion, research into the corrosion resistance of materials has attracted intense attention in recent years. Currently, superhydrophobic surfaces have been widely fabricated on a variety of metal and alloy substrates, such as Al, Cu, Fe, Ti, steel, and so on, to provide adequate anti-corrosion protection [87,88,89]. The corrosion resistance mechanism of superhydrophobic surfaces can be expounded as follows: an air cushion between the surface and the corrosive medium is formed due to the micro-nano-level rough structure and low surface energy property of superhydrophobic surfaces, which significantly reduces the contact area between the corrosive medium and the surface, effectively increasing the substrate surface resistance against invading corrosion particles [90]. Liu et al. presented another important factor: ‘capillarity’, in anti-corrosion improvement, where water transport against gravity is easy in porous structures due to capillarity so that the seawater can be pushed out from the pores of the superhydrophobic surfaces by the Laplace pressure. Thus, the substrates could be protected perfectly from corrosion [91]. Li et al. compared Fe film and FAS thin film coated on the surface of Ca60Mg15Zn25 BMGs [67]. The result showed that Fe coated Ca60Mg15Zn25 BMGs demonstrated excellent corrosion resistance with an average hydrogen generation rate of 0.1076 ml cm−2 h−1. In comparison, the untreated sample showed a higher average hydrogen generation rate of 7.509 ml cm−2 h−1. Similarly, to improve the corrosion resistance of Mg-based MGs, Yan et al. constructed a MgO nanoplate array film on the surface of MGs [75]. As shown in Fig. 10, the composite hydrophobic film exhibited better corrosion resistance when compared with the untreated MGs and the crystal material with the same composition.
a BSE image and (b) TEM image of the hydrophobic film; (c) polarization curves and (d) open circuit potential of samples treated in different ways [75]
Huang et al. [79], Qiao et al. [82] and Cheng et al. [83] prepared hydrophobic MG coatings through the thermal spray method. All coatings displayed excellent corrosion resistance. However, these coatings only exhibited hydrophobicity with the WCA greater than 90° and less than 150°. In 2014, Ma et al. reported a superhydrophobic Pd-based MG surface with superior corrosion resistance [28]. Recently, Xiao et al. fabricated a superhydrophobic Zr-based BMG surface [26]. Electrochemical tests were performed with a scanning rate of 0.33 mV/s in a 3.5 wt% NaCl solution to determine the corrosion resistance of superhydrophobic Zr-based BMG. The result showed that the corrosion current density (Icorr) of the original alloy surface and the superhydrophobic Zr-based BMG surface were 4.50 × 10−8 A/cm2 and 1.14 × 10−8 A/cm2, respectively. Furthermore, the Icorr of the superhydrophobic Zr-based BMG surface was more than an order of magnitude lower than superamphiphobic stainless steel with an Icorr of 1.04 × 10−7A/cm2, indicating the excellent corrosion resistance of the superhydrophobic Zr-based BMG surface.
5.2 Self-cleaning
Self-cleaning, as a distinctive attribute of material surfaces, has various applications in our daily life. This phenomenon is usually interpreted as the removal of dirt from the surface. There are two primary manufacturing methods to obtain materials with self-cleaning property [92]. The first method is to create super hydrophilic surfaces, where it is cleaned through decomposing organic pollutants under light. Typically, TiO2 and related compounds are used as photocatalysts. The second strategy is to construct bio-inspired superhydrophobic surfaces. In the case of superhydrophobic surfaces, a WCA large than 150°, and a WSA less than 10° allows the droplet to easily roll off from the surface and take away the dirt particles. Here, we will mainly discuss the metallic glass superhydrophobic surfaces-induced self-cleaning. He et al. reported a self-cleaning BMG material constructed using the TPF process followed by SiO2/soot deposition (Fig. 11) [61]. The surface structure is composed of micro-pits covered by random nanostructures, which is similar to the lotus leaf.
ESEM images of the silicon mould surface and the resultant bulk metallic glass replica surface at different magnification; Demonstration of self-cleaning and low adhesion ability of the superhydrophobic BMGs [61]
In comparison to the original smooth BMGs, water droplets can easily remove the glass powder from the superhydrophobic BMGs. Similarly, Liu et al. adopted a biomimetic method to construct Micro-nanoscale hierarchical structures on Ce-based BMG surfaces with further modification using FAS [29]. Such BMG surfaces exhibited self-cleaning behavior, while self-cleaning lotus leaf-like porous morphology was fabricated through HCl-treatments. Zhang et al. showed a one-step method on the preparation of the robust superhydrophobic self-cleaning Fe-based amorphous coating [80]. The coating also showed super-hard, robust, and highly corrosion-resistant properties.
5.3 Oil/water separation
In recent years, the discharge of enormous volumes of oily wastewater from industry and the increase in offshore oil spills presents severe threats to the aquatic environment and also pose a massive challenge to sewage treatment technology. Compared with the traditional low-efficiency and high-cost methods of oil–water separation (such as ultrasonic separation, air flotation, electric field, etc.), technology using a significant number of unique wetting materials to separate the oil/water mixture efficiently have attracted full attention [93,94,95]. Generally, the wetting materials can be divided into two kinds: one type of surfaces integrated with both hydrophobicity and oleophilicity, and the other integrated with both hydrophilicity and oleophobicity. A series of functional wetting materials, such as cotton fabric, silica aerogel, Nanocellulose, etc. have been manufactured through different synthetic strategies to achieve the oil and water mixture separation [5, 96, 97]. In 2004, Feng et al. first reported the successful preparation of a novel coating mesh film, which can separate oil and water adequately [98]. The hard coating mesh film was fabricated through a spray-and-dry method and had both superhydrophobic and super-oleophilic properties. Based on this strategy, separation techniques developed rapidly and have been employed in industrial machines that require efficient oil–water separation capabilities.
The first work concerning metallic glass for oil–water separation was reported by Kassa et al., in 2019. The specific details have been described in the previous section [77]. This study overcame the difficulty of metallic glass applied in membranes for oil–water separation. The results showed that it was highly possible for the resultant film to take full advantage of the strong oil displacement effect of the surfactant sodium dodecyl sulfate (SDS) (95% to 100%), and could be used for altering the dimensions of the oil particles. Furthermore, the resulting TFMG-coated membranes showed stable chemical stability in N-dimethylacetamide (DMAc) at 166 °C, which is significant for application in industrial fields. Recently, the fabrication of Zr-based BMG with superhydrophobic/ superoleophilic surfaces made it possible for bulk metallic glasses to be used in oil/water separation [26].
5.4 Other potential applications
In addition to the above-mentioned applications, metallic glass with special wettability also shows great potential in biomedical application [99]. Sun et al. [100] have evaluated the chemical stability and biological safety of the Zr61Ti2Cu25Al12 BMG for clinical requirements, which shows that the Zr61Ti2Cu25Al12 BMG has good wettability and is expected to be used in biomedical applications. Antibacterial activity studies were employed to inspect the biocompatibility of Zr based TFMGs [101, 102], and the results show that Zr based TFMG with Ag and Cu have good antibacterial properties. The surfaces combined with both strong adhesion and superhydrophobicity are a suitable contestant for dry adhesives and transport of liquid micro-droplets due to the high adhesive force of liquids on such surfaces [103]. Li et al. fabricated a Zr-based metallic glass superhydrophobic surface, which can be used in droplet transmission systems [60]. A superamphiphobic CaLi-based bulk metallic glass surface was prepared by Zhao et al., which showed broad application prospects for usage such as oil transportation, oil storage, and oil crawling [66].
Many MGs have been used in high-temperature applications [104,105,106]. The behavior of brazing joints with nickel-silicon-based amorphous alloys ribbons was analyzed and Ni78Si8B15 MG had a good castability, wettability and flowability [104]. A series of sessile droplet wetting experiments was carried out to study the wettability MG melt-droplets on the Cu or Mo plates at high temperatures, which provide the basis for selecting the roller materials for producing MG by twin-roll casting [106]. Superhydrophobic surfaces have another extraordinary capability to delay and reduce the accumulation/adhesion of wet snow, ice, or frost. Recently, usage of numerous superhydrophobic surfaces prepared on metal substrates in the anti-icing field have been proposed. For example, Xing et al. fabricated a superhydrophobic anti-icing aluminum alloy surface through picosecond laser processing [107]. The result showed that compared with a hydrophobic surface, the enhanced anti-icing ability of the superhydrophobic surface was due to the reduction of the surface liquid–solid contact area, which resulted in cutback of the electrostatic force & van der Waals force, decreased the solid–liquid adhesion, and subdued the heat loss by way of heat transfer. Moreover, Jung et al. investigated the influence of environmental conditions such as humidity and flowing gas on the ice crystallization mechanism through experiments, nucleation theory, and heat transfer physics [108]. Bai et al. proved the existence of a critical ice nucleus through theoretical calculations and experimental analysis [109]. These findings could provide novel ideas to superhydrophobic anti-icing materials. However, reports on the potential anti-icing applications of the metallic glass are quite rare. Lu et al. fabricated FeMnCrB metallic glass with low curie point via plasms spray, which could be applied in anti-icing in transmission lines [110]. The potential applications of MG with superhydrophobic surfaces have been shown in Table 3.
6 Outlook and conclusions
The development of superhydrophobic metallic glass surfaces, including wetting theory, fabrication methods, and promising applications have been systematically reviewed in this paper. The report demonstrates metallic glass with special wettability’s potential to become the future generation of advanced functional materials. Inspired by nature, various bionic superhydrophobic surfaces with similar or better properties have been constructed by combining surface roughness structures and surface free energy. Strategies for the preparation of superhydrophobic surfaces can be simply divided into two types: one with pre-roughening and post-modification, and the other with one-step roughening and modification. In recent years, superhydrophobic surfaces prepared with metallic glasses are widely investigated not only for their superior properties such as ultrahigh strength, excellent corrosion and wear resistance, etc., but also for the easy construction of micro-nano structures. The potential applications are also reviewed, including corrosion resistance, self-cleaning, oil/water separation, biomedical application, transport of liquid micro-droplets, superamphiphobicity (high and low temperature), and anti-icing.
Although researchers have achieved a lot so far, there are still certain difficulties and challenges to overcome, and there is still a long way to go from the laboratory experimental stage to actual industrial applications:
-
1.
Surface energy is one of the most important factors to measure wettability. Although metallic glass has the advantage of lower free energy compared to metal, the static water CA of metallic glass is mostly less than 90°, which is worse than fluoride or fluoropolymers. At the same time, the free energy of different kinds of metallic glass is quite different, so it is necessary to develop metallic glass with lower surface energy.
-
2.
Due to the lack of systematic summary of the effect of structure on wettability, the design of metal–glass layered structures should be thoroughly analyzed.
-
3.
When constructing metal glass surface, due to the limitation of preparation methods, such as thermoplastic forming, laser etching, etc. Different degrees of crystallization and oxidation occur rapidly, which inevitably affects the original properties of metallic glass. Because the larger grain boundaries in metallic glass are short, laser etching has been recognized as an ideal candidate for machining micro and nano structures. But more laser system parameters need to be determined to ensure that material removal is carefully controlled without damaging the area around the material.
-
4.
Due to the low surface free energy, a variety of modifiers, such as HF, FAS, etc., are usually used to provide water resistance by chemically modifying the surface of metallic glass. However, it may become damaged after wear or corrosion, resulting in reduced wettability. Considering this problem, it is necessary to design ultra-wetting metallic glass without modifier coating. It is clear that these studies of metallic glass surfaces with ultra-wettability open new doors for the manufacture of surfaces with excellent physical and chemical properties. It can also be used to guide the surface modification of metallic glass and more engineering applications.
Availability of data and materials
The datasets used during the current study will be made available on request.
References
Ollivier H (1907) Recherches sur la capillarité. J Phys: Theor Appl 6(1):757–782. https://doi.org/10.1051/jphystap:019070060075700
Barthlott W, Neinhuis C (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202:1–8. https://doi.org/10.1007/s004250050096
Zhang X, Shi F, Niu J et al (2008) Superhydrophobic surfaces: from structural control to functional application. J Mater Chem 18(6):621–633. https://doi.org/10.1039/b711226b
Hongbo Q (2022) Research progress in superhydrophobic wood surface based on bionic technology. China Plast 36(2):182. https://doi.org/10.19491/j.issn.1001-9278.2022.02.024
Li S, Huang J, Chen Z et al (2017) A review on special wettability textiles: theoretical models, fabrication technologies and multifunctional applications. J Mater Chem A 5(1):31–55. https://doi.org/10.1039/c6ta07984a
Zhang C, Zhang X, Shen H, et al. Superior self-cleaning surfaces via the synergy of superhydrophobicity and photocatalytic activity: Principles, synthesis, properties, and applications. J Clean Prod 2023:139430. https://doi.org/10.1016/j.jclepro.2023.139430
Feng L, Li S, Li Y et al (2002) Super-hydrophobic surfaces: from natural to artificial. Adv Mater 14(24):1857–1860. https://doi.org/10.1002/adma.200290020
Zheng Y, Gao X, Jiang L (2007) Directional adhesion of superhydrophobic butterfly wings. Soft Matter 3(2):178–182. https://doi.org/10.1039/b612667g
Liu F, Wang L, Sun Q et al (2012) Transesterification catalyzed by ionic liquids on superhydrophobic mesoporous polymers: heterogeneous catalysts that are faster than homogeneous catalysts. J Am Chem Soc 134(41):16948–16950. https://doi.org/10.1021/ja307455w
Xu M, Feng Y, Li Z et al (2019) A novel, efficient and cost-effective synthesis technique for the development of superhydrophobic glass surface. J Alloy Compd 781:1175–1181. https://doi.org/10.1016/j.jallcom.2018.12.084
Li Z, Wang B, Qin X et al (2018) Superhydrophobic/superoleophilic polycarbonate/carbon nanotubes porous monolith for selective oil adsorption from water. ACS Sustain Chem Eng 6(11):13747–13755. https://doi.org/10.1021/acssuschemeng.8b01637
Choi CH, Ko DH, Park B et al (2019) Air-water interfacial fluidic sonolysis in superhydrophobic silicon-nanowire-embedded system for fast water treatment. Chem Eng J 358:1594–1600. https://doi.org/10.1016/j.cej.2018.10.126
Muangnapoh T, Janampansang N, Chuphong S et al (2023) The study of the anti-icing performance of superhydrophobic silica-nanostructured metal substrates. Coll Interface Sci Commun 57:100745. https://doi.org/10.1016/j.colcom.2023.100745
Adel-Mehraban F, Raeissi K, Karimzadeh F (2023) Grafting aryldiazonium cations to copper: insights into wettability behavior of a highly hydrophobic coating with enhanced corrosion performance. J Ind Eng Chem 123:488–499. https://doi.org/10.1016/j.jiec.2023.04.002
Birkett M, Dover L, Cherian Lukose C et al (2022) Recent advances in metal-based antimicrobial coatings for high-touch surfaces. Int J Mol Sci 23(3):1162. https://doi.org/10.3390/ijms23031162
Myronyuk O, Baklan D, Rodin AM (2023) UV resistance of super-hydrophobic stainless steel surfaces textured by femtosecond laser pulses. Photonics MDPI 10(9):1005. https://doi.org/10.3390/photonics10091005
Ashby MF, Cebon D (2005) Materials selection in mechanical design. MRS Bull 30(12):995. https://doi.org/10.1051/jp4:1993701
Korkmaz S, Kariper İA (2020) Glass formation, production and superior properties of Zr-based thin film metallic glasses (TFMGs): a status review. J Non-Cryst Solids 527:119753. https://doi.org/10.1016/j.jnoncrysol.2019.119753
Li N, Chen W, Liu L (2016) Thermoplastic micro-forming of bulk metallic glasses: a review. Jom 68:1246–1261. https://doi.org/10.1007/s11837-016-1844-y
Meagher P, O’Cearbhaill ED, Byrne JH et al (2016) Bulk metallic glasses for implantable medical devices and surgical tools. Adv Mater 28(27):5755–5762. https://doi.org/10.1002/adma.201505347
Khan MM, Nemati A, Rahman ZU et al (2018) Recent advancements in bulk metallic glasses and their applications: a review. Crit Rev Solid State Mater Sci 43(3):233–268. https://doi.org/10.1080/10408436.2017.1358149
Klement W, Willens RH, Duwez POL (1960) Non-crystalline structure in solidified gold–silicon alloys. Nature 187(4740):869–870. https://doi.org/10.1038/187869b0
Hirata A, Guan P, Fujita T et al (2011) Direct observation of local atomic order in a metallic glass. Nat Mater 10(1):28–33. https://doi.org/10.1038/NMAT2897
Wang WH, Dong C, Shek CH (2004) Bulk metallic glasses. Mater Sci Eng R Rep 44(2–3):45–89. https://doi.org/10.1016/j.mser.2004.03.001
Williams E, Lavery N (2017) Laser processing of bulk metallic glass: a review. J Mater Process Technol 247:73–91. https://doi.org/10.1016/j.jmatprotec.2017.03.034
Xiao S, Zhang H, Guo S (2020) Fabrication of a Zr-based bulk metallic glass surface with extreme wettability. J Non-Cryst Solids 536:120001. https://doi.org/10.1016/j.jnoncrysol.2020.120001
Xia T, Li N, Wu Y et al (2012) Patterned superhydrophobic surface based on Pd-based metallic glass. Appl Phys Lett 101(8):169902. https://doi.org/10.1063/1.4747327
Ma J, Zhang XY, Wang DP et al (2014) Superhydrophobic metallic glass surface with superior mechanical stability and corrosion resistance. Appl Phys Lett 104(17):173701. https://doi.org/10.1063/1.4874275
Liu K, Li Z, Wang W et al (2011) Facile creation of bio-inspired superhydrophobic Ce-based metallic glass surfaces. Appl Phys Lett 99(26):261905. https://doi.org/10.1063/1.3672036
Feng XQ, Gao X, Wu Z et al (2007) Superior water repellency of water strider legs with hierarchical structures: experiments and analysis. Langmuir 23(9):4892–4896. https://doi.org/10.1021/la063039b
Gao X, Yan X, Yao X et al (2007) The dry-style antifogging properties of mosquito compound eyes and artificial analogues prepared by soft lithography. Adv Mater 19(17):2213–2217. https://doi.org/10.1002/adma.200601946
Feng L, Zhang Y, Xi J et al (2008) Petal effect: a superhydrophobic state with high adhesive force. Langmuir 24(8):4114–4119. https://doi.org/10.1021/la703821h
Liu K, Du J, Wu J et al (2012) Superhydrophobic gecko feet with high adhesive forces towards water and their bio-inspired materials. Nanoscale 4(3):768–772. https://doi.org/10.1039/c1nr11369k
Young T. An essay on the cohesion of fluids. In: Abstracts of the Papers Printed in the Philosophical Transactions of the Royal Society of London. London: The Royal Society 1832(1):171–172. https://doi.org/10.1098/rspl.1800.0095
Berg JM, Eriksson LGT, Claesson PM et al (1994) Three-component Langmuir-Blodgett films with a controllable degree of polarity. Langmuir 10(4):1225–1234. https://doi.org/10.1021/la00016a041
Yoon RH, Flinn DH, Rabinovich YI (1997) Hydrophobic interactions between dissimilar surfaces. J Colloid Interface Sci 185(2):363–370. https://doi.org/10.1006/jcis.1996.4583
Guo C, Wang S, Liu H et al (2008) Wettability alteration of polymer surfaces produced by scraping. J Adhes Sci Technol 22(3–4):395–402. https://doi.org/10.1163/156856108X304832
Wenzel RN (1936) Resistance of solid surfaces to wetting by water. Ind Eng Chem 28(8):988–994. https://doi.org/10.1021/ie50320a024
Cassie ABD, Baxter S (1944) Wettability of porous surfaces. Trans Faraday Soc 40:546–551. https://doi.org/10.1039/TF9444000546
Fihri A, Bovero E, Al-Shahrani A et al (2017) Recent progress in superhydrophobic coatings used for steel protection: a review. Colloids Surf, A 520:378–390. https://doi.org/10.1016/j.colsurfa.2016.12.057
Souza AM, Ferreira R, Barragán G et al (2021) Effects of laser polishing on surface characteristics and wettability of directed energy-deposited 316L stainless steel. J Mater Eng Perform 30(9):6752–6765. https://doi.org/10.1007/s11665-021-05991-y
Vishnu J, Manivasagam G (2021) High-surface-energy nanostructured surface on low-modulus Beta titanium alloy for orthopedic implant applications. J Mater Eng Perform 30(6):4370–4379. https://doi.org/10.1007/s11665-021-05769-2
Zhou Y, Ye Q, Han Y et al (2023) Fabrication of superhydrophobic porous brass by chemical dealloying for efficient emulsion separation. Molecules 28(18):6509. https://doi.org/10.3390/molecules28186509
Wu LK, Hu JM, Zhang JQ (2013) One step sol–gel electrochemistry for the fabrication of superhydrophobic surfaces. J Mater Chem A 1(46):14471–14475. https://doi.org/10.1039/c3ta13459h
Taurino R, Cannio M, Boccaccini DN et al (2023) Preliminary study on the design of superhydrophobic surface by 3D inkjet printing of a sol-gel solution. J Sol-Gel Sci Technol 108(2):368–376. https://doi.org/10.1007/s10971-023-06193-9
Zhai Y, Yuan X (2023) Superhydrophobic, magnetic aerogels based on nanocellulose fibers derived from harakeke for oily wastewater remediation. Polymers 15(19):3941. https://doi.org/10.3390/polym15193941
Liu J, Qiu C, Xu Z et al (2023) Tailoring hydrophobic-aerophilic microenvironment for robust electrochemical ozone production. Chem Eng J 468:143504. https://doi.org/10.1016/j.cej.2023.143504
Ahmadizadeh N, Sobhani M, Habibolahzadeh A (2023) Enhancement of hydrophobic properties of HTV silicone rubber by CF4 plasma treatment. Appl Surf Sci 641:158534. https://doi.org/10.1016/j.apsusc.2023.158534
Zhang F, Qian H, Wang L et al (2018) Superhydrophobic carbon nanotubes/epoxy nanocomposite coating by facile one-step spraying. Surf Coat Technol 341:15–23. https://doi.org/10.1016/j.surfcoat.2018.01.045
Guo X, Shao Z, Wang H et al (2024) Mechanically durable superhydrophobic coating of poly (ethyl cyanoacrylate)/SiO2/polydimethylsiloxane with superior abrasion resistance. Prog Org Coat 186:108044. https://doi.org/10.1016/j.porgcoat.2023.108044
Owens DK, Wendt RC (1969) Estimation of the surface free energy of polymers. J Appl Polym Sci 13(8):1741–1747. https://doi.org/10.1002/app.1969.070130815
Altay BN, Ma R, Fleming PD et al (2020) Surface free energy estimation: a new methodology for solid surfaces. Adv Mater Interfaces 7(6):1901570. https://doi.org/10.1002/admi.201901570
Chang CH, Li CL, Yu CC et al (2018) Beneficial effects of thin film metallic glass coating in reducing adhesion of platelet and cancer cells: clinical testing. Surf Coat Technol 344:312–321. https://doi.org/10.1016/j.surfcoat.2018.03.040
Zhang C, Guo RQ, Yang Y et al (2011) Influence of the size of spraying powders on the microstructure and corrosion resistance of Fe-based amorphous coating. Electrochim Acta 56(18):6380–6388. https://doi.org/10.1016/j.electacta.2011.05.020
Meng Y, Cheng J, Zhou C (2023) Superhydrophobic and stretchable carbon nanotube/thermoplastic urethane-based strain sensor for human motion detection. ACS Applied Nano Mater 6(7):5871–5878. https://doi.org/10.1021/acsanm.3c00246
Saotome Y, Itoh K, Zhang T et al (2001) Superplastic nanoforming of Pd-based amorphous alloy. Scripta Mater 44(8–9):1541–1545. https://doi.org/10.1016/S1359-6462(01)00837-5
Leamy HJ, Wang TT, Chen HS (1972) Plastic flow and fracture of metallic glass. Metall Mater Trans B 3:699–708. https://doi.org/10.1007/BF02642754
Cui X, Li JJ, Qiao JC et al (2020) Relating melting temperature with structure heterogeneity and plasticity of Zr57Cu20Al10Ni8Ag5 bulk metallic glass. J Non-Cryst Solids 543:120100. https://doi.org/10.1016/j.jnoncrysol.2020.120100
Yuan H, Pan Y, Wang X et al (2020) Simple water tunable polyurethane microsphere for super-hydrophobic dip-coating and oil-water separation. Polymer 204:122833. https://doi.org/10.1016/j.polymer.2020.122833
Li N, Xia T, Heng L et al (2013) Superhydrophobic Zr-based metallic glass surface with high adhesive force. Appl Phys Lett 102(25):251603. https://doi.org/10.1063/1.4812480
He Y, Peng Y, Li Z et al (2016) Bio-inspired multifunctional metallic glass. Sci China Chem 59:271–276. https://doi.org/10.1007/s11426-015-5496-5
Hasan M, Schroers J, Kumar G (2015) Functionalization of metallic glasses through hierarchical patterning. Nano Lett 15(2):963–968. https://doi.org/10.1021/nl504694s
Arora HS, Xu Q, Xia Z et al (2013) Wettability of nanotextured metallic glass surfaces. Scripta Mater 69(10):732–735. https://doi.org/10.1016/j.scriptamat.2013.08.014
Hasan M, Warzywoda J, Kumar G (2018) Decoupling the effects of surface texture and chemistry on the wetting of metallic glasses. Appl Surf Sci 447:355–362. https://doi.org/10.1016/j.apsusc.2018.03.205
Huang Z, Geyer N, Werner P et al (2011) Metal-assisted chemical etching of silicon: a review: in memory of Prof. Ulrich Gösele. Adv Mater 23(2):285–308. https://doi.org/10.1002/adma.201001784
Zhao K, Liu KS, Li JF et al (2009) Superamphiphobic CaLi-based bulk metallic glasses. Scripta Mater 60(4):225–227. https://doi.org/10.1016/j.scriptamat.2008.10.009
Li H F, Wang Y B, Cheng Y, et al. Surface modification of Ca60Mg15Zn25 bulk metallic glass for slowing down its biodegradation rate in water solution[J]. Materials Letters. 2010;64(13):1462–4. https://doi.org/10.1016/j.matlet.2010.03.060.
Zhang L, Huang H (2019) Micro machining of bulk metallic glasses: a review. Int J Adv Manufact Technol 100(1–4):637–661. https://doi.org/10.1007/s00170-018-2726-y
Jiao Y, Brousseau E, Han Q et al (2019) Investigations in nanosecond laser micromachining on the Zr52.8Cu17.6Ni14.8Al9.9Ti4.9 bulk metallic glass: Experimental and theoretical study. J Mater Process Technol. 273:116232. https://doi.org/10.1016/j.jmatprotec.2019.05.013
Fornell J, Pellicer E, Garcia-Lecina E et al (2014) Structural and mechanical modifications induced on Cu47.5Zr47.5Al5 metallic glass by surface laser treatments. Appl Surf Sci 290:188–193. https://doi.org/10.1016/j.apsusc.2013.11.032
Jiao Y, Brousseau E, Shen X et al (2020) Investigations in the fabrication of surface patterns for wettability modification on a zr-based bulk metallic glass by nanosecond laser surface texturing. J Mater Process Technol 283:116714. https://doi.org/10.1016/j.jmatprotec.2020.116714
Huang H, Yan J (2017) Surface patterning of zr-based metallic glass by laser irradiation induced selective thermoplastic extrusion in nitrogen gas. J Micromech Microeng 27(7):075007. https://doi.org/10.1088/1361-6439/aa71d5
Chen F, Zhang D, Yang Q et al (2013) Bioinspired wetting surface via laser microfabrication. ACS Appl Mater Interfaces 5(15):6777–6792. https://doi.org/10.1021/am401677z
Yusen H, Tao Y, Zhengyang X et al (2021) Electrochemical micromachining of ZrCu-based amorphous alloy in ethylene glycol solution. Intermetallics 132:107155. https://doi.org/10.1016/j.intermet.2021.107155
Yan Y, Liu X, Xiong H et al (2020) Stearic acid coated MgO nanoplate arrays as effective hydrophobic films for improving corrosion resistance of Mg-based metallic glasses. Nanomaterials 10(5):947. https://doi.org/10.3390/nano10050947
Gao W, Ma F, Yin Y et al (2023) Robust and durable transparent superhydrophobic F-TNTs/TiN coating fabricated by structure tuning on surface of TiN hard coating. Appl Surf Sci 613:155967. https://doi.org/10.1016/j.apsusc.2022.155967
Kassa ST, Hu CC, Liao YC et al (2019) Thin film metallic glass as an effective coating for enhancing oil/water separation of electrospun polyacrylonitrile membrane. Surf Coat Technol 368:33–41
Bansal P, Singh G, Sidhu HS (2021) Plasma-sprayed hydroxyapatite-strontium coating for improved corrosion resistance and surface properties of biodegradable AZ31 Mg alloy for biomedical applications. J Mater Eng Perform 30(3):1768–1779. https://doi.org/10.1007/s11665-021-05490-0
Huang B, Zhang C, Zhang G et al (2019) Wear and corrosion resistant performance of thermal-sprayed Fe-based amorphous coatings: a review. Surf Coat Technol 377:124896. https://doi.org/10.1016/j.surfcoat.2019.124896
Zhang C, Wu Y, Liu L (2012) Robust hydrophobic Fe-based amorphous coating by thermal spraying. Appl Phys Lett 101(12):44. https://doi.org/10.1063/1.4754140
Zhang LM, Zhang SD, Ma AL et al (2018) Influence of sealing treatment on the corrosion behavior of HVAF sprayed Al-based amorphous/nanocrystalline coating. Surf Coat Technol 353:263–273. https://doi.org/10.1016/j.surfcoat.2018.08.086
Qiao J, Jin X, Qin J et al (2018) A super-hard superhydrophobic Fe-based amorphous alloy coating. Surf Coat Technol 334:286–291. https://doi.org/10.1016/j.surfcoat.2017.11.046
Cheng J, Feng Y, Yan C et al (2020) Development and characterization of Al-based amorphous coating. Jom 72:745–753. https://doi.org/10.1007/s11837-019-03966-y
Xu J, Kang J, Yue W et al (2021) High-temperature tribological property of Fe-based amorphous alloy coating. J Non-Cryst Solids 573:121136. https://doi.org/10.1016/j.jnoncrysol.2021.121136
Wang S, Ling Y, Zhang J et al (2014) Microstructure and properties of hydrophobic films derived from Fe-W amorphous alloy. Int J Miner Metall Mater 21:395–400. https://doi.org/10.1007/s12613-014-0921-x
Gao M, Wang DP, Huang YF et al (2016) Tunable hydrophobicity on fractal and micro-nanoscale hierarchical fracture surface of metallic glasses. Mater Des 95:612–617. https://doi.org/10.1016/j.matdes.2016.01.145
Vazirinasab E, Jafari R, Momen G (2018) Application of superhydrophobic coatings as a corrosion barrier: a review. Surf Coat Technol 341:40–56. https://doi.org/10.1016/j.surfcoat.2017.11.053
Chobaomsup V, Metzner M, Boonyongmaneerat Y (2020) Superhydrophobic surface modification for corrosion protection of metals and alloys. J Coat Technol Res 17:583–595. https://doi.org/10.1007/s11998-020-00327-2
Bi P, Li H, Zhao G et al (2019) Robust super-hydrophobic coating prepared by electrochemical surface engineering for corrosion protection. Coatings 9(7):452. https://doi.org/10.3390/coatings9070452
Wu LK, Zhang XF, Hu JM (2014) Corrosion protection of mild steel by one-step electrodeposition of superhydrophobic silica film. Corros Sci 85:482–487. https://doi.org/10.1016/j.corsci.2014.04.026
Liu T, Chen S, Cheng S et al (2007) Corrosion behavior of super-hydrophobic surface on copper in seawater. Electrochim Acta 52(28):8003–8007. https://doi.org/10.1016/j.electacta.2007.06.072
Ganesh VA, Dinachali SS, Raut HK et al (2013) Electrospun SiO2 nanofibers as a template to fabricate a robust and transparent superamphiphobic coating. RSC Adv 3(12):3819–3824. https://doi.org/10.1039/c3ra22968h
Rubio J, Souza ML, Smith RW (2002) Overview of flotation as a wastewater treatment technique. Miner Eng 15(3):139–155. https://doi.org/10.1016/S0892-6875(01)00216-3
Lin Z, Wang W, Huang R (2017) Study of oily sludge treatment by centrifugation. Desalin Water Treat 68:99–106. https://doi.org/10.5004/dwt.2017.20192
Yan L, Wang Y, Li J et al (2014) Comparative study of different electrochemical methods for petroleum refinery wastewater treatment. Desalination 341:87–93. https://doi.org/10.1016/j.desal.2014.02.037
Saeed A, Rehan ZA, Zhan D et al (2023) A facile approach for making superhydrophobic cotton fabric membrane for oil water separation. Coll Surf A: Physicochem Eng Aspects. 678:132478. https://doi.org/10.1016/j.colsurfa.2023.132478
Liu B, Shan W, Ding X et al (2023) Superhydrophobic PODS-modified nickel foam with reversible wettability for oil-water separation. J Water Process Eng 56:104562. https://doi.org/10.1016/j.jwpe.2023.104562
Feng L, Zhang Z, Mai Z et al (2004) A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew Chem 116(15):2046–2048. https://doi.org/10.1002/anie.200353381
Antonini LM, Takimi AS, Amaral VP, Camassola M, Malfatti C (2021) Cold-plasma-sterilized nanostructured Ti6Al4V: effect on nanostructured surface morphology and osteogenic differentiation of bone-marrow-derived mesenchymal stem cells. Mater Eng Perform 30(10):7236–7246. https://doi.org/10.1007/s11665-021-05903-0
Sun K, Fu R, Liu XW et al (2022) Osteogenesis and angiogenesis of a bulk metallic glass for biomedical implants. Bioact Mater 8:253–266. https://doi.org/10.1016/j.bioactmat.2021.06.018
Bouala GIN, Etiemble A, Der Loughian C et al (2018) Silver influence on the antibacterial activity of multi-functional Zr-Cu based thin film metallic glasses. Surf Coat Technol 343:108–114. https://doi.org/10.1016/j.surfcoat.2017.10.057
Liu Y, Padmanabhan J, Cheung B et al (2016) Combinatorial development of antibacterial Zr-Cu-Al-Ag thin film metallic glasses. Sci Rep 6(1):26950. https://doi.org/10.1038/srep26950
Heng L, Su J, Zhai J et al (2011) Dual high adhesion surface for water in air and for oil underwater. Langmuir 27(20):12466–12471. https://doi.org/10.1021/la202692c
Vodă M, Codrean C, Chicot D et al (2019) Characterization of brazed joints by electrical resistance spot brazing with Ni-based amorphous self-flux alloys. J Manuf Process 37:617–627. https://doi.org/10.1016/j.jmapro.2018.10.029
Shen P, Yang J, Wang Y, Guo RF, Jiang QC (2014) Effect of structural transition in an amorphous Ni80P20 alloy on the wetting by a eutectic Sn-Bi solder. Mater Sci 49(7):2932–2942. https://doi.org/10.1007/s10853-013-8004-3
Wu Y, Zhang L, Zhang H et al (2022) Wettability and interfacial reactions between metallic glass melts and Cu/Mo used as roller materials for twin-roll casting. Acta Metallurgica Sinica (English Letters) 35(7):1221–1230. https://doi.org/10.1007/s40195-021-01356-5
Xing W, Li Z, Yang H et al (2019) Anti-icing aluminum alloy surface with multi-level micro-nano textures constructed by picosecond laser. Mater Des 183:108156. https://doi.org/10.1016/j.matdes.2019.108156
Jung S, Tiwari MK, Doan NV et al (2012) Mechanism of supercooled droplet freezing on surfaces. Nat Commun 3(1):615. https://doi.org/10.1038/ncomms1630
Bai G, Gao D, Liu Z et al (2019) Probing the critical nucleus size for ice formation with graphene oxide nanosheets. Nature 576(7787):437–441. https://doi.org/10.1038/s41586-019-1827-6
Gao X, Bao X (2012) Magnetocaloric effect of FeCrB amorphous alloys with low Curie point. Beijing Keji Daxue Xuebao J Univ Sci Technol Beijing 34(8):915–920. https://doi.org/10.13374/j.issn1001-053x.2012.08.010
Acknowledgements
This project is supported by the Pre-Research Program in National 14th Five-Year Plan (Grant No. 80923010602), the National Natural Science Foundation of China (Grant Nos. 52175196).
Funding
Funding was provided by the Pre-Research Program in National 14th Five-Year Plan (Grant No. 80923010602), the National Natural Science Foundation of China (Grant Nos. 52175196).
Author information
Authors and Affiliations
Contributions
The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
This article has not been published previously; is not under consideration elsewhere; has the full consent of all authors; and that, if accepted, will not be published elsewhere in the same form, in English or in any other language, without the written consent of the Publisher. We deeply appreciate your consideration of our manuscript and we look forward to receiving comments from the reviewers. If you have any queries, please don’t hesitate to contact me.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, Yy., Xu, Jj., Zhu, Ln. et al. The wettability of metallic glasses: a review. Surf. Sci. Tech. 2, 5 (2024). https://doi.org/10.1007/s44251-024-00035-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44251-024-00035-8