Abstract
Due to its deleterious and large-scale effects on the ecosystem and long-range transboundary nature, acid rain has attracted the attention of scientists and policymakers. Acid rain (AR) is a prominent environmental issue that has emerged in the last hundred years. AR refers to any form of precipitation leading to a reduction in pH to less than 5.6. The prime reasons for AR formation encompass the occurrence of sulfur dioxide (SO2), nitrogen oxides (NOx), ozone (O3), and organic acids in air produced by natural as well as anthropogenic activities. India, the top SO2 emitter, also shows a continuous increase in NO2 level responsible for AR formation. The plants being immobile unavoidably get exposed to AR which impacts the natural surrounding negatively. Plants get affected directly by AR due to reductions in growth, productivity, and yield by damaging photosynthetic mechanisms and reproductive organs or indirectly by affecting underground components such as soil and root system. Genes that play important role in plant defense under abiotic stress gets also modulated in response to acid rain. AR induces soil acidification, and disturbs the balance of carbon and nitrogen metabolism, litter properties, and microbial and enzymatic activities. This article overviews the factors contributing to AR, and outlines the past and present trends of rainwater pH across the world, and its effects on plants and soil systems.
Similar content being viewed by others
1 Introduction
A worldwide increase in globalization and urbanization had augmented the consumption of energy from various sources. The use of fossil fuels mainly coal for the generation of electricity, oil in transport services, and the impact of industrialization has caused a higher degree of concentration of pollutants and particulate matter in the atmosphere, thus enhancing air pollution (Singh and Agrawal 2005). Greater access to energy improves both the economic growth and human development of a country, but this increase in energy demand also causes several environmental problems (Liu et al. 2019). Although the growth in renewables has been seen in all forms of energy since 2010, the proportion of fossil fuels in global primary energy demand remains above 80% (World Energy Outlook 2019). Table 1 shows the scenario of world primary energy demand (past, present, and estimated) by regions from 2000 to 2040. In India, energy demand outpaced global energy growth and oil demand grew by 5% in 2018 (Global Energy and CO2 Status Report 2019). Fossil fuel consumption in India has increased from 208 million tons per year in 2000 to 708 million tons in 2017 (World Energy Outlook 2019).
Acid rain (AR) can be defined as a combination of dry and wet deposition from the atmosphere having higher than normal concentrations of nitric (HNO3), sulfuric acids (H2SO4), and acidifying compounds which lead to a decrease in the pH of rainwater to less than 5.61. In 1845, AR was first been mentioned by Ducros, although a detailed study of AR was conducted by Robert Angus Smith (1872) and potentially harmful effects were described. There are various sources and precursors of AR formation that result both from natural and man-made activities. Natural sources are volcanic eruption, decay of vegetation, lightening, and other biogenic activities, while human-induced sources include the burning of coal, natural gas, oil in thermal power plants, and agricultural emissions resulting from the use of fertilizers, pesticides, intensive farming of paddy, and stubble burning (Zhang et al. 2007).
AR had arisen as one of the major environmental disasters in countries such as North America, Europe, and East Asia (Singh and Agrawal 2007). China suffered from a high frequency of events of acid deposition (Zhou et al. 2019). India is the second known emitter of SO2, and emissions of both SO2 and NOx, the major sources of AR, are expected to grow at least until 2030 (Li et al. 2017; Andrade et al. 2020). The events for the occurrence of acid rain (pH < 5.6) across India showed an increasing trend over the past four decades (Bhaskar and Rao 2017). Moreover, emissions from agricultural activities due to excessive use of fertilizers and pesticides add ammonia (NH3) and reactive nitrogen (Nr) species to the atmosphere, which further enhances the acidity of depositions (Sutton et al. 2017).
Worldwide occurrence of AR could negatively affect ecosystem components causing forest declines (Zheng et al. 2019) and loss of biodiversity, altering litter properties and enhancing soil acidification (Fei et al. 2020), leading to declining in soil microbial communities (Wei et al. 2020). One of the essential components of terrestrial ecosystems, plant productivity, is also negatively affected by AR pollution (Liu et al. 2018a, b), leading to loss of leaves, inhibition of growth, premature defoliation or premature aging, necrotic spots, and other visible symptoms (Bobbink et al. 2010; Du et al. 2017). The aboveground parts get directly affected by AR, thus inhibiting the functions of wax biosynthesis, accumulation of intracellular H+ ions, and other harmful ions in mesophyll cells (Shu et al. 2019). The excessive accumulation of intracellular H+ ions can induce oxidative stress due to the generation of reactive oxygen species (ROS) (Neves et al. 2009). Acidification of water bodies makes the environment uninhabitable for plants and local animals and thus causes risks to their survival (Singh and Agrawal 2007).
Due to lots of repercussions on the ecosystem, controlling the emissions of acidic depositary compounds in the atmosphere can be one of the best solutions that can be prioritized. Several steps were employed globally to decrease the emissions of SO2 and NOx like the use of cleaning technologies and equipment such as efficient boilers, oxy furnaces, and fluidized combustion beds (FBC or circulation dry scrubber) in power plants and industries to control pollution, reducing the sulfur content of the fuel by using scrubbers such as lime injection multi-stage burning (LIMB) and flue gas desulfurization (FGS) (Ahmadi 2020). The use of selective catalytic reduction process (SCR), electrochemical reduction, selective non-catalytic reduction (SNCR), and wet scrubber to reduce NOx emission (Gholami et al. 2020) were other control measures adopted to reduce acidic components in the emission. The expansion of renewable energy capacities (sources), such as hydroelectric projects, solar cells, nuclear power, windmills, and biofuels, for the production of electricity was enhanced instead of dependency on coal (Mohajan 2018). In India, vehicular emission is one of the prime contributors leading to the worsening of the air quality of cities (WHO 2018). Steps taken to tackle the emissions are switching to low sulfur fuel (10 ppm) and implementing Bharat VI standards for engines; the introduction of a National Automobile Scrappage Policy (2021) which ensures fleet modernization; increasing the distribution of electric and hybrid vehicles; and use of anti-smog guns and smog towers which helps to reduce pollution in the atmosphere.
This review focuses on the prevailing trend of decrease in pH of rainwater in the world and India as compared to earlier decades ago and the effects of AR on plant growth characteristics, its physiology, biochemistry, gene regulation, and soil system.
2 Methodology
For the literature survey, 180 papers were selected for relevant information by browsing the World Wide Web, PubMed, Google Scholar, and ResearchGate. For finding related papers, keywords such as acid rain, acidic deposition, simulated acid rain, emission from agriculture, effects of acid rain on plants, acid rain and reproductive organs, fertilizers, and acidic soil, were used. Finally, 150 articles published from August 1980 to October 2021 were considered. Data from Global Energy and CO2 Status Report, Central Pollution Control Board (CPCB), World Energy Outlook, etc. were also used.
3 Acid Rain Formation
Uncontrolled emissions of SO2 and NOx from various sources are the main constituents leading to AR. The emitted pollutants dissolve in atmospheric water vapor and turn into acids like H2SO4 and HNO3. The interaction of SO2, NOx, and O3 in the atmosphere leads to many chemical reactions which finally form H2SO4 and HNO3 mists (Calvert et al. 1985). Figure 1 depicts the schematic representation of the pathway of AR formation and consequent effects.
Poor quality coal contains 0.5% of sulfur (S) with 35–40% of ash, which gets emitted into the environment after getting burned in thermal power plants. This converts S into SO2. Furthermore, it gets gradually oxidized into sulfite ion (SO32−).
However, SO32− gets oxidized into SO42− in the atmosphere due to the presence of NH3 and O3, which finally get converted to H2SO4 in clouds.
A recent study by Mallick et al. (2021) suggested a process that can increase SO2 concentrations in the atmosphere where HOSO* can act as a source of S. The HOSO* is generated as an intermediate in the combustion condition from the oxidation of S and was found to be quite stable in the atmospheric condition. The new reaction path of HOSO* with NH2* has been identified which caused the in situ generation of SO2 in the atmosphere.
Nitrogen (N) released from vehicular exhaust undergoes oxidation which after gradual oxidation turns into NO2. Photochemical conversion takes place which leads to the formation of different forms of oxides of N that ultimately result in the formation of HNO3.
The formation of AR involving O3 is the most common reaction in the atmosphere. Photolysis of O3 into nascent oxygen occurs which then reacts with H2O and forms OH− which then reacts with SO2 and gets transformed into HSO3−.
Ozone plays an important role as an oxidant up to pH 5.0. In the liquid phase, H2O2 is considered the most dominant oxidant for the conversion of dissolved SO2 to H2SO4 at pH range from 2 to 5 in the atmosphere, which is the main contributor for the acidification of cloudwater, fog, and rainwater (Gonçalves et al. 2010).

4 The Trend of Acid Rain Scenario
Countries like North America, Europe, and China are facing a huge number of problems due to acid rain in particular (Abbasi et al. 2013). The first evidence of AR was observed in the mid-nineteenth century. In 1972, United Nations held a conference in Sweden on the subject of the human environment which concluded that AR is a serious international pollution problem (Kowalok 1993). The pH of AR in Europe was reported to increase by 10% over the last 20 years. Presently, the acidity of rainwater in the countries of Europe such as Canada, Denmark, and Germany was observed to be between 4.2 and 4.5 whereas it was 4.8 in the USA (Abbasi et al. 2013). In 2018, the pH of Poland’s rainwater lies between 3.64 and 7.36 with mean values ranging between 4.52 and 6.58 (Diatta et al. 2021). According to Piñeiro et al. (2014), the pH of rainwater in Coruña (Spain) is found to be 5.55. The pH values of precipitation of several European countries including Austria, Belarus, Croatia, Finland, Ireland, Italy, Norway, Switzerland, and the UK were reported by Keresztesi et al. (2019) to be between 4.19 and 5.82 with a mean of 4.80. The higher concentration of acidic anions (SO42−, Cl−, NO3−) compared to neutralizing cations (Ca2+, Mg2+, NH4+) can be considered a reason for the lower values of pH as reported.
As per US EPA (2013), the USA and Canada have decreased the near boundary activities of releases due to the implementation of the Cross-State Air Pollution Rule and litigation (CSAPR 2011) which reduced the sulfur and nitrogen deposition. This report further stated that the major decrease in the SO2 and NOx emissions and deposition of acid is due to the implementation of programs such as the Clean Air Interstate Rule (CAIR), Acid Rain Program (ARP), and NOx Budget training Program (NBP). It was also mentioned that the present emission levels were still not acceptable, and complete recovery of acid-sensitive ecosystems is not possible in near future (Ahmadi 2020).
Andrade et al. (2020) identified AR as a rising issue in several major cities of Brazil such as São Paulo and Rio de Janeiro where low pH values of 3.5 and 4.0 were reported. Akpo et al. (2015) reported a pH of 5.19 in Djougou, West Africa. At present, SO2 emissions in the western parts of the world are decreasing and the ecosystem of these regions is improving (Shah et al. 2000), whereas the situation in the eastern parts of the world, especially in the regions of south-central Asia, has continuously deteriorated over the years due to the growing size of the industrial sector and population boom. AR has affected around two million square kilometers in China and this area is also continuously expanding. Also, in around 44 cities in China, the pH values of rainwater lie between 3.8 and 4.5 while the mean value was around 5.6 (Sun et al. 2016). According to Watanabe and Honoki (2013), the mean rainwater pH was found to be 4.7–5.3 in the Mt. Tateyama region near the Japan Sea. The presence of CaCO3 in dust particles leads to the neutralization of acidic species of rainwater. The Japan Environment Agency reported an average pH of 5.2 in the 1970s and below 4.7 in 2000 at Ryori on the Pacific coast which showed a fivefold increase in acidity (Shah et al. 2000). Table 2 shows the variations in the values of pH of various regions of the globe from 1980 to 2016.
In 2018, a World Health Organization (WHO) report has stated that many Indian cities including Kanpur, Faridabad, Gaya, Varanasi, and Patna are some of the most polluted cities around the globe in terms of air pollution. Studies on rainwater in India showed a range of pH from alkaline to acidic (Table 3). Metropolitan cities such as Mumbai, Delhi, Kolkata, and Chennai, as well as cities located close to industrial areas, show evidence of AR. According to data from CPCB, there was a significant 2–threefold increase in NO2 level as compared to SO2 from 2004 to 2020 (Table 4) which has led to a rise in the frequency of AR. In India, AR is often ruled out due to the abundance of alkaline particles (Ca2+, NH4+, and Mg2+) in the atmosphere, but with increasing emissions from vehicles and industries, the contribution of acidic components has increased in rainwater (Bisht et al. 2015; Rao et al. 2016).
Events of AR in India have increased since the last decade. During 1970–1990, in India, the regions with lower pH values of rainwater have been increasing gradually but AR has still not been considered a threat in the country (Sridharan and Saksena 1990). Datar et al. (1996) assessed the annual precipitation volume-weighted means of monthly collected rainwater samples from 10 Background Air Pollution Monitoring Network (BAPMoN) stations between 1973 and 1990. The study revealed that the pH of rainwater is decreasing in almost all stations but reductions in mean values were not significant (Datar et al. 1996) (Table 5). Based on sensitivity calculations done by using the RAIN-ASIA model, it was also predicted that the southeast coastal regions are considered most susceptible to AR (Foell et al. 1995). According to Bhaskar and Rao (2017), Global Atmospheric Watch (GAW) stations reported that the mean pH of rainwater was highest and lowest at Jodhpur and Mohanbari while the values vary from 5.25 to 6.91. During 1981–2012, all stations recorded a decrease in the mean pH of the precipitation. It was also observed that the probability of rainfall with low pH has decreased in Srinagar during 2001–2012 but in all other stations, acidic rainfall percentage has increased from 1981–1990 to 2001–2012 (Table 5). A rainfall of pH 3.67 has been reported from Allahabad. The mean pH value of rainwater was 5.32 during 2003–2005 at Dhanbad, the coal city of India. Singh et al. (2007) stated that this part of the country has been dealing with large quantities of suspended particulate matter due to various activities such as mining, untreated outlets from the industrial sector, loading and unloading of coal, and vehicular emissions. At Mahabaleshwar, a hill station located in Peninsular India, a study assessed that there was a significant concentration of SO42− and NO3− ions in the samples taken during the summer monsoon between 2016 and 2017, and about 23% of the rainfall occurrences were acidic in nature (Waghmare et al. 2021).
Apart from inorganic acids (H2SO4, HNO3, HCl), organic acids (weak acids) can cause the acidity of rainwater. Organic acids (OCs) are a pervasive component of the troposphere and present in gaseous form in the atmosphere (Sun et al. 2016). Acetic (CH3COOH) and formic acids (HCOOH), as well as dicarboxylic acids such as oxalic acids (C2H2O4), are most abundant in the atmosphere (Avery et al. 1991; Legrand et al. 2005). Yearly, in the extratropical northern hemisphere, carboxylic acid accounts for < 25% of rainwater H+, 50% in the southern tropical continents, and around 25 to 50% in the southern hemisphere, causing the rainwater pH below 4.5 (Shah et al. 2000, 2020). It was estimated that the presence of these compounds in urban environments leads to 16 to 35% of the free acidity in rainwater and 65% in remote areas (Paulot et al. 2011).
Avery et al. (2006) reported different types of OCs in the rainwater of North Carolina, USA. Formic and acetic acids were the most abundant which comprised approximately 75% of total OCs. The presence of OCs is also reported in marine areas of Puerto Rico of the Caribbean Sea (Gioda et al. 2011). The sources of OCs can either be direct or indirect which include incomplete combustion of fuels in vehicles, biomass burning, biofuels, fossil fuel, and vegetation, or formed in the atmosphere by photochemical reactions. A study by Cruz et al. (2019) reported that on average, 89% of acidity in the Brazilian city Salvador was caused by OCs (48% of acetic acid and 41% of formic acid) in contrast to 11% by inorganic acids. A study of rainwater chemistry carried out in Spain by Peña et al. (2002) reported that formic and acetic acids are dominant carboxylic acids in rainwater and led to 90 and 89% of acidity while oxalic and citric acids were present in lower percentages. A study carried out by Sun et al. (2016) in the area of Mount Lu in south China showed a significant amount of OCs in rainwater which contributed to 17.66% acidity. Kumar et al. (2014) suggested that the presence of OCs led to an increase in the acidity of rainwater in Delhi. Khare et al. (1997) reported the presence of aldehyde (HCHO), formic, and acetic acid in rainwater was reported during the monsoon period at a rural site in Agra.
4.1 The Annual Trend of SO2 and NO2 Concentrations Across the World
SO2 and NO2 concentrations depict significant spatial variations throughout the world. Higher percentage changes were recorded from tropical and subtropical countries including India, Bangladesh, Pakistan, and Thailand (Table 6). Variations in SO2 and NO2 levels depend on sources and prevailing local, regional, and global meteorological conditions (Swartz et al. 2020). Krotkov et al. (2015) examined the long-term (2005–2015) spatial and temporal trends of SO2 and NO2 pollution around the globe by retrieving data from the satellite-borne Ozone Monitoring Instrument (OMI) of NASA’s Aura satellite. It was reported that in many regions, pollution levels showed dramatic upward and downward trends while others showed opposite trends of SO2 and NO2. The period of 2005–2015 evidenced a drastic decrease in SO2 and NO2 levels in the eastern USA by 80 and > 40%, respectively, as a result of stricter emission regulations and technological advancements. Similarly, as per the data of EEA (European Environment Agency 2013), ~ 80% reduction in SO2 emissions was observed in Europe during 1990–2011. Between 1980 and 1990s, a remarkable reduction of SO2 emissions was recorded in western European countries after which SO2 levels dropped below the detection limit of the OMI, while insignificant changes have been reported for NO2 on a regional level (Krotkov et al. 2015, 2016).
Swartz et al. (2020) assessed the long-term inter-annual and seasonal trends of atmospheric O3, SO2, and NO2 for 21 years at the Cape Point Global Atmosphere Watch (CPT GAW) station, South Africa. The analysis revealed a constant trend of NO2 and SO2 concentrations for long-term average (1995–2015); however, a nominal decrease was noticed in SO2 levels between 1995 and 2004 and then a steady rise from 2005 to 2009. The annual average concentrations of NO2 declined from 1996 to 2002 after which a consistent increment was observed with maximum concentrations in 2011 (Swartz et al. 2020).
Although being the world’s most severe SO2 polluter, the North China Plain (NCP) experienced a decreasing trend of SO2 since 2011, with about a 50% reduction from 2012 to 2015. In contrast, NO2 peaked in 2011, after a substantial increase of ~ 50% since 2009, which further showed a reduction of 40% between 2014 and 2015 due to the stagnant economic growth (Krotkov et al. 2015, 2016). Similarly, a study by Zhao et al. (2021) reveals that the annual average concentrations of SO2 and NO2 throughout China decreased by 67.9 and 24.9%, respectively, in 2019 as compared to 2014. On contrary, from the period 2005 to 2015, India experienced escalating levels of SO2 and NO2 of more than 100 and 50%, respectively, emitted from fossil-fuelled power plants and smelters (Krotkov et al. 2015, 2016). However, a significant reduction in the annual mean concentration of SO2 in 2020 (approx.7–8%) was observed as compared to 2010–2020. This change was evident due to the COVID-19 pandemic-led national lockdown and the shutdown of industries as well as the implementation of effective control technologies such as the flue gas desulfurization (FGD) and scrubber (Kuttippurath et al. 2022).
Irie et al. (2016) investigated the annual trend analysis of NO2 levels in East Asia and found that in Japan, NO2 levels decreased from 2005 to 2013 including a larger decrease that tended to occur in metropolitan areas of Tokyo and Fukuoka. However, the NO2 level increased by ~ 13% year−1 from 2013 to 2015. As per the observation of Ito et al. (2021), a significant reduction in (~ 75%) SO2 concentrations has been detected in Japan over 30 years (1990–2018). Jang et al. (2017) observed an increasing trend of SO2 levels in the rural and commercial sites of Busan, South Korea, throughout the period from 2005 to 2014 due to local emissions from shipping industries, while NO2 levels remain constant.
4.2 The Annual Trend of Rainfall pH
A comprehensive assessment of rainwater chemistry between 1978 and 2017 collected from proximal areas of the USA showed that 87.90% of samples have an acidic composition with pH values under 5.6, including 49.12% of pH values ranging between 3.04 and 5, while 34.97% and 15.91% of the pH values were between 5–5.6 and > 5.6, respectively (Keresztesi et al. 2020). European countries also recorded acidic to slightly acidic pH of rainwater ranging from 4.19 to 5.82 over two decades (Keresztesi et al. 2019). In a long-term analysis of precipitation from 2018 to 2022 at Mt. Lushan located in South China, the pH of rainwater ranged from 4.9 to 7.9, having values of 5.8 as the annual volume-weighted mean pH of 87.7% of rainwater (Li et al. 2022). The study also recorded an increasing trend in the annual flux of wet deposition during the entire experimental period with 3 times higher wet flux of nitrate (76.3 kg/ha/year) than the annual wet deposition flux of sulfate (21.7 kg/ha/year), indicating that acidic deposition is still a serious environmental issue in the region. Similarly, the period 2000–2018 marked a significant increase in the pH of annual mean precipitation from 4.96 in 2000 to 6.88 in 2018 across the western Pearl River Delta region, south China (Liu et al. 2021). The annual mean pH of precipitation for 20 years (1994–2013) at Fushan Experimental Forest, northeastern Taiwan, was 4.62 ± 0.62, having ~ 77% of the rainwater considered acidic with a pH of 5.0 (Chang et al. 2017). Itahashi et al. (2021) reported an increase in the annual mean pH of precipitation from 4.7 to 4.8 between 2000 and 2011 at the WMO-GAW station, Ryori, northeastern Japan.
5 Effects of Acid Rain on Plants
5.1 Growth and Yield
Acid rain causes deleterious effects on the agricultural ecosystem by retarding the growth of crops and affecting their production (Singh and Agrawal 2004). It has been well established that as compared to woody plants, herbaceous plants are more sensitive to direct injury by AR (Heck et al. 1986). As compared to monocotyledons, dicotyledons are more sensitive toward AR (Evans 1988; Knittel and Pell 1991). Anatomical alterations produced by AR are modification in the thickness of cuticle (Cape 1986), loss of trichomes in the epidermis, cellular deformation, collapse of the mesophyll cell, occlusion of stomatal cells, and the formation of scar tissue (Da Silva 2005). The detrimental effects of simulated acid rain (SAR) on morphology include chlorosis, necrosis, dehydration, wilting, early senescence, stunting, pathogen infection, and death (Fig. 2) (Milton and Abigail 2015). A study by Milton and Abigail (2015) investigated the impact of SAR on the morphology of okra at pH 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, and 7.0 (control) from seed to maturity. It was found that plants wilted when SAR of pH 1.0 was applied. Yellow coloration and early leaf senescence were observed at pH 2.0. At pH 3.0, plants exhibited mild and marginal chlorosis while at pH 4.0 and 6.0 chlorosis, black spots and white powdery growth all over leaves due to fungal infection were found.
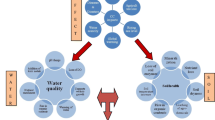
Underground regulation of soil microbes and fungi and effects on plant growth under AR stress: a high acidity, b moderate acidity. Abbreviations—(1) downregulation of the soil microbial community structure, decrease in the abundance of soil nitrogen-fixing bacteria, and decelerating the soil nutrient mineralization; (2) increase in mycorrhizal fungi which helps in remediation of heavy metals; (3) promoting pathogen infection, changing root physiological conditions; PM, plasma membrane; Ca2+, calcium ion; Mg2+, magnesium ion; Al3+, aluminum ion
The deleterious effects of AR have been reported on several agricultural and horticultural crops such as broad bean (Singh et al. 1992), tomato (Debnath et al. 2020), soybean (Pham et al. 2021), maize (Papova et al. 2019), spinach, bush bean, radish (Hosono and Nouchi 1993), and wheat (Singh and Agrawal 1996, 2004). Haruna et al. (2016) found that the SAR caused severe symptoms on leaves of papaya (Carica papaya), and small lesions were observed after the second spray of SAR of pH 4.5. However, after the 5th and 8th spray, broad lesions, big necrotic spots on the lamina, and marginal necrosis appeared on the leaves at pH levels 4.5, 5.0, and 5.5 respectively.
A study by Andrade et al. (2020) evaluated the effects of SAR on the leaf blade surface of Joannesia princeps, a tree species of rainforest. It was found that when the seedlings were subjected to SAR of pH 4.5 (H2SO4) compared to pH 6.0 (control), microstructural damage was detected only in the youngest leaves, which led to wilting of epidermal cells. Structural alterations in stomatal guard cells were also recorded. Rodríguez-Sánchez et al. (2020) found that when two tree species Liquidambar styraciflua and Fraxinus uhdei were exposed to SAR of pH 2.5, 3.8, and 5.6 (control), visible leaf damage and cuticle alteration were only found at pH 2.5 in both the species.
Neufeld et al. (1985) examined the effects of foliar applications of SAR of pH 2.0, 3.0, 4.0, and 5.6 on seedlings of four deciduous tree species native of the eastern USA (Liriodendron tulipifera, Liquidambar styraciflua, Platanus occidentalis, and Robinia pseudoacacia). SAR-induced foliar damage was only found at pH 2.0. P. occidentalis was found to be the most sensitive and L. tulipifera was the least, whereas old leaves of both species showed more damage than young leaves. Da Silva et al. (2005) screened the response of the tropical tree species (Gallesia integrifolia, Genipa americana, Joannesia princeps, Mimosa artemisiana, Spondias dulcis) under SAR treatments of pH 3.0 and 6.0 (control) by evaluating foliar injury, growth, and anatomical alterations in the leaves. It was found that all species showed chlorosis, necrotic spots, and curling of leaf blade after the first application of SAR, but J. princeps was found to be the most sensitive and S. dulcis was the least for foliar injury and seedling growth. In most sensitive species, necroses showed accretion of phenolic compounds, hypertrophy, and collapsed cells (Da Silva et al. 2005).
A pioneering study by Evans and Lewin (1981) established a relation between rainfall acidity and plant response which predicted the overall impact of the ambient level of AR on yield or productivity. Evans et al. (1982) studied the effects of different concentrations of SAR (pH 2.7, 3.1, 4.0, and 5.7) on the yield of alfalfa (Medicago sativa), garden beet (Beta vulgaris), kidney bean (Phaseolus vulgaris), and radish (Raphanus sativus). It was found that there were no significant differences observed in root mass of radish, kidney bean, and alfalfa, while a significant reduction in yield of beetroot was observed at SAR of pH 2.7, 3.1, and 4.0. The SAR treatments caused reductions in plant growth and yield of corn (Banwart et al. 1988), coriander (Dursun et al. 2000), green pepper (Shripal et al. 2000), pinto beans (Evans and Lewin 1981), and soybean (Evans et al. 1981a, b). A control field experiment using greenhouse chambers was conducted to determine the effect of SAR of sulfuric acid rain of pH 3.0, 3.5, 4.0, and 5.6 (control) on the yield of several crops such as beet, broccoli, carrot, cabbage, cucumber, radish, mustard greens, spinach, tobacco, cauliflower, potato, green pea, peanut, soybean, alfalfa, red clover, strawberry, tomato, green pepper, onion, corn, wheat, oats, barley, orchardgrass, bluegrass, ryegrass, and timothy (Lee et al. 1981). It was found that marketable yield production, i.e., total aboveground portion and root weight, was inhibited in the case of beet, carrot, radish, mustard greens, and broccoli while stimulated for alfalfa, green pepper, orchardgrass, tomato, strawberry, and timothy when exposed to pH 3.0–4.0. Potato yield was also inhibited at pH 3.0 while stimulated at pH 3.5 and 4.0. No significant effects on the yield of other crops were reported. Similar results found in tomato when treated by SAR treatment of pH 2.5 showed that the growth parameters including plant height, the number of leaves, shoot weight, and stem girth were reduced significantly (Debnath et al. 2020).
Singh and Agrawal (1996) conducted a field experiment on two cultivars of wheat (Triticum aestivum var. Malviya 206 and 234) to assess the effects of SAR of pH 5.6 (control), 5.0, 4.5, 4.0, and 3.0. It was found that leaf area, shoot and root lengths, total biomass, no. of grains per plant, grain weight per plant, and yield m−2 were decreased significantly at all levels of SAR as compared to control. Similar results were observed when two different cultivars of wheat (Malviya 213 and Sonalika) were applied with SAR of pH 5.6, 5.0, 4.5, 4.0, and 3.0. The reduction in yield of Malviya 213 is observed at pH 3.0 and 4.0, whereas only at pH 3.0 in Sonalika as compared to control (Singh and Agrawal 2004).
One of the important forages used in China, Lolium perenne, when exposed to SAR of different pH 7.0, 6.0, 5.0, 4.0, 3.5, and 3.0, showed increments in the root-shoot ratio and total biomass between pH 4.0 and 7.0 with the maximum value at pH 5.0, indicating that moderate acidity promoted the growth of leaves, while strong AR impaired the leaves and suppresses the growth of seedlings (Yin et al. 2021). The growth decreased below pH 5.0, with the greatest reduction occurred at pH 3.5. Several studies have also reported that the low acidity of rain improves seed germination, promotes the aboveground biomass, and increases overall biomass accumulation in the plants (Ramlall et al. 2015).
Pham et al. (2021) exposed soybean (Glycine max) plants to SAR of pH 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, and 6.0 (control). It was found that SAR of low pH decreased the germination rate, leaf area index, shoot length, and the number of main branches of the plants. The components of yield and actual yield also decreased especially in the plants treated with pH 3.0. A similar result was obtained in Manihot esculenta when subjected to SAR of pH 2.0, 3.0, 4.0, 5.0, 6.0, and 7.0 (control) (Odiyi and Bamidele 2014). It was found that high acidity of SAR (pH 2.0 and 3.0) led to the significant reduction of plant height, leaf area, total biomass, relative growth rate (RGR), and the harvest index (HI) (Odiyi and Bamidele 2014).
SAR induced browning of leaves, with 70% leaf abscission in Vigna unguiculata when exposed to SAR of pH 2.0, 3.0, and 4.0 as compared to pH 7.0 (control) (Odiyi and Eniola 2015). The RGR and HI were lowest compared to pH 7.0. Liang et al. (2015) reported that SAR at pH 5.5 did not affect the RGR of rice seedlings as compared to the control. However, the maximum decrease of 79 and 57% in RGR of seedlings was observed when exposed with SAR of pH 3.5 and 2.5.
AR affects the plants either by damaging the foliage leading to a reduction in canopy cover and their growth or increasing susceptibility to drought as well as diseases (Aber et al. 2001). Acidic deposition impacted eastern USA red spruce and sugar maple through loss of Ca from cell membrane due to direct leaching from foliage or reduction in uptake of Ca from soil or due to losses of available Ca and Mg which made the trees more susceptible to winter injury. The Black Forest in Germany and Bavaria, Poland, the Czech Republic, and Switzerland are the areas in Europe most vulnerable under AR. Similar reports of a decline in the health of pine species have been reported in Asia (Driscoll and Wang 2019). Asian pine species suffered negative effects due to soil acidification which results from nutrient inequality caused due to high Al and low Ca in soil (Driscoll and Wang 2019).
A field investigation on the seedlings of four tree species from south China (Cunninghamia lanceolata, Fokienia hodginsii, Phoebe zhennan, and Pinus massoniana) revealed that SAR of high acidity (pH 2.5) significantly reduced the germination of F. hodginsii and P. zhennan, while SAR of pH 2.5, 3.5, 4.5, and 5.5 increased the germination of P. massoniana and had no effect on the germination of C. lanceolata seeds (Gilani et al. 2021). The results further demonstrated that seedling germination is more resistant than seed emergence, and seed germination in conifer species is less sensitive under SAR of pH 4.5 and 5.5 as compared to broad-leaved species. As a whole, AR of pH 3.5 was found to be the threshold level, and below this value, detrimental effects on seed germination and seedling emergence were recorded (Gilani et al. 2021). In contrast, Lee and Weber (1979) found that SAR of pH 2.3 to 4.0 promotes seedling emergence and growth of woody tree species (Fig. 2b).
In nature, plants are rarely exposed to anyone kind of stress. Invasion by alien plant species causes a significant effect on the ecosystem. An experiment performed by Cheng et al. (2021) using four Asteraceae alien invasive plants (AIP), i.e., Conyza canadensis, Erigeron annuus, Aster subulatus, and Bidens pilosa, on germination of Lactuca sativa revealed that SAR of high acidity (pH 4.5) increases the process of invasion and allelopathy on the germination and root length of L. sativa.
5.2 Physiological and Biochemical Performances
Plant’s various physiological and biochemical traits were found to be negatively damaged by AR (Lee et al. 1981). The photosynthetic pigments in plants are most sensitive to air pollutants and are also identified as an indicator of the physiological status of plants stressed by AR. As shown in Table 7, different plants responded differently to acid deposition, but there was a common response of reduction in foliar chlorophyll content of different plant species under SAR treatments. Likewise, AR hampered the photosynthetic activity; nonetheless, the effects of SAR on photosynthetic activities varied depending on the plant species, stage, pH of the acid rain, and environmental conditions (Tong and Zhang 2014). Copolovici et al. (2017) showed that the photosynthetic parameters including stomatal conductance and assimilation rate of Phaseolus vulgaris decreased drastically when sprayed with acidic solutions of pH 4.0 and 4.5. Assimilation rate recovered at the initial values after 2 h of treatments, while stomatal conductance increased as acidity increased. Similarly, Odiyi and Eniola (2015) reported that in Vigna unguiculata (cowpea) leaves, SAR of pH 2.0 and 3.0 leads to reduced chlorophyll content as compared to the pH 7.0 (control).
The maximal photochemical quantum efficiency of Photosystem II (PSII) represented by Fv/Fm is widely used as a sensitive stress indicator of photosynthetic performance in plants. The decline in Fv/Fm in plants indicates an increase in non-photochemical quenching processes or photo-inactivation of PSII reaction centers (Liu et al 2018b). In rice, when leaves are subjected to SAR of pH 3.5 and 2.5, it was found that Fv/Fm showed reductions but did not show any difference at pH 5.5 from control (Wen et al. 2011). It indicates that the extremely high acidity SAR not only affects photosynthetic components but destroys chloroplast structure (Wen et al. 2011).
Sun et al. (2016) studied the impact of AR on chloroplast and its ultrastructure, photosynthesis, ATP synthase activity, gene expression, intracellular H+ level, and water content of rice seedlings. It was found that at pH 4.5, 4.0, or less, chloroplast structure remained unchanged but got destroyed. It was also reported that SAR of pH 4.0 or less decreased the leaf water content, inhibits the expression of chloroplast ATP sythase subunits which caused decreased activity of chloroplast ATP synthase, reduced photosynthesis, and damage the integrity of chloroplast structure, while at pH 4.5, the expression of ATP synthase subunits and activity got increased and promoted. It shows that AR influences the plant growth and development by changing the acidity of the cells which in turn affects the chloroplast ATPase transcription and net photosynthetic rate.
Foliar application of SAR of pH 3.0, 3.5, 4.0, 4.5, 5.0, and 5.5 on green leaves of 13 deciduous species (Acacia, Acer, Betula spp., Carpinus betulus, Castanea spp., Fagus, Juglans sp., Malus domestica, Populus, Quercus robur, Salix, Tilia europaea, Ulmus minor) and 10 species of dicotyledonous plants (Bellis perennis, Beta vulgaris, Brassica oleracea, Cucumis sativas, Lactuca sativa, Lycopersicon esculentum, Phaseolus spp., Petroselinum crispim, Solanum tubersum, Vitis) resulted into leaching of Ca, Mg, Fe, and Zn from the photosynthetic organs (Diatta et al. 2021). Intra-species variations were found in deciduous trees and dicotyledonous plants with more pronounced leakage of alkaline elements (Ca, Mg) and Zn. It was found that 77% of deciduous species showed very low to intermediate photosynthetic recovery implying that highly AR impacted trees have lower survival whereas, and dicotyledonous plants showed 70% (high to very high) survival. Mineral nutrients particularly Ca and Mg increased plants’ resistance to AR (Diatta et al. 2021). Zhou et al. (2020) found that SAR of pH 2.5 and 3.5 severely damaged the root plasma membrane (PM) permeability in Masson pine (Pinus massoniana) seedlings, while pH 4.5 and 5.6 lowered the PM permeability, thus indicating that SAR can destroy the integrity of plant PM.
5.3 Effect of AR on Plants at the Genetic Level
A recent study by Raju et al. (2021) on Allium cepa roots revealed that SAR of sulfuric acid of pH 3.8, 4.08, and 4.4 showed adverse effects on the morphological aspects of root and altered the root cells genetically compared to pH 4.63, 5.32, and 7.0. The SAR of sulfuric acid of pH 3.8 and 4.08 led to low root growth which is accompanied by a shorter root length in comparison with pH 7.0. Table 8 shows the mean root length and numbers of roots grown under different pH of SAR. It was found that the SAR of sulfuric acid of lower pH values (pH 3.8) significantly decreased the number of cells in prophase, metaphase, anaphase, and telophase, thus restraining cell division which led to lower mitotic index, causing the chromosome reorganization and thus led to modification in the number or structure of chromosomes. The chromosomal aberrations such as chromosomal bridges and fragments, nuclear lesions, micronucleus, polyploidy, binucleated nucleus, vagrant chromosomes, and sticky chromosomes were also recorded.
A proteomic study on Arabidopsis thaliana using 2-D gel electrophoresis revealed that several genes that are involved in the light reaction of photosynthesis such as photosynthetic electron transport chain-related genes and light-harvesting complex in photosystem I (PSI)- and PSII-related gene were repressed, while genes related to cell defense were upregulated under SAR (Liu et al. 2013). A study on Camellia sinensis using transcriptomic analysis reported the expression of multiple genes associated with photosynthesis, N, and S, and carbohydrate metabolisms were altered under SAR treatments (Zhang et al. 2020). A total of six genes that are involved in light reactions are repressed which include two genes encoding the protein of the light-harvesting complex of PSII, two genes involved in the PSII subunit, and one of PSI subunit and of ferredoxin-NADP ( +) reductase (FNR). This suggests that SAR directly damages the leaves, thus disturbing the light-harvesting and electron transfer process of PSI and PSII which in turn decreases the carbon assimilation efficiency of plants (Zhang et al. 2020). Genes involved in metabolism pathways of starch and sucrose as well in glycolysis such as phosphoglycerate kinase gene (PGK3), pectin methylesterase genes (PMEPCRA), enolase gene (LOS2), phosphoglycerate dehydrogenase gene (EDA9), and amidophosphoribosyl transferase gene (ASE2) were downregulated under high acidic treatment of pH 2.5.
Debnath et al. (2020) analyzed the transcriptomic profile of greenhouse-grown tomato plants exposed to SAR of pH 2.5 and 5.6 (control) and found that 182 genes were upregulated, while 1046 genes were downregulated and 17,486 genes showed no differential expression. The qPCR results used 15 genes to confirm the consistency and reliability of the profile, and among these genes, 11 genes which are related to plant secondary metabolites and 4 genes related to stress-responsive including bZIP, ERF, MYB, and WRKY family protein got downregulated in treated plants (Debnath et al. 2020).
A recent study by Yang et al. (2018) on soybean seedlings by using the next-generation sequencing platform has identified 416 genes that are related to the regulation of N, S, and photosynthesis, and carbohydrate metabolism showed alteration in expression when exposed to SAR. Moreover, different transcription factors that are related to abiotic and biotic stress such as WRKY, zinc finger proteins, MYB, and Ca signal pathway-associated genes were induced after SAR treatment (Liu et al. 2013).
6 Effects of Acid Rain on Soil
Being dynamic and complex in nature, soil can be easily affected by AR, which results in soil acidification and an increase in the exchange between H+ and nutrient cations (Mg, K, and Ca) in the soil and results in leaching (Breemen et al. 1984). The growth of plants and soil fertility are affected indirectly by deficiency of these nutrients (Mishima et al. 2013). Nutrient deficiency inhibits nodulation in plants by limiting legumes’ ability to transmit signals that attract the rhizobia (Sullivan et al. 2017) and indirectly inhibits ectomycorrhizal fungal association with plants (Maltz et al. 2019), which results in reduced plant vigor and productivity (Fig. 2).
Ma et al. (2020) found that AR influenced the soil’s chemical properties under Chinese cabbage cultivation. It was observed that spraying of SAR of pH 3.5 reduced the soil pH by 0.21, 0.19, and 0.15 units at a depth of 0, 4, and 8 cm as compared to the pH 7.0 (control). However, no significant difference was found in soil pH between treatments at pH 4.5, 5.5, and 7.0. Similarly, Zhou et al. (2020) found that SAR caused a lowering of both rhizosphere and non-rhizosphere soil pH with the decrease of SAR pH in Masson pine (Pinus massoniana) seedlings. Wei et al. (2020) also showed that SAR of pH 5.5, 4.5, 3.5, and 2.5 reduced the soil pH by 5.1, 6.8, and 7.0% in latosols, lateritic red soils, and red soils, respectively. Soils having a high cation exchange capacity (CEC) and clay content showed more resistance to SAR at low acidity levels of pH 5.5 and 4.5. The maximum decline of soil pH has been observed in the soil having the lowest CEC and clay content under SAR of pH 2.5. Latosols are found to be more resistant to AR and lateritic red soils are the least as the lateritic red soil contains the lowest soil CEC and clay content. The CEC of soil mainly rely on various physical, chemical, and biological properties of soil such as soil pH, clay, and soil organic matter, which helps to mitigate the effects of acidity on the soil. Pedogenic acidification also affects water holding capacity, porosity, and soil structure (Yadav et al. 2020). Furthermore AR composition also has an immense impact on soil chemical and biological properties.
AR negatively regulates litter decomposition and soil respiration (Mo et al. 2008), but hardly affects soil temperature and soil moisture (Wu et al. 2016). It was reported that SAR of pH 2.0, 3.0, and 4.0 decelerates the litter decomposition in birch, spruce, and pine (Francis 1982). The deposition of N is suggested to be one of the key drivers of C storage in the forest (Wei et al. 2012). The increase in the amount of N deposition could increase sequestration of soil C by suppressing the decomposition of litter and soil organic carbon (Frey et al. 2014), and can decrease soil microbial biomass C. AR increases dissolved organic carbon in soil (Fang et al. 2009). Wu et al. (2016) reported an increase in soil total organic carbon in topsoil (upper 10 cm) by 24.5% at SAR treatment of pH 3.0 compared to pH 4.5. Tang et al. (2019) also found that litter decomposition significantly decelerated in needle of Cunninghamia lanceolata and leaf of Cinnamomum camphora under AR treatments.
A study reported that SAR treatments of pH 2.5, 3.5, 4.5, and 6.4–6.6 (control) on C. sinensis (tea) cultivated on red soil decreased the levels of both available soil Mg and Ca, while SAR of pH 2.5 leads to increase in ratios of Al/Mg and Al/Ca, but decrease N/Al in twigs and roots (Hu et al. 2019). When SAR of pH 3.0 along with earthworm and mycorrhizal fungi (MF) treatments were applied on seedling of maize, significant increments in shoot biomass, nutrient uptake, an abundance of functional nitrogen-fixing bacteria, activation of soil nutrients, and promotion of transfer to the root system were found (Wang et al. 2021). The study also suggests that soil acid-neutralizing capacity can be improved by the use of earthworm and MF which helps them to combat the low pH levels (Fig. 2) (Wang et al. 2021).
AR has a severe effect on the activity, mobility, and environmental behavior of heavy metals (HMs) (Hernandez et al. 2003). AR after falling on the ground may lead to the release of HMs from soil and thus alters the soil chemical status, groundwater contamination, and function of the decomposer community (Ding et al. 2011). Kim et al. (2010) reported that under acidic conditions, HMs such as Cd, Co, Cr, Cu, Mn, Ni, Pb, and Zn become more soluble and mobile. Accumulation of HMs in the soil also affects its fertility by forming ion complexes with toxic metal ions such as Al3+, Pb2+, Hg2+, and Cd+ (Ling et al. 2010). AR leads to an increase in levels of soluble Ni as well as Zn in soil except of Cu, Cd, or Pb as they are considered to interact with organic matter (Merino et al. 1994).
An increase in soil acidity also enhances the extractable aluminum (Al3+) in the soil leading to Al toxicity (Hu et al. 2017). Mannings et al. (1996) reported that low acid treatments of pH 5.6 and 4.0 caused increased mobilization of Al and Zn in soil, while Cu, Mn, and Pb were observed only at high acid treatments of less than pH 2.5.
Li et al. (2015) found that SAR of pH 4.0 resulted in the release of HMs in the soil in decreasing order of Cd > Zn > Cu > Pb. In addition, HMs released after AR leaching was strongly associated with HM speciation and soil properties such as pH, texture, and organic matter. Ma et al. (2021) reported a significant reduction in the total concentration of Pb and Zn in soil when treated with SAR of pH 3.0, 4.0, and 5.6. The study revealed that high acidity contributes to the release of soil colloidal particles, and significantly enhanced the mobilization of Pb and Zn in soils due to the formation of organic–inorganic complexes with colloidal particles that are covered with organic matter, oxides of Fe and Al, and microbial cells in soil, which provide strong adsorption surface to these metal ions (Sen and Khilar 2006). Kim et al. (2010) studied the effects of SAR of pH 3.0, 4.5, and 5.6 on the transfer and phytoavailability of HMs in soil collected from a paddy field near a smelter in China. It was found that phytoavailability of HMs was strongly controlled by the pH of AR and lower pH can elevate the plant uptake of HMs, except Pb. After SAR treatments, total HM concentrations in soil were increased twice under pH 3.0 compared to pH 5.6. The concentrations of Cu and Zn were highest at pH 3.0 and lowest at pH 5.6. However, Cd was found to be highest and lowest at pH 4.5 and 5.6 respectively. In the case of Pb, decreasing acidity led to increased availability in exchangeable and carbonate forms because Pb changed to an available phase only after desorption with strong acid.
AR causes changes in the micro-environment of the soil, thus resulting in inhibition of the soil micro-organism activities and enzymes of soil nutrient cycling which in turn negatively affects the conversion efficiency of soil nutrients such as N, P, and S (Wang et al. 2018). Killham et al. (1983) reported that when the Sierran forest soil planted with Ponderosa pine seedlings were sprayed with SAR of pH 2.0, 3.0, 4.0, and 5.6, changes in microbial activity were most significant in surface soil. Soil respiration, dehydrogenase, and microbial activity were simulated under pH 3.0 and 4.0, while SAR of pH 2.0 shows inhibition of respiration and enzymatic activities. Soils receiving SAR of pH 3.0 showed increased arylsulfatase and decreased phosphatase activity, while urease was unaffected (Killham et al. 1983). Sinsabaugh et al. (2010) reported that AR affects soil hydrolase activity, while the activity of phosphatase shows an increasing trend with decreasing soil pH.
6.1 Effect on Soil due to Transition in Composition of AR
As the chemical composition of rainwater has been gradually changing, the shifting of AR from sulfuric to mixed and then nitric type has impacted soil enzymatic activity and microbial biomass differently (Li et al. 2021). When 2-year-old seedlings of Cunninghamia lanceolata, Cyclobalanopsis glauca, Pinus massoniana, and Phyllostachys edulis were exposed with SAR of sulfuric acid (S/N = 5), mixed acid (S/N = 1), and nitric (S/N = 0.2) acid of pH 2.5, 3.5, and 4.5, it was found that enzymatic activities decreased significantly under high- and mild-intensity AR treatments, and were lower than that under pH 7.0 (control). At lower acidity of all treatments, the soil rhizosphere enzyme activity was higher as compared to the control. The activity in P. massoniana, C. lanceolata, and P. edulis was inhibited more by nitric acid, while C. glauca was more inhibited by sulfuric acid (Li et al. 2021). Liu et al. (2020) also found that the activities of phosphatase, sucrase, and urease were higher under nitric acid as compared with sulfuric acid. Moreover, Liu et al. (2017) reported that increasing acidity of sulfuric acid (pH 3.5, 2.5) and nitric acid (pH 3.5, 2.5) leads to a decline in soil pH as compared to the pH 6.6 (control). However, no significant difference was observed among the same acidity of sulfuric and nitric acid.
Lv et al. (2014) found that a decrease of SO42−/NOx− in the AR led to decrease of soil pH. The soil pH of the broad-leaved forest showed significant reduction only under mixed and nitric acids (S/N = 0:1), while the coniferous forest showed a decrease in soil pH in all AR types. Under nitric acid treatment, most soil enzyme activities except phosphatase were significantly lower than that in mixed acid (S/N = 5:1, 1:1, 1:5) and sulfuric acid (S/N = 1:0). The negative effects of nitric acid were more pronounced than those of sulfuric and mixed acid. The results revealed that the SO42−/NO3− ratio in AR is an important factor that has a profound impact on litter decomposition, soil microbial biomass, and soil enzyme activities. Liu et al. (2021a, b) reported that AR of different S/N ratios (sulfuric acid = 5:1, mixed acid = 1:1, and nitric acid = 1:5) did not have a significant effect on soil pH at the initial period of the experiment except for nitric acid pH 2.5. Soil enzymatic activity of urease and phosphatase was affected when subjected to AR with higher acidity. The activities of soil urease were highly intensified, and conversely, phosphatase activity decreased when exposed to nitric acid of pH 2.5.
7 Effects of Acid Rain on Reproductive Structures
The impact of AR is not limited to vegetative organs of the plants but also affects generative parts which include structures such as pollens and ovules. AR results in inhibition of pollen germination and pollen tube elongation and as a result affects pollination and fertilization and changes the quality and quantity of seeds (Fig. 2). Acidity (pH < 3.1) causes morphological alterations in pollens below pH 3.0. The pollen germination was completely stopped in apple (Malva sylvestris) at pH 2.9 (Munzuroglu et al. 2003). AR reduced the sucrose permeability in pollen (Renzoni and Veigi 1991). Wertheim and Craker (1988) also found a reduction in pollen germination in corn (Zea mays) by 25% at pH 2.6 compared to 5.6. It was shown that pollen tube length decreased in date palm and rice with an increase in acidity of rainwater (Ismail and Zohair 2013). Nandlal and Sachan (2017) conducted a field study to assess the effects of SAR of 7.0, 5.7, 4.5, and 3.0 on pollen germination of sunflowers which showed significant reductions of 71, 51, and 43% in pollen germination at 5.7, 4.5, and 3.0 respectively.
Microscopic studies in bean plants reported variations in the ovule’s formation, development, structure, and protein content (Majd and Chehregani 1992). Plants grown in pots when subjected to SAR of pH 2.0, 3.0, 4.0, and 4.5 showed a reduction in the size of the embryo sac (34%), poor penetration of embryo sac into nucellar tissue, increase in the volume of the vacuole in nucellar cells, accumulation of starch-like particles in the embryo sac, and overgrowth of ovule integuments leading to early blockage of micropyle canal (Majd and Chehregani 1992). Alterations in ovules resulted in abnormalities in seed formation and seed protein. The bean plants when exposed to an acidic solution of pH 2.0 set an average of 3 seeds as compared to 5–6 seeds in normal plants. There was no change in protein pattern and band numbers when seed storage protein was extracted and run on SDS-PAGE. Acidification of rain hampers gene regulation which may decrease protein production and cause modification of the quantity of protein bands (Chehregani and Kavianpour 2007).
8 Conclusion
Acid rain is one of the global-scale environmental challenges that have caused widespread negative effects on ecosystems during the last several decades. Gradual increase in emissions of major acid rain precursors (SO2 and NO2) in the atmosphere has resulted in view of tremendous economic development and industrial growth throughout the world. Acid rain, earlier identified as a problem of developed countries, has now spread in developing countries. The most economically developing countries like India, China, and Brazil are experiencing increased instances of AR frequency. Emission patterns of tropical and subtropical countries revealed the threats by AR are going to be more adverse in the near future as evidenced from decreasing trend of pH of rainwater. AR has potential short-term as well as long-term negative effects on plant integrity, forest and grassland ecosystems, and soil chemistry and biology. Acid rain affects plants’ biochemical, physiological, and cellular processes and causes alteration in gene expression. It enhances the chance of invasion of alien plant species through allelopathy. Soil physical and chemical properties and microbial community structure and functions are also negatively altered under AR influence.
Complications of acid rain have been tackled to some extent in the developed world by implementing the emission norms for the gases effectuating acid rain. To avoid such problems, robust and effective monitoring of emissions along with stringent regulation policies is required to be adopted by the developing world. Additionally, increasing NOx pollution around the globe changes the chemical composition of AR. A comprehensive assessment and prediction of the impacts of changing types of acid rain on plant growth and function, biodiversity, and soil properties are needed in view of scarce studies conducted on such aspects. Further investigations are also needed to assess the futuristic impacts of acid rain with a dynamically changing environment on different facets of plants and ecosystems in India and around the world which may give valuable insights into differential plant responses under AR stress.
References
Abbasi T, Poornima P, Kannadasan T, Abbasi SA (2013) Acid rain: past, present, and future. Int J Environ Eng 5:229–272. https://doi.org/10.1504/IJEE.2013.054703
Aber J, Neilson RP, McNulty S, Lenihan JM, Bachelet D, Drapek RJ (2001) Forest processes and global environmental change: predicting the effects of individual and multiple stressors: we review the effects of several rapidly changing environmental drivers on ecosystem function, discuss interactions among them, and summarize predicted changes in productivity, carbon storage, and water balance. J Biosci 51:735–751. https://doi.org/10.1641/0006-3568(2001)051[0735:FPAGEC]2.0.CO;2
Ahmadi GM (2020) Acid rain, causes, effects and control strategies. Int J All Res Writings 1:219–225
Akpo AB, Galy-Lacaux C, Laouali D, Delon C, Liousse C, Adon M, Darakpa C (2015) Precipitation chemistry and wet deposition in a remote wet savanna site in West Africa: Djougou (Benin). Atmos Environ 115:110–123. https://doi.org/10.1016/j.atmosenv.2015.04.064
Ambient air quality monitoring data for the year 2004 and 2020 (Manual Monitoring under National Ambient Air Quality Monitoring Programme) CPCB.https://cpcb.nic.in/manual-monitoring/ and http://www.cpcbenvis.nic.in/airpollution/bulletin/del/2004/maindata2004.htm
Andrade GC, Castro LN, & da Silva LC (2020) Micromorphological alterations induced by simulated acid rain on the leaf surface of Joannesia princeps Vell. (Euphorbiaceae). Ecol Indic 116:106526 https://doi.org/10.1016/j.ecolind.2020.106526
Atkins DH, Irwin JG, Tuck AF, Scriven RA, Barrett CF, Cape JN, Fowler D, Kallend AS, Martin A, Pitman JI (1983) Warren Spring Lab., Stevenage (United Kingdom): Acid deposition in the United Kingdom
Avery GB Jr, Kieber RJ, Witt M, Willey JD (2006) Rainwater monocarboxylic and dicarboxylic acid concentrations in southeastern North Carolina, USA, as a function of air-mass back-trajectory. Atmos Environ 40:1683–1693. https://doi.org/10.1016/j.atmosenv.2005.10.058
Avery GB Jr, Willey JD, Wilson CA (1991) Formic and acetic acids in coastal North Carolina rainwater. Environ Sci Technol 25:1875–1880. https://doi.org/10.1021/es00023a005
Bamidele JF, Eguagie MO (2015) Ecophysiological response of Capsicum annuum L. exposed to simulated acid rain. Niger J Biotechnol 30:48–52. https://doi.org/10.4314/njb.v30i1.6
Banwart WL, Porter PM, Ziegler EL, Hassett JJ (1988) Growth parameter and yield component response of field corn to simulated acid rain. Environ Exp Bot 28:43–51. https://doi.org/10.1016/0098-8472(88)90045-7
Behera S, Mishra PC, Ghosh S, Goswami C, Mallick B (2019) Microscopic Observation of Acid Rain Induced Bacopa monnieri L. Microsc Res 7:11–25. https://doi.org/10.4236/mr.2019.72002
Bhaskar VV, Rao SP (2017) Annual and decadal variation in chemical composition of rain water at all the ten GAW stations in India. J Atmos Chem 74:23–53. https://doi.org/10.1007/s10874-016-9339-3
Bhatti N, Streets DG, Foell WK (1992) Acid rain in Asia. Environ Manage 16:541–562. https://doi.org/10.1007/BF02394130
Bisht DS, Dumka UC, Kaskaoutis DG, Pipal AS, Srivastava AK, Soni VK, Tiwari S (2015) Carbonaceous aerosols and pollutants over Delhi urban environment: temporal evolution, source apportionment and radiative forcing. Sci Total Environ 521:431–445. https://doi.org/10.1016/j.scitotenv.2015.03.083
Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, De Vries W (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20:30–59. https://doi.org/10.1890/08-1140.1
Breemen NV, Driscoll CT, Mulder J (1984) Acidic deposition and internal proton sources in acidification of soils and waters. Nature 307:599–604. https://doi.org/10.1038/307599a0
Calvert JG, Lazrus A, Kok GL, Heikes BG, Walega JG, Lind J, Cantrell CA (1985) Chemical mechanisms of acid generation in the troposphere. Nature 317:27–35. https://doi.org/10.1038/317027a0
Cape JN (1986) Effects of air pollution on the chemistry of surface waxes of Scots pine. Wat Air Soil Poll 31:393–399. https://doi.org/10.1007/BF00630856
Chandravanshi CK, Patel VK, Patel KS (1997) Acid rain in Korba city of India. Indian J Environ Prot 17:658–661
Chang CT, Wang CP, Huang JC, Wang LJ, Liu CP, Lin TC (2017) Trends of two decadal precipitation chemistry in a subtropical rainforest in East Asia. Sci Total Environ 605:88–98. https://doi.org/10.1016/j.scitotenv.2017.06.158
Chehregani Abdolkarim, Kavianpour Farideh (2007) Effects of Acid Rain on the Developmental Stages of Ovules and Seed Proteins in Bean Plants (Phaseolus vulgaris L.). American Journal of Plant Physiology 2:367–372. https://doi.org/10.3923/ajpp.2007.367.372
Cheng H, Wang S, Wei M, Yu Y, Wang C (2021) Effect of leaf water extracts of four Asteraceae alien invasive plants on germination performance of Lactuca sativa L. under acid deposition. Plant Ecol 222:433–443. https://doi.org/10.1007/s11258-021-01117-5
Copolovici L, Ban A, Faur I, Copolovici D (2017) The influence of simulated acidic rain on plants volatile organic compounds emission and photosynthetic parameters. AgroLife, 73.
Cruz LP, Mota ER, Campos VP, Santana FO, Luz SR, Santos DF (2019) Inorganic and organic acids in the atmosphere of the urban area of the city of Salvador, Brazil. J Braz Chem Soc 30:904–914. https://doi.org/10.21577/0103-5053.20180227
da Silva LC, Azevedo AA, da Silva EAM, Oliva MA (2005) Effects of simulated acid rain on the growth of five Brazilian tree species and anatomy of the most sensitive species (Joannesia princeps). Aust J Bot 53:789–796. https://doi.org/10.1071/BT04096
Datar SV, Mukhopadhyay B, Srivastava HN (1996) Trends in background air pollution parameters over India. Atmos Environ 30:3677–3682. https://doi.org/10.1016/1352-2310(96)00052-0
Debnath B, Hussain M, Irshad M, Mitra S, Li M, Liu S, Qiu D (2018) Exogenous melatonin mitigates acid rain stress to tomato plants through modulation of leaf ultrastructure, photosynthesis and antioxidant potential. Molefw 23:388. https://doi.org/10.3390/molecules23020388
Debnath B, Li M, Liu S, Pan T, Ma C, Qiu D (2020) Melatonin-mediate acid rain stress tolerance mechanism through alteration of transcriptional factors and secondary metabolites gene expression in tomato. Ecotoxicol Environ Saf 200:110720. https://doi.org/10.1016/j.ecoenv.2020.110720
Diatta J, Youssef N, Tylman O, Grzebisz W, Markert B, Drobek L, Lejwoda P (2021) Acid rain induced leakage of Ca, Mg, Zn, Fe from plant photosynthetic organs–Testing for deciduous and dicotyledons. Ecol Indic 121:107210. https://doi.org/10.1016/j.ecolind.2020.107210
Ding Z, Wang Q, Hu X (2011) Fractionation of Zn and Pb in bulk soil and size fractions of water-stable micro-aggregates of lead/zinc tailing soil under simulated acid rain. Procedia Environ Sci Eng Manag 10:325–330. https://doi.org/10.1016/j.proenv.2011.09.053
Dolatabadian A, Sanavy SAMM, Gholamhoseini M, Joghan AK, Majdi M, Kashkooli AB (2013) The role of calcium in improving photosynthesis and related physiological and biochemical attributes of spring wheat subjected to simulated acid rain. Physiol Mol Biol Plants 19:189–198. https://doi.org/10.1007/s12298-013-0165-7
Dörter M, Mağat-Türk E, Döğeroğlu T, Özden-Üzmez Ö, Gaga EO, Karakaş D, Yenisoy-Karakaş S (2022) An assessment of spatial distribution and atmospheric concentrations of ozone, nitrogen dioxide, sulfur dioxide, benzene, toluene, ethylbenzene, and xylenes: ozone formation potential and health risk estimation in Bolu city of Turkey. Environ Sci Pollut Res 1-15.https://doi.org/10.1007/s11356-022-19608-x
Driscoll CT, Wang Z (2019) Ecosystem effects of acidic deposition. In Encyclopedia of Water; American Cancer Society: New York, NY, USA, 1–12. https://doi.org/10.1002/9781119300762.wsts0043
Du E, Dong D, Zeng X, Sun Z, Jiang X, de Vries W (2017) Direct effect of acid rain on leaf chlorophyll content of terrestrial plants in China. Sci Total Environ 605:764–769. https://doi.org/10.1016/j.scitotenv.2017.06.044
Duncan BN, Lamsal LN, Thompson AM, Yoshida Y, Lu Z, Streets DG, Pickering KE (2016) A space-based, high-resolution view of notable changes in urban NOx pollution around the world (2005–2014). J Geophys Res Atmos 121:976–996. https://doi.org/10.1002/2015JD024121
Dursun A, Yildirim E, Güvenc I, Kumlay AM (2000) Effects of simulated acid rain on plant growth and yield of tomato (Lycopersicon esculentum). In II Balk Symp Vegetables Potatoes 579:245–248.https://doi.org/10.17660/ActaHortic.2002.579.40
EEA (2013) European Union emission inventory report 1990–2011 under the UNECE Convention on Long-range Transboundary Air Pollution (LRTAP), European Environment Agency (EEA), Technical report No 10/2013. https://doi.org/10.2800/44480
Eguagie MO, Aiwansoba RP, Omofomwan KO, Oyanoghafo OO (2016) Impact of simulated acid rain on the growth, yield and plant component of Abelmoschus caillei. J Adv Biol Biotechnol 6:1–6. https://doi.org/10.9734/JABB/2016/24804
Evans LS (1988) Effect of acidic decomposition on vegetation: State of science. In: Rao D.N., M. Yunus, K.J. Ahmad, S.N. Singh, (eds) Perspectives in environmental botany, today and tomorrows. Printers and Publishers, New Delhi, pp 73–119
Evans LS, Curry TM, Lewin KF (1981) Responses of leaves of Phaseolus vulgaris L. to simulated acidic rain. New Phytol 88:403–420. https://doi.org/10.1111/j.1469-8137.1981.tb04089.x
Evans LS, Gmur NF, Mancini D (1982) Effects of simulated acidic rain on yields of Raphanus sativus, Lactuca sativa, Triticum aestivum and Medicago sativa. Environ Exp Bot 22:445–453. https://doi.org/10.1016/0098-8472(82)90055-7
Evans LS, Lewin KF (1981) Growth, development and yield responses of pinto beans and soybeans to hydrogen ion concentrations of simulated acidic rain. Environ Exp Bot 21:103–113. https://doi.org/10.1016/0098-8472(81)90015-0
Evans LS, Lewin KF, Conway CA, Patti MJ (1981) Seed yields (quantity and quality) of field-grown soybeans exposed to simulated acidic rain. New Phytol 89:459–470. https://doi.org/10.1111/j.1469-8137.1981.tb02327.x
Fang HJ, Yu GR, Cheng SL, Mo JM, Yan JH, Li S (2009) 13 C abundance, water-soluble and microbial biomass carbon as potential indicators of soil organic carbon dynamics in subtropical forests at different successional stages and subject to different nitrogen loads. Plant Soil 320:243–254. https://doi.org/10.1007/s11104-009-9890-7
Fei J, Ma J, Yang J, Liang Y, Ke Y, Yao L, Min X (2020) Effect of simulated acid rain on stability of arsenic calcium residue in residue field. Environ Geochem Health 42:769–780. https://doi.org/10.1007/s10653-019-00273-y
Foell W (1995) RAINS-Asia: an assessment model for air pollution in Asia. Report on the World Bank Sponsored Project" Acid Rain and Emission Reduction in Asia"
Francis AJ (1982) Effects of acidic precipitation and acidity on soil microbial processes. Water Air Soil Poll 18:375–394. https://doi.org/10.1007/BF02419425
Frey SD, Ollinger S, Nadelhoffer KE, Bowden R, Brzostek E, Burton A, Wickings K (2014) Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 121:305–316. https://doi.org/10.1007/s10533-014-0004-0
Gao YF, Rong LP, Zhao DH, Zhang JQ, Chen JS (2021) Effects of simulated acid rain on the photosynthetic physiology of Acer ginnala seedlings. Can J for Res 51:18–24. https://doi.org/10.1139/cjfr-2020-0091
Garaga R, Chakraborty S, Zhang H, Gokhale S, Xue Q, Kota SH (2020) Influence of anthropogenic emissions on wet deposition of pollutants and rainwater acidity in Guwahati, a UNESCO heritage city in Northeast India. Atmos Res 232:104683. https://doi.org/10.1016/j.atmosres.2019.104683
Gholami F, Tomas M, Gholami Z, Vakili M (2020) Technologies for the nitrogen oxides reduction from flue gas: A review. Sci Total Environ 714:136712. https://doi.org/10.1016/j.scitotenv.2020.136712
Gilani MM, Tigabu M, Liu B, Farooq TH, Rashid MHU, Ramzan M, Ma X (2021) Seed germination and seedling emergence of four tree species of southern China in response to acid rain. J for Res 32:471–481. https://doi.org/10.1007/s11676-020-01102-0
Gioda A, Reyes‐Rodríguez GJ, Santos‐Figueroa G, Collett Jr JL, Decesari S, Ramos MDCK, Bezerra Netto HJ, de Aquino Neto FR, Mayol‐Bracero OL (2011) Speciation of water‐soluble inorganic, organic, and total nitrogen in a background marine environment: Cloud water, rainwater, and aerosol particles. J Geophys Res Atmos. https://doi.org/10.1029/2010JD015010
Global energy and CO2 status report (2019). https://www.iea.org/reports/global-energy-co2-status-report-2019
Gonçalves C, Santos MAD, Fornaro A, Pedrott JJ (2010) Hydrogen peroxide in the rainwater of Sao Paulo megacity: measurements and controlling factors. J Braz Chem Soc 21:331–339. https://doi.org/10.1590/S0103-50532010000200020
Guo Y, Zhang J, Wang S, She F, Li J (2011) Chemical characteristics of atmospheric precipitation in spring and summer in Toyama, Japan. In 2011 Int Conf Remote Sens Environ Transp Eng 3917–3920. IEEE.https://doi.org/10.1109/RSETE.2011.5965175
Haruna MY, Khan AA, Darma ZU (2016) Effect of acid rain on growth of Papaya (Carica papaya) and Castor (Ricinus communis) plants. J Sci Technol 9:43–47. https://doi.org/10.18780/e-jst.v9i1.738
Heck WW, Heagle AS, Shriner DS (1986) Hordeum vulgare exposed to long term fumigation with low concentration of SO2. Physiol Plant 76:445–450
Hernandez L, Probst A, Probst JL, Ulrich E (2003) Heavy metal distribution in some French forest soils: evidence for atmospheric contamination. Sci Total Environ 312:195–219. https://doi.org/10.1016/S0048-9697(03)00223-7
Hosono T, Nouchi I (1993) Effects of simulated acid rain on the growth of agricultural crops. J Agric Meteorol 48:743–746. https://doi.org/10.2480/agrmet.48.743
Hu H, Hua W, Shen A, Zhou H, Sheng L, Lou W, Zhang G (2021) Photosynthetic rate and chlorophyll fluorescence of barley exposed to simulated acid rain. Environ Sci Pollut Res 28:42776–42786. https://doi.org/10.1007/s11356-021-13807-8
Hu XF, Chen FS, Wine ML, Fang XM (2017) Increasing acidity of rain in subtropical tea plantation alters aluminum and nutrient distributions at the root-soil interface and in plant tissues. Plant Soil 417:261–274. https://doi.org/10.1007/s11104-017-3256-3
Hu XF, Wu AQ, Wang FC, Chen FS (2019) The effects of simulated acid rain on internal nutrient cycling and the ratios of Mg, Al, Ca, N, and P in tea plants of a subtropical plantation. Environ Monit Assess 191:1–14. https://doi.org/10.1007/s10661-019-7248-z
Irie H, Muto T, Itahashi S, Kurokawa JI, Uno I (2016) Turnaround of tropospheric nitrogen dioxide pollution trends in China, Japan, and South Korea. Sola 12:170–174. https://doi.org/10.2151/sola.2016-035
Ismail OM, Zohair MM (2013) Date palm pollen germination and growth susceptibility to different pH medium. J Agr Food Technol 3:26–30
Itahashi S, Kurokawa J, Ohara T, Uno I, Fujita SI (2021) The 36-Year Historical Variation of Precipitation Chemistry during 1976–2011 at Ryori WMO-GAW Station in Japan. SOLA. https://doi.org/10.2151/sola.2021-032
Ito A, Wakamatsu S, Morikawa T, Kobayashi S (2021) 30 years of air quality trends in Japan. Atmosphere 12(8):1072. https://doi.org/10.3390/atmos12081072
Jang E, Do W, Park G, Kim M, Yoo E (2017) Spatial and temporal variation of urban air pollutants and their concentrations in relation to meteorological conditions at four sites in Busan, South Korea. Atmos Pollut Res 8:89–100. https://doi.org/10.1016/j.apr.2016.07.009
Janta R, Sekiguchi K, Yamaguchi R, Sopajaree K, Plubin B, Chetiyanukornkul T (2020) Spatial and temporal variations of atmospheric PM10 and air pollutants concentration in upper Northern Thailand during 2006–2016. Appl Sci Eng Prog 13:256–267. https://doi.org/10.14416/j.asep.2020.03.007
Jiao R, Zhang M, Wei Z, Xu J, Zhang H (2021) Alleviative effects of nitric oxide on Vigna radiata seedlings under acidic rain stress. Mol Biol Rep 48:2243–2251. https://doi.org/10.1007/s11033-021-06244-w
Keresztesi Á, Birsan MV, Nita IA, Bodor Z, Szép R (2019) Assessing the neutralisation, wet deposition and source contributions of the precipitation chemistry over Europe during 2000–2017. Environ Sci Eur 31:1–15. https://doi.org/10.1186/s12302-019-0234-9
Keresztesi Á, Nita IA, Boga R, Birsan MV, Bodor Z, Szép R (2020) Spatial and long-term analysis of rainwater chemistry over the conterminous United States. Environ Res 188:109872. https://doi.org/10.1016/j.envres.2020.109872
Khare P, Satsangi GS, Kumar N, Kumari KM, Srivastava SS (1997) HCHO, HCOOH and CH3COOH in air and rain water at a rural tropical site in north central India. Atmos Environ 31:3867–3875. https://doi.org/10.1016/S1352-2310(97)00263-X
Khemani LT, Momin GA, Rao PSP, Pillai AG, Safai PD, Mohan K, Rao MG (1994) Atmospheric pollutants and their influence on acidification of rain water at an industrial location on the west coast of India. Atmos Environ 28:3145–3154. https://doi.org/10.1016/1352-2310(94)00148-E
Killham K, Firestone MK, Mc Coll JG (1983) Acid rain and soil microbial activity: effects and their mechanisms. J Environ Qual 12:133–137. https://doi.org/10.2134/jeq1983.00472425001200010024x
Kim AY, Kim JY, Ko MS, Kim KW (2010) Acid rain impact on phytoavailability of heavy metals in soils. Geosystem Eng 13:133–138. https://doi.org/10.1080/12269328.2010.10541320
Knittel R, Pell EJ (1991) Effects of drought stress and simulated acidic rain on foliar conductance of Zea mays L. Environ Exp Bot 31:79–90. https://doi.org/10.1016/0098-8472(91)90010-L
Kowalok ME (1993) Common threads: Research lessons from acid rain, ozone depletion, and global warming. Environ Sci Policy Sustain Dev 35:12–38. https://doi.org/10.1080/00139157.1993.9929107
Krotkov NA, McLinden CA, Li C, Lamsal LN, Celarier EA, Marchenko SV, Swartz WH, Bucsela EJ, Joiner J, Duncan BN, Boersma KF (2016) Aura OMI observations of regional SO2 and NO2pollution changes from 2005 to 2015.Atmos Chem Phys 16:605-4629. https://doi.org/10.5194/a5-2016
Kumar P, Yadav S, Kumar A (2014) Sources and processes governing rainwater chemistry in New Delhi, India. Nat Hazards 74:2147–2162. https://doi.org/10.1007/s11069-014-1295-0
Kuttippurath J, Patel VK, Pathak M, Singh A (2022) Improvements in SO2 pollution in India: role of technology and environmental regulations. Environ Sci Pollut Res 1–13. https://doi.org/10.1007/s11356-022-21319-2
Le Bolloch O, Guerzoni S (1995) Acid and alkaline deposition in precipitation on the Western coast of Sardinia, Central Mediterranean (40 N, 8 E). Wat Air Soil Poll 85:2155–2160. https://doi.org/10.1007/BF01186153
Lee JJ, Neely GE, Perrigan SC, Grothaus LC (1981) Effect of simulated sulfuric acid rain on yield, growth and foliar injury of several crops. Environ Exp Bot 21:171–185. https://doi.org/10.1016/0098-8472(81)90024-1
Lee JJ, Weber DE (1979) The effect of simulated acid rain on seedling emergence and growth of eleven woody species. For Sci 25:393–398. https://doi.org/10.1093/forestscience/25.3.393
Legrand M, Preunkert S, Galy‐Lacaux C, Liousse C, Wagenbach D (2005) Atmospheric year‐round records of dicarboxylic acids and sulfate at three French sites located between 630 and 4360 m elevation. J Geophys Res Atmos 110:D13302. https://doi.org/10.1029/2004JD005515
Li C, McLinden C, Fioletov V, Krotkov N, Carn S, Joiner J, Streets D, He H, Ren X, Li Z, Dickerson RR (2017). India is overtaking China as the world’s largest emitter of anthropogenic sulfur dioxide. Sci Rep 7:1-7. https://doi.org/10.1038/s41598-017-14639-8
Li HR, Xiang HM, Zhong JW, Ren XQ, Wei H, Zhang JE, Zhao BL (2020) Acid Rain Increases Impact of Rice Blast on Crop Health via Inhibition of Resistance Enzymes. Plants 9:881. https://doi.org/10.3390/plants9070881
Li J, Jia C, Lu Y, Tang S, Shim H (2015) Multivariate analysis of heavy metal leaching from urban soils following simulated acid rain. Microchem J 122:89–95. https://doi.org/10.1016/j.microc.2015.04.015
Li J, Wu H, Jiang P, Fu C (2022) Rainwater chemistry in a subtropical high-altitude mountain site, South China: Seasonality, source apportionment and potential factors. Atmos Environ 268:118786. https://doi.org/10.1016/j.atmosenv.2021.118786
Li X, Wang Y, Zhang Y, Wang Y, Pei C (2021) Response of soil chemical properties and enzyme activity of four species in the Three Gorges Reservoir area to simulated acid rain. Ecotoxicol Environ Saf 208:111457. https://doi.org/10.1016/j.ecoenv.2020.111457
Liang Chanjuan, Ge Yuqing, Su Lei, Bu Jinjin (2015) Response of plasma membrane H+-ATPase in rice (Oryza sativa) seedlings to simulated acid rain. Environmental Science and Pollution Research 22(1):535–545. https://doi.org/10.1007/s11356-014-3389-3
Ling DJ, Huang QC, Ouyang Y (2010) Impacts of simulated acid rain on soil enzyme activities in a latosol. Ecotoxicol Environ Saf 73:1914–1918. https://doi.org/10.1016/j.ecoenv.2010.07.024
Liu H, Ren X, Zhu J, Wu X, Liang C (2018) Effect of exogenous abscisic acid on morphology, growth and nutrient uptake of rice (Oryza sativa) roots under simulated acid rain stress. Planta 248:647–659. https://doi.org/10.1007/s00425-018-2922-x
Liu TW, Niu L, Fu B, Chen J, Wu FH, Chen J, Zheng HL (2013) A transcriptomic study reveals differentially expressed genes and pathways respond to simulated acid rain in Arabidopsis thaliana. Genome 56:49–60. https://doi.org/10.1139/gen-2012-0090
Liu X, Fu Z, Zhang B, Zhai L, Meng M, Lin J, Zhang J (2018) Effects of sulfuric, nitric, and mixed acid rain on Chinese fir sapling growth in Southern China. Ecotoxicol Environ Saf 160:154–161. https://doi.org/10.1016/j.ecoenv.2018.04.071
Liu X, Li C, Meng M, Zhai L, Zhang B, Jia Z, Zhang J (2020) Comparative effects of the recovery from sulfuric and nitric acid rain on the soil enzyme activities and metabolic functions of soil microbial communities. Sci Total Environ 714:136788. https://doi.org/10.1016/j.scitotenv.2020.136788
Liu X, Meng M, Zhang Y, Li C, Ma S, Li Q, Zhang J (2021) Effects of sulfuric, nitric, and mixed acid rain on the decomposition of fine root litter in Southern China. Ecol Process 10:1–13. https://doi.org/10.1186/s13717-021-00334-0
Liu X, Zhang B, Zhao W, Wang L, Xie D, Huo W, Zhang J (2017) Comparative effects of sulfuric and nitric acid rain on litter decomposition and soil microbial community in subtropical plantation of Yangtze River Delta region. Sci Total Environ 601:669–678. https://doi.org/10.1016/j.scitotenv.2017.05.151
Liu XD, Feng YJ, Mo QF, Chu GW, Li YL, Zhang QM, Zhou Q (2021) Long-term changes of water acidity in an intact forested watershed in south China. J Mt Sci 18(12):3138–3146. https://doi.org/10.1007/s11629-021-6929-6
Liu Z, Yang J, Zhang J, Xiang H, Wei H (2019) A bibliometric analysis of research on acid rain. Sustainability 11:3077. https://doi.org/10.3390/su11113077
Lv Y, Wang C, Jia Y, Wang W, Ma X, Du J, Tian X (2014) Effects of sulfuric, nitric, and mixed acid rain on litter decomposition, soil microbial biomass, and enzyme activities in subtropical forests of China. Appl Soil Ecol 79:1–9. https://doi.org/10.1016/j.apsoil.2013.12.002
Ma J, Zhong B, Khan MA, Wu D, Zhu Y, Wang Y, Xie X, Liu H, Liu D (2021) Transport of mobile particles in heavy metal contaminated soil with simulated acid rain leaching. Bull Environ Contam Toxicol 106:965-969. https://doi.org/10.1007/s00128-021-03269-6
Ma S, Chen W, Zhang J, Shen H (2020) Influence of simulated acid rain on the physiological response of flowering Chinese cabbage and variation of soil nutrients. Plant Soil Environ 66:648–657.https://doi.org/10.17221/469/2020-PSE
Ma Y, Wang B, Zhang R, Gao Y, Zhang X, Li Y, Zuo Z (2019) Initial simulated acid rain impacts reactive oxygen species metabolism and photosynthetic abilities in Cinnamonum camphora undergoing high temperature. Ind Crops Prod 135:352–361. https://doi.org/10.1016/j.indcrop.2019.04.050
Majd A, Chehregani AH (1992) Studies on developmental processes in ovules of soya Glycine max L. Plants and effects of certain toxins and environmental pollutants. In: Int Symp Transplant Prod Syst 319:431–436.https://doi.org/10.17660/ActaHortic.1992.319.67
Malaysia SA (1983) The state of the Malaysian environment 1983–1984. SAM, Penang, Malaysia (Original not seen: cited from McCormick, J. 1985. Acid earth. Earthscan Pub, International Institute for Environment and Development, London, UK.)
Mallick S, Kumar A, Kumar P (2021) Oxidation of HOSO[rad] by NH2[rad]: A new path for the formation of an acid rain precursor. Chem Phys Lett 773:138536. https://doi.org/10.1016/j.cplett.2021.138536
Maltz MR, Chen Z, Cao J, Arogyaswamy K, Shulman H, Aronson EL (2019) Inoculation with Pisolithus tinctorius may ameliorate acid rain impacts on soil microbial communities associated with Pinus massoniana seedlings. Fungal Ecol 40:50–61. https://doi.org/10.1016/j.funeco.2018.11.011
Mannings S, Smith S, Bell JNB (1996) Effect of acid deposition on soil acidification and metal mobilisation. Appl Geochemistry 11:139–143. https://doi.org/10.1016/0883-2927(95)00077-1
Meenakshi S, Sharma VP (2011) Effect of acid rain on the contents of leaves of Solanum melongena. J Plant Dev Sci 3:169–173
Merino A, Fernandez V, Garcia-Rodeja E (1994) Effects of experimental acidification of acid soils from Galicia (NW Spain). II. Heavy metal mobilization, 3rd Int Symp Environ Geochem Kraków, Book of Abstracts, 280–281.
Milton MB, Abigael TO (2015) Effects of simulated acid rain on the morphology, phenology and yield of Okra (Abelmoschus esculentus (L). Moench). J Environ Sci Comp Sci Engg Technol 4:501–518
Mishima SI, Kimura SD, Eguchi S, Shirato Y (2013) Changes in soil available-nutrient stores and relationships with nutrient balance and crop productivity in Japan. Soil Sci Plant Nutr 59:371–379. https://doi.org/10.1080/00380768.2013.786646
Mo J, Zhang W, Zhu W, Gundersn P, Fang Y, Li D, Wang H (2008) Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob Chang Biol 14:403–412. https://doi.org/10.1111/j.1365-2486.2007.01503.x
Mohajan H (2018) Acid rain is a local environment pollution but global concern. 47–55
Moreda-Piñeiro J, Alonso-Rodríguez E, Moscoso-Pérez C, Blanco-Heras G, Turnes-Carou I, López-Mahía P, Prada-Rodríguez D (2014) Influence of marine, terrestrial and anthropogenic sources on ionic and metallic composition of rainwater at a suburban site (northwest coast of Spain). Atmos Environ 88:30–38. https://doi.org/10.1016/j.atmosenv.2014.01.067
Munzuroglu O, Obek E, Geckil H (2003) Effects of simulated acid rain on the pollen germination and pollen tube growth of apple (Malus sylvestris miller cv. golden). Acta Biol Hung 54:95–103. https://doi.org/10.1556/ABiol.54.2003.1.10
Nandlal Hemlata Singh, Sachan Priti (2017) Simulated Acid Rain-induced Alterations in Flowering, Leaf Abscission and Pollen Germination in Sunflower (Helianthus annuus L.). Journal of Applied Sciences and Environmental Management 21(2):290. https://doi.org/10.4314/jasem.v21i2.9
National Automobile Scrappage Policy (2021) Ministry of road transport and highways website. https://morth.nic.in/vehiclescrapping-policy-overview
Neufeld HS, Jernstedt JA, Haines BL (1985) Direct foliar effects of simulated acid rain: I. Damage, growth and gas exchange. New Phytol 99:389–405. https://doi.org/10.1111/j.1469-8137.1985.tb03667.x
Neves NR, Oliva MA, da Cruz CD, Costa AC, Ribas RF, Pereira EG (2009) Photosynthesis and oxidative stress in the restinga plant species Eugenia uniflora L. exposed to simulated acid rain and iron ore dust deposition: potential use in environmental risk assessment. Sci Total Environ 407:3740–3745. https://doi.org/10.1016/j.scitotenv.2009.02.035
Odiyi BO, Bamidele JF (2014) Effects of simulated acid rain on growth and yield of Cassava Manihot esculenta (Crantz). J Agric Sci 6:96. https://doi.org/10.5539/jas.v6n1p96
Odiyi BO, Eniola AO (2015) The effect of simulated acid rain on plant growth component of cowpea (Vigna unguiculata) L. Walps Jordan J Biol Sci 8:51–54
Paulot F, Wunch D, Crounse JD, Toon GC, Millet DB, DeCarlo PF, Wennberg PO (2011) Importance of secondary sources in the atmospheric budgets of formic and acetic acids. Atmos Chem Phys 11:1989–2013. https://doi.org/10.5194/acpd-10-24435-2010
Peña RM, Garcı́a S, Herrero C, Losada M, Vázquez A, Lucas T (2002) Organic acids and aldehydes in rainwater in a northwest region of Spain. Atmos Environ 36:5277–5288. https://doi.org/10.1016/S1352-2310(02)00648-9
Pham Ha T. T., Nguyen An Thinh, Do Anh T. Ngoc., Hens Luc (2021) Impacts of Simulated Acid Rain on the Growth and the Yield of Soybean (Glycine max (L.) Merr.) in the Mountains of Northern Vietnam. Sustainability 13(9):4980. https://doi.org/10.3390/su13094980
Polishchuk OV, Vodka MV, Belyavskaya NA, Khomochkin AP, Zolotareva EK (2016) The effect of acid rain on ultrastructure and functional parameters of photosynthetic apparatus in pea leaves. Cell Tissue Biol 10:250–257. https://doi.org/10.1134/S1990519X16030093
Popova T, Petrova T, Petrichev M, Valyova M (2019) Action of activated waters on plants after adverse chemical effects, imitating acid rain. Bulg J Agric Sci 25:638–645
Prathibha P, Kothai P, Saradhi IV, Pandit GG, Puranik VD (2010) Chemical characterization of precipitation at a coastal site in Trombay, Mumbai, India. Environ Monit Assess 168:45–53. https://doi.org/10.1007/s10661-009-1090-7
Rahman MM, Mahamud S, Thurston GD (2019) Recent spatial gradients and time trends in Dhaka, Bangladesh, air pollution and their human health implications. J Air Waste Manag Assoc 69:478–501. https://doi.org/10.1080/10962247.2018.1548388
Raju R, Paul AG, Aguilor UP, Capili JT (2021) The Effect of Induced Acid Rain; Allium cepa Chromosomal Aberration Assay. Sch Acad J Biosci 9:89–97. https://doi.org/10.36347/sajb.2021.v09i03.005
Ramlall C, Varghese B, Ramdhani S, Pammenter NW, Bhatt A, Berjak P (2015) Effects of simulated acid rain on germination, seedling growth and oxidative metabolism of recalcitrant-seeded Trichilia dregeana grown in its natural seed bank. Physiol Plant 153:149–160. https://doi.org/10.1111/ppl.12230
Renzoni, GC, Viegi, L (1991) In vitro sensitivity of Pinus pinaster and P. pinea pollen grains to different pH values. Ann Bot Fenn 28(2):135–142
Rao PSP, Tiwari S, Matwale JL, Pervez S, Tunved P, Safai PD, Hopke PK (2016) Sources of chemical species in rainwater during monsoon and non-monsoonal periods over two mega cities in India and dominant source region of secondary aerosols. Atmos Environ 146:90–99. https://doi.org/10.1016/j.atmosenv.2016.06.069
Rodríguez-Sánchez VM, Rosas U, Calva-Vásquez G, Sandoval-Zapotitla E (2020) Does acid rain alter the leaf anatomy and photosynthetic pigments in urban trees? Plants 9:862. https://doi.org/10.3390/plants9070862
Roy A, Chatterjee A, Tiwari S, Sarkar C, Das SK, Ghosh SK, Raha S (2016) Precipitation chemistry over urban, rural and high altitude Himalayan stations in eastern India. Atmos Res 181:44–53. https://doi.org/10.1016/j.atmosres.2016.06.005
Sen TK, Khilar KC (2006) Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Adv Colloid Interface Sci 119:71–96. https://doi.org/10.1016/j.cis.2005.09.001
Shah J, Nagpal T, Johnson T, Amann M, Carmichael G, Foell W, Streets D (2000) Integrated analysis for acid rain in Asia: policy implications and results of RAINS-ASIA model. Annu Rev Environ Resour 25:339–375. https://doi.org/10.1146/annurev.energy.25.1.339
Shah V, Jacob D, Moch JM, Wang X, Zhai S (2020). Global modeling of cloudwater acidity, rainwater acidity, and acid inputs to ecosystems. In AGU Fall Meeting Abstracts A74–13.
Shivashankara GP, Ranga K, Ramalingaiah M (1999) Characterisation of bulk precipitation in industrial areas of Bangalore city. Indian J Environ Health 41:229–238
Shripal N, Pal KS, Kumar N (2000) Effects of simulated acid rain on yield and carbohydrate contents of green pepper. Advan Plant Sci 13:85–88
Shu X, Zhang K, Zhang Q, Wang W (2019) Ecophysiological responses of Jatropha curcas L. seedlings to simulated acid rain under different soil types. Ecotoxicol Environ Saf 185:1-12. https://doi.org/10.1016/j.ecoenv.2019.109705
Singh A, Agrawal M (1996) Response of two cultivars of Triticum aestivum L. to simulated acid rain. Environ Pollut 91:161–167. https://doi.org/10.1016/0269-7491(95)00056-9
Singh A, Agrawal M (2007) Acid rain and its ecological consequences. J Environ Biol 29:15
Singh AK, Mondal GC, Kumar S, Singh KK, Kamal KP, Sinha A (2007) Precipitation chemistry and occurrence of acid rain over Dhanbad, coal city of India. Environ Monit Assess 125:99–110. https://doi.org/10.1007/s10661-006-9243-4
Singh B, Agrawal M (2004) Impact of simulated acid rain on growth and yield of two cultivars of wheat. Wat Air Soil Poll 152:71–80. https://doi.org/10.1023/B:WATE.0000015331.02874.df
Singh N, Yunus M, Singh SN, Ahmad KJ (1992) Performance of Vicia faba plants in relation to simulated acid rain and/or endosulphan treatment. Bull Environ Contam Toxicol 48:243–248. https://doi.org/10.1007/BF00194378
Singh RK, Agrawal M (2005) Atmospheric depositions around a heavily industrialized area in a seasonally dry tropical environment of India. Environ Pollut 138:142–152. https://doi.org/10.1016/j.envpol.2005.02.009
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404. https://doi.org/10.1016/j.soilbio.2009.10.014
Smith RA (1872) Air and rain: the beginnings of a chemical climatology. Longmans, Green, and Company, 600pp, London.
Sridharan PV, Saksena S (1990) Description of acid rain problem in India. In: Second Annual Workshop on Acid Rain and Emissions in Asia. Bangkok, Thailand
Sullivan TS, Barth VP, Lewis RW (2017) Soil acidity impacts beneficial soil microorganisms. Washington State University Extension and the U.S. Department of Agriculture 1-6.
Sun X, Wang Y, Li H, Yang X, Sun L, Wang X, Wang W (2016) Organic acids in cloud water and rainwater at a mountain site in acid rain areas of South China. Environ Sci Pollut Res 23:9529–9539. https://doi.org/10.1007/s11356-016-6038-1
Sutton MA, Drewer J, Moring A, Adhya TK, Ahmed A, Bhatia A, Brownlie W, Dragosits U, Ghude SD, Hillier J, Hooda S (2017) The Indian nitrogen challenge in a global perspective. The Indian Nitrogen Assessment. https://doi.org/10.1016/B978-0-12-811836-8.00002-1
Swartz JS, Van Zyl PG, Beukes JP, Labuschagne C, Brunke EG, Portafaix T, Pienaar JJ (2020) Twenty-one years of passive sampling monitoring of SO2, NO2 and O3 at the Cape Point GAW station. South Africa Atmos Environ 222:117128. https://doi.org/10.1016/j.atmosenv.2019.117128
Tang L, Lin Y, He X, Han G (2019) Acid rain decelerates the decomposition of Cunninghamia lanceolata needle and Cinnamomum camphora leaf litters in a karst region in China. Ecol Res 34:193–200. https://doi.org/10.1016/B978-0-12-811836-8.00002-1
Tiwari S, Hopke PK, Thimmaiah D, Dumka UC, Srivastava AK, Bisht DS, Tripathi SN (2016) Nature and sources of ionic species in precipitation across the Indo-Gangetic Plains, India. Aerosol Air Qual Res 16:943–957. https://doi.org/10.4209/aaqr.2015.06.0423
Tiwari S, Srivastava MK, Bisht DS (2008) Chemical composition of rainwater in Panipat, an industrial city in Haryana. Indian J Radio Space Phys 37:443–449.
Tong S, Zhang L (2014) Differential sensitivity of growth and net photosynthetic rates in five tree species seedlings under simulated acid rain stress. Pol J Environ Stud 23(6): 2259-2264. https://doi.org/10.15244/pjoes/24930
US EPA progress report (2013) 2012 Progress report, clean air markets. https://www3.epa.gov/airmarkets/progress/reports/, https://www.epa.gov/sites/default/files/2015-08/documents/arpcair12_02.pdf
Varma GS (1989) Impact of soil-derived aerosols on precipitation acidity in India. Atmos Environ 23:2723–2728. https://doi.org/10.1016/0004-6981(89)90552-0
Varma GS (1989) Background trends of pH of precipitation over India. Atmos Environ 23:747–751. https://doi.org/10.1016/0004-6981(89)90476-9
Waghmare VV, Aslam MY, Yang L, Safai PD, Pandithurai G (2021) Inorganic Ionic Composition of Rainwater at a High Altitude Station over the Western Ghats in Peninsular India. J Atmos Chem 78:59–76. https://doi.org/10.1007/s10874-021-09416-x
Wang B, Wang C, Xue R, Liu M, Xu G, Li Z (2021). Earthworm and arbuscular mycorrhizal fungi improve plant hormones and antioxidant enzymes to alleviate simulated acid rain stress. https://doi.org/10.21203/rs.3.rs-438862/v1
Wang Y, Xu Y, Li D, Tang B, Man S, Jia Y, Xu H (2018) Vermicompost and biochar as bio-conditioners to immobilize heavy metal and improve soil fertility on cadmium contaminated soil under acid rain stress. Sci Total Environ 621:1057–1065. https://doi.org/10.1016/j.scitotenv.2017.10.121
Watanabe K, Honoki H (2013) Measurements of aerosol number concentrations and rainwater chemistry at Mt. Tateyama, near the coast of the Japan sea in central Japan: On the influence of high-elevation Asian dust particles in autumn. J Atmos Chem 70:115–129. https://doi.org/10.1007/s10874-013-9258-5
Wei H, Liu Y, Xiang H, Zhang J, Li S, Yang J (2020) Soil pH responses to simulated acid rain leaching in three agricultural soils. Sustainability 12:280. https://doi.org/10.3390/su12010280
Wei X, Blanco JA, Jiang H, Kimmins JH (2012) Effects of nitrogen deposition on carbon sequestration in Chinese fir forest ecosystems. Sci Total Environ 416:351–361. https://doi.org/10.1016/j.scitotenv.2011.11.087
Wen K, Liang C, Wang L, Hu G, Zhou Q (2011) Combined effects of lanthanum ion and acid rain on growth, photosynthesis and chloroplast ultrastructure in soybean seedlings. Chemosphere 84:601–608. https://doi.org/10.1016/j.chemosphere.2011.03.054
Wertheim FS, Craker LE (1988) Effects of acid rain on corn silks and pollen germination. J Environ Qual 17:135–138. https://doi.org/10.2134/jeq1989.00472425001800010023x
World energy outlook 2019 assessed from https://www.iea.org/reports/world-energy-outlook-2019
World Health Organization (WHO), world air quality report 2018. https://www.iqair.com/world-most-polluted. cities/world-air-quality-report-2018-en.pdf
Wu J, Liang G, Hui D, Deng Q, Xiong X, Qiu Q, Zhang D (2016) Prolonged acid rain facilitates soil organic carbon accumulation in a mature forest in Southern China. Sci Total Environ 544:94–102. https://doi.org/10.1016/j.scitotenv.2015.11.025
Xu Z, Wu Y, Liu WJ, Liang CS, Ji J, Zhao T, Zhang X (2015) Chemical composition of rainwater and the acid neutralizing effect at Beijing and Chizhou city, China. Atmos Res 164:278–285. https://doi.org/10.1016/j.atmosres.2015.05.009
Yadav DS, Jaiswal B, Gautam M, Agrawal M (2020) Soil Acidification and its Impact on Plants. In: Plant Responses to Soil Pollution. Springer, Singapore, pp 1–26 https://doi.org/10.1007/978-981-15-4964-9_1
Yang L, Xu Y, Zhang R, Wang X, Yang C (2018) Comprehensive transcriptome profiling of soybean leaves in response to simulated acid rain. Ecotoxicol Environ Saf 158:18–27. https://doi.org/10.1016/j.ecoenv.2018.04.015
Yin P, Peng YJ, Song CC, Liu XJ, Huang J, Lv X (2021). Effects of simulated acid rain on Growth of Lolium perenne. In: IOP Conf Ser: Earth Environ Sci. IOP Publishing, pp 022078. https://doi.org/10.1088/1755-1315/714/2/022078
Zhang C, Yi X, Zhou F, Gao X, Wang M, Chen J, Shen C (2020) Comprehensive transcriptome profiling of tea leaves (Camellia sinensis) in response to simulated acid rain. Sci Hortic 272:109491. https://doi.org/10.1016/j.scienta.2020.109491
Zhang JE, Ouyang Y, Ling DJ (2007) Impacts of simulated acid rain on cation leaching from the Latosol in south China. Chemosphere 67:2131–2137. https://doi.org/10.1016/j.chemosphere.2006.12.095
Zhao C, Sun Y, Zhong Y, Xu S, Liang Y, Liu S, He M (2021) Spatio-temporal analysis of urban air pollutants throughout China during 2014–2019. Air Qual Atmos Health 14:1619–1632. https://doi.org/10.1007/s11869-021-01043-5
Zheng W, Li R, Yang Q, Zhang W, Huang K, Guan X, Wang S (2019) Short-term response of soil respiration to simulated acid rain in Cunninghamia lanceolata and Michelia acclurei plantations. J Soils Sediments 19:1239–1249. https://doi.org/10.1007/s11368-018-2148-3
Zhou S, Zhang M, Chen S, Xu W, Zhu L, Gong S, Wang P (2020) Acid resistance of Masson pine (Pinus massoniana Lamb.) families and their root morphology and physiological response to simulated acid deposition. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-79043-1
Zhou X, Xu Z, Liu W, Wu Y, Zhao T, Jiang H, Zhang X, Zhang J, Zhou L, Wang Y (2019) Chemical composition of precipitation in Shenzhen, a coastal mega-city in South China: Influence of urbanization and anthropogenic activities on acidity and ionic composition. Sci Total Environ 662:218–226. https://doi.org/10.1016/j.scitotenv.2019.01.096
Acknowledgements
The authors are thankful to the Head, Department of Botany, and the Coordinators, CAS in Botany, Institute of Eminence and Interdisciplinary School of Life Sciences, Banaras Hindu University, for providing necessary facilities to carry out a systematic literature review. MA is also thankful to SERB, New Delhi for providing JC Bose Fellowship.
Funding
The Council of Scientific and Industrial Research (CSIR), New Delhi, India, provided financial support in the form of CSIR-UGC JRF (file no. 09/013(0934)/2020-EMR-I).
Author information
Authors and Affiliations
Contributions
Jigyasa Prakash: conceptulization, visualization, writing original draft. Shashi Bhushan Agrawal: visualization, writing—review and editing. Madhoolika Agrawal: writing—review and editing and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prakash, J., Agrawal, S.B. & Agrawal, M. Global Trends of Acidity in Rainfall and Its Impact on Plants and Soil. J Soil Sci Plant Nutr 23, 398–419 (2023). https://doi.org/10.1007/s42729-022-01051-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-01051-z