Abstract
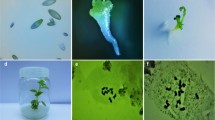
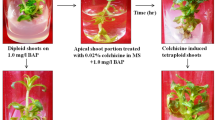
A protocol for in vitro polyploidy induction was developed in Mini Red cultivar of African daisy (Gerbera jamesonii). Various concentrations of colchicine (0, 0.05, 0.1, 0.2, 0.5 and 1%) were applied with different exposure durations (2, 4 and 8 h) on 2 weeks old explants originated from in vitro induced Gerbera shoots. Murashige and Skoog (MS) medium supplemented with 8.8 μM 6-benzyladenine (BAP) plus 11.4 μM indole-3-acetic acid (IAA) were used for proliferation of the explants. Root induction occurred when proliferated colchicine-treated shoots transferred to the half strength MS medium supplemented with 3.99 μM IAA. The results of analysis based on the comparison of means revealed that increasing concentrations of colchicine led to significant reduction in survival rate of Gerbera in longer exposure durations; however we achieved the highest survival rate with polyploidy induction by using 0.1% (w/v) colchicine for 8 h. We obtained the highest percentage of tetraploid plants (53.3%) by applying 0.2% colchicine for 2 h. Direct chromosome counting method verified the duplicated chromosome number in colchicine-induced tetraploid plants (2n = 4x = 48) in comparison with intact diploid plants of Gerbera (2n = 2x = 24). Colchicine-induced tetraploid plants of Gerbera represented reduced stomata density with wider size than their diploid relatives. In vitro tetraploidy induction led to larger flower size, longer stalks, broader leaf length and width and lower shoots in Mini Red cultivar of Gerbera. The optimized polyploidy induction protocol has high commercial value and can be also applicable for in vitro micropropagation of Gerbera.

Similar content being viewed by others
Abbreviations
- ADS:
-
Adenine sulphate
- ANOVA:
-
Analysis of variance
- BAP:
-
6-benzyladenine
- CRD:
-
Completely randomized design
- DMRT:
-
Duncan’s multiple range test
- IAA:
-
Indole-3-acetic acid
- MS:
-
Murashige and Skoog medium
- PPFD:
-
Photosynthetic photon flux density
References
Allum JF, Bringloe DH, Roberts AV. Chromosome doubling in a Rosa rugosa Thunb. hybrid by exposure of in vitro nodes to oryzalin: the effects of node length, oryzalin concentration and exposure time. Plant Cell Rep. 2007;26(11):1977–84.
Ascough GD, Van Staden J, Erwin JE. Effectiveness of colchicine and oryzalin at inducing polyploidy in Watsonia lepida NE Brown. HortScience. 2008;43(7):2248–51.
Caperta AD, Delgado M, Ressurreição F, Meister A, Jones RN, Viegas W, Houben A. Colchicine-induced polyploidization depends on tubulin polymerization in c-metaphase cells. Protoplasma. 2006;227(2):147–53.
Cardoso JC, da Silva JAT. Gerbera micropropagation. Biotechnol Adv. 2013;31(8):1344–57.
Chakraborti SP, Vijayan K, Roy BN, Qadri SMH. In vitro induction of tetraploidy in mulberry (Morus alba L.). Plant Cell Rep. 1998;17(10):799–803.
Chauvin JE, Label A, Kermarrec MP. In vitro chromosome-doubling in tulip (Tulipa gesneriana L.). J Hortic Sci Biotechnol. 2005;80(6):693–8.
Chulalaksananukul W, Chimnoi W. Polyploid induction in Centella asiatica (L.) Urban by colchicine treatment. J Sci Res Chula Univ. 1999;24:56–65.
Cohen D, Yao JL. In vitro chromosome doubling of nine Zantedeschia cultivars. Plant Cell, Tissue Organ Cult. 1996;47(1):43–9.
Dhooghe E, Denis S, Eeckhaut T, Reheul D, Van Labeke MC. In vitro induction of tetraploids in ornamental Ranunculus. Euphytica. 2009;168(1):33–40.
Dhooghe E, Grunewald W, Leus L, Van Labeke MC. In vitro polyploidisation of helleborus species. Euphytica. 2009;165(1):89–95.
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult. 2011;104(3):359–73.
Dole JM, Wilkins HF. Floriculture: principles and species. Upper Saddle River: Prentice-Hall Inc.; 2005.
Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11(1):1–42.
Eeckhaut T, Samyn G, Van Bockstaele E (2001) In vitro polyploidy induction in Rhododendron simsii hybrids. In: XX International Eucarpia symposium, section ornamentals, strategies for new ornamentals-part II 572; 2001. p. 43–9.
Eeckhaut T. Ploidy breeding and interspecific hybridization in Spathiphyllum and woody ornamentals. Doctoral dissertation. Ghent University: Ghent; 2003.
Gallone A, Hunter A, Douglas GC. Polyploid induction in vitro using colchicine and oryzalin on Hebe ‘Oratia Beauty’: production and characterization of the vegetative traits. Sci Hortic. 2014;179:59–66.
Gantait S, Mandal N, Bhattacharyya S, Das PK. An elite protocol for accelerated quality-cloning in Gerbera jamesonii Bolus cv. Sciella. In Vitro Cell Dev Biol Plant. 2010;46(6):537–48.
Gantait S, Mandal N, Bhattacharyya S, Das PK. Induction and identification of tetraploids using in vitro colchicine treatment of Gerbera jamesonii Bolus cv. Sciella. Plant Cell Tissue Organ Cult. 2011;106(3):485–93.
Ghani A, Neamati SH, Azizi M, Saharkhiz MJ, Farsi M. Artificial autotetraploidy induction possibility of two Iranian endemic mint. Notulae Scientia Biologicae. 2014;6(2):185–91.
Harding J, Huang H, Byrne T. Maternal, paternal, additive, and dominance components of variance in Gerbera. Theor Appl Genet. 1991;82(6):756–60.
Hasbullah NA, Taha RM, Awal A. Growth optimization and organogenesis of Gerbera jamesonii Bolus ex. Hook f. in vitro. Pak J Biol Sci PJBS. 2008;11(11):1449–54.
Henny RJ, Holm JR, Chen J, Scheiber M. In vitro induction of tetraploids in Dieffenbachia × ‘Star Bright M-1’ by colchicine. HortScience. 2009;44(3):646–50.
Kanwar JK, Kumar S. In vitro propagation of Gerbera: a review. Hort Sci (Prague). 2008;35(1):35–44.
Kermani MJ, Sarasan V, Roberts AV, Yokoya K, Wentworth J, Sieber VK. Oryzalin-induced chromosome doubling in Rosa and its effect on plant morphology and pollen viability. Theor Appl Genet. 2003;107(7):1195–200.
Khosravi P, Kermani MJ, Nematzadeh GA, Bihamta MR, Yokoya K. Role of mitotic inhibitors and genotype on chromosome doubling of Rosa. Euphytica. 2008;160(2):267–75.
Li H, Yan B, Zhang T, Jiang Y, Zhang H, Yu L, Li S. Preliminary studies on polyploidy mutation of cut flower Gerbera jamesonii Bolus. Acta Hortic Sin. 2009;36(4):605–10.
Lin H, Jian M, Liang LY, Pei WJ, Liu XZ, Zhang HY. Production of polyploids from cultured shoot tips of Eucalyptus globulus Labill by treatment with colchicine. Afr J Biotech. 2010;9(15):2252–5.
Liu Z, Gao S. Micropropagation and induction of autotetraploid plants of Chrysanthemum cinerariifolium (Trev) Vis. In Vitro Cell Dev Biol Plant. 2007;43(5):404–8.
Lu C, Bridgen MP. Chromosome doubling and fertility study of Alstroemeria aurea × A. caryophyllaea. Euphytica. 1997;94(1):75–81.
Meyer EM, Touchell DH, Ranney TG. In vitro shoot regeneration and polyploid induction from leaves of Hypericum species. Hort Sci. 2009;44(7):1957–61.
Minerva G, Kumar S. Micropropagation of gerbera (Gerbera jamesonii Bolus). In protocols for micropropagation of selected economically-important horticultural plants. Totowa: Humana Press; 2013. p. 305–16.
Mitrofanova IV, Zilbervarg IR, Yemets AI, Mitrofanova OV, Blume YB. The effect of dinitroaniline and phosphorothioamidate herbicides on polyploidisation in vitro of Nepeta plants. Cell Biol Int. 2003;27(3):229–31.
Nhut DT, An TTT, Huong NTD, Don NT, Hai NT, Thien NQ, Vu NH. Effect of genotype, explant size, position, and culture medium on shoot generation of Gerbera jamesonii by receptacle transverse thin cell layer culture. Sci Hortic. 2007;111(2):146–51.
Nimura M, Kato J, Horaguchi H, Mii M, Sakai K, Katoh T. Induction of fertile amphidiploids by artificial chromosome-doubling in interspecific hybrid between Dianthus caryophyllus L. and D. japonicus Thunb. Breed Sci. 2006;56(3):303–10.
Noori SAS, Norouzi M, Karimzadeh G, Shirkool K, Niazian M. Effect of colchicine-induced polyploidy on morphological characteristics and essential oil composition of Ajowan (Trachyspermum ammi L.). Plant Cell Tissue Organ Cult. 2017;130:543–51.
Omidbaigi R, Mirzaee M, Hassani ME, Sedghi Moghadam M. Induction and identification of polyploidy in basil (Ocimum basilicum L.) medicinal plant by colchicine treatment. Int J Plant Prod. 2012;4(2):87–98.
Omran A, Mohammad B. Polyploidization effect in two diploid cotton (Gossypium herbaceum L. and G. arboreum L.) species by colchicine treatments. Afr J Biotechnol. 2008;7(2):102–8.
Pande S, Khetmalas M. Biological effect of sodium azide and colchicine on seed germination and callus induction in Stevia rebaudiana. Asian J Exp Biol Sci. 2012;3:93–8.
Parthasarathy VA, Nagaraju V. In-vitro propagation in Gerbera jamesonii Bolus. Indian J Hortic. 1999;56(1):82–5.
Petersen KK, Hagberg P, Kristiansen K. Colchicine and oryzalin mediated chromosome doubling in different genotypes of Miscanthus sinensis. Plant Cell Tissue Organ Cult. 2003;73(2):137–46.
Pickens KA, Kania SA. Effects of colchicine and oryzalin on callus and adventitious shoot formation of Euphorbia pulchurrima Winter Rose’. HortScience. 2006;41(7):1651–5.
Sajjad YA, Jaskani MJ, Mehmood A, Ahmad I, Abbas HA. Effect of colchicine on in vitro polyploidy induction in African marigold (Tagetes erecta). Pak J Bot. 2013;45(3):1255–8.
Silva PAKX, Callegari-Jacques S, Bodanese-Zanettini MH. Induction and identification of polyploids in Cattleya intermedia Lindl.(Orchidaceae) by in vitro techniques. Ciência Rural. 2000;30(1):105–11.
Singh S, Ram R, Kaundal S, Sharma A, Kumar A, Dhyani D. Field performance and differential response of micro-propagated potential F1 genotypes of Gerbera jamesonii. Am J Exp Agric. 2016;10(1):1–11.
Suzuki K, Takatsu Y, Gonai T, Kasumi M. Plant regeneration and chromosome doubling of wild Gladiolus species. Acta Hortic. 2005;673:175–81.
Takamura T, Miyajima I. Colchicine induced tetraploids in yellow-flowered cyclamens and their characteristics. Sci Hortic. 1996;65(4):305–12.
Takamura T, Lim KB, Van Tuyl JM. Effect of a new compound on the mitotic polyploidization of Lilium longiflorum and Oriental hybrid lilies. In XX International Eucarpia symposium, section ornamentals, strategies for new ornamentals-part II 572. 2001; p. 37–42.
Teng ES, Leonhardt KW. In vitro and in vivo polyploidization of Dracaena with oryzalin VI. Int Symp New Floric Crops. 2007;813:509–16.
Tosca A, Pandolfi R, Citterio SA, Fasoli A, Sgorbati S. Determination by flow cytometry of the chromosome doubling capacity of colchicine and oryzalin in gynogenetic haploids of Gerbera. Plant Cell Rep. 1995;14(7):455–8.
Urwin NA. Generation and characterisation of colchicine-induced polyploid Lavandula × intermedia. Euphytica. 2014;197(3):331–9.
Wang C, Lei J. In vitro induction of tetraploids from immature embryos through colchicine treatments in Clivia miniata Regel. Afr J Agric Res. 2012;7(25):3712–8.
Widoretno W. In vitro induction and characterization of tetraploid Patchouli (Pogostemon cablin Benth.) plant. Plant Cell Tissue Organ Cult. 2016;125(2):261–7.
Zhang Q, Luo F, Liu L, Guo F. In vitro induction of tetraploids in crape myrtle (Lagerstroemia indica L.). Plant Cell Tissue Organ Cult. 2010;101(1):41–7.
Zhang Z, Dai H, Xiao M, Liu X. In vitro induction of tetraploids in Phlox subulata L. Euphytica. 2008;159(1–2):59–65.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khalili, S., Niazian, M., Arab, M. et al. In vitro chromosome doubling of African daisy, Gerbera jamesonii Bolus cv. Mini Red. Nucleus 63, 59–65 (2020). https://doi.org/10.1007/s13237-019-00282-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-019-00282-3