Abstract
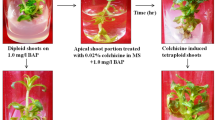
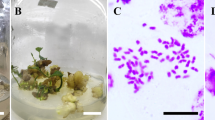
Patchouli (Pogostemon cablin Benth.) is one kind of plant from Indonesia which is producing oil. In vitro induction of tetraploid in Patchouli plant and cytological and morphological characterization were conducted. Induced polyploidy was done by culturing leaf explant on MS medium added with a varying concentration of colchicine. It was observed that colchicine on MS medium mostly affect to growth of explant and shoot regeneration. Tetraploid plants were determined by observation of chromosome number. The tetraploid plants have chromosome number (2n = 4x = 128), twice higher than those of diploid plants (2n = 2x = 64). The tetraploid plants exhibited much larger stomata size but less than diploid plants. The tetraploid plants also showed more glandular hairs than diploid plants. Significant different in morphological characters between the diploid and tetraploid plants were noted. Leaf size and stem diameter of the tetraploids plant are larger than the diploid plants. In this study, the tetraploids Patchouli have a promising potential as a superior variety.





Similar content being viewed by others
References
Blasco M, Badenes MA, Naval MDM (2015) Colchicine-induced polyploidy in loquat (Eriobotrya japonica (Thunb.) Lindl.). Plant Cell Tissue Organ Cult 120:453–461
Caperta AD, Delgado M, Ressurreicao F, Meister A, Jones RN, Viegas W, Houben A (2012) Colchicine-induced polyploidization depends on tubulin polymerization in c-metaphase cells. Protoplasma 227:147–153
Chakraborti SP, Vijayan K, Roy BN, Quari SMH (1998) In vitro induction of tetraploidi mulberry. Plant Cell Rep 17:799–803
Chen LP, Wang YJ, Zhao M (2006) In vitro induction and characterization of tetraploid Lychnis senno Siebold et Zucc. HortScience 41:759–761
Cheng ZH, Zhou XJ, Khan MA, Su L, Meng HW (2012) In vitro induction of tetraploid garlic with trifluralin. Genet Mol Res 11:2620–2628
De Jesus-Gonzales L, Weather PJ (2003) Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep 21:809–813
Donelian A, Carlson LHC, Lopes TJ, Machado RAF (2009) Comparison of extraction of patchouli (Pogostemon cablin) essential oil with supercritical CO2 and by steam distillation. J Supercrit Fluid 48:15–20
Gao SL, Zhu DN, Cai ZH, Xu DR (1996) Autotetraploid plants from colchicine-treated bud culture of Salvia miltiorrhiza Bge. Plant Cell Tissue Organ Cult 47:73–77
Ghani A, Neamati SH, Azizi M, Saharkhiz MJ, Farsi M (2014) Artificial autotetraploidy induction possibility of two Iranian EndemicMint (Mentha mozaffarianii) ecotypes. Not Sci Biol 6:185–191
Huang HP, Gao SL, Chen LL, Wei KH (2010) In vitro tetraploid induction and generation of tetraploids from mixoploidy in Dioscorea zingiberensis. Pharmacogn Mag 6:51–56
Jones JR, Ranney TG, Eaker TA (2008) A novel method for inducing polyploidy in rhododendron seedlings. J Am Rhododendron Soc 62:130–135
Kerdsuwan N, Te-chato S (2012) Effects of colchicine on survival rate, morphological, physiological and cytological character of Chang Daeng orchid (Rhynchostylis gigantean var. rubrum Sagarik) in vitro. J Agric Technol 8:1451–1460
Maeda E, Miyake H (1997) Leaf anatomy of patchouli (Pogostemon patchouli) with reference to the disposition of mesophyll glands. Jpn J Crop Sci 66:307–317
Maruska A, Proscevicius J, Bimbiraite-Surviliene K, Kornysova O, Ragazinskiene O, Ratautaite V (2010) Comparison of phytochemical composition of medicinal plants by means of chromatographic and related techniques. Procedia Chem 2:83–91
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays for tobacco tissue cultures. Physiol Plant Int J Plant Biol 15:473–497
Nilanthi D, Chen XL, Zhao FC, Yang YS, Wu H (2009) Induction of tetraploids from petiole explants through colchicine treatments in Echinacea purpurea L. J Biomed Biotechnol 2009:7
Omidbaigi R, Mirzaee M, Hassani ME, Sedghi Moghadamc M (2010) Induction and identification of polyploidy in basil (Ocimum basilicum L.) medicinal plant by colchicine treatment. IJPP 4:1735–6814
Pande S, Khetmalas M (2012) Biological effect of sodium azide and colchicine on seed germination and callus induction in Stevia rebaudiana. Asian J Exp Biol Sci 3:93–98
Petersen KK, Hagberg P, Kristiansek K (2003) Colchicine and oryzalin mediated chromosome doubling in different genotypes of Miscanthus sinensis. Plant Cell Tissue Organ Cult 73:137–146
Piromya R, Kermanee P (2013) Occurrence of tetraploidy in colchicine-treated physic nut (Jatropha curcas Linn.). J Nat Sci 47:23–29
Sajjad Y, Jaskani MJ, Mehmood A, Ahmad I, Abbas H (2013) Effect of colchicine on in vitro polyploidy induction in African marigold (Tagetes erecta). Pak J Bot 45:1255–1258
Saradhuldhat P, Silayai B (2001) Some chemical treatments on Kluai Khai through tissue culture for mutation breeding. Kasetsart J (Nat Sci) 35:231–241
Shi H, Yu W, Zhang G, Tsang PE, Chow CF (2014) Induction of polyploid hairy roots and its plant regeneration in Pogostemon cablin. Chin J Biotechnol 30(8):1235–1246
Silva PAKXM, Jacques SC, Zanettini MHB (2000) Induction and identification of polyploids in Cattleya intermedia Lindl. (Orchidaceae) Atraves de Technicas in vitro. Cienc Rural Santa Maria 3:105–111
Song C, Liu SJ, Xiao J, He WG, Zhou Y, Qin QB, Zhang C, Liu Y (2012) Polyploid organisms. Sci China Life Sci 55:301–311
Sun Q, Sun H, Li L, Bell RL (2009) In vitro colchicine-induced polyploid plantlet production and regeneration from leaf explants of the diploid pear (Pyrus communis L.) cultivar, ‘Fertility’. J Hortic Sci Biotechnol 84:548–552
Tulay E, Unal M (2010) Production of colchicine induced tetraploids in Vicia villosa roth. Caryologia 63:292–303
Wu Y, Li M (2013) Induction of tetraploid plants of Pogostemon cablin (Blanco) and its quality evaluation. Pharmacogn J 5(6):281–285
Zeng SH, Chen CW, Liu H, Liu JH, Deng XX (2006) In vitro induction, regeneration and analysis of autotetraploids derived from protoplasts and callus treated with colchicine in Citrus. Plant Cell Tissue Organ Cult 87:85–93
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Widoretno, W. In vitro induction and characterization of tetraploid Patchouli (Pogostemon cablin Benth.) plant. Plant Cell Tiss Organ Cult 125, 261–267 (2016). https://doi.org/10.1007/s11240-016-0946-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-0946-0