Abstract
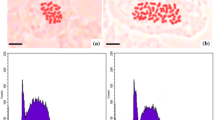
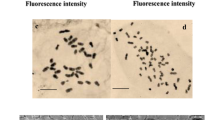
Polyploidy is an amazing evolutionary event that can be used in plant breeding to improve plant material. The present study was conducted to assess the effect of in vitro-induced polyploidy on different properties of Ajowan medicinal plant. Five different concentrations of colchicine, including 0.025, 0.05, 0.1, 0.2, and 0.5% (w/v), were applied to germinating seeds of ajowan in six different durations of exposure, including 6, 12, 24, 36, and 48 h. Chromosome counting showed successful duplication of chromosome number in tetraploid plants (2n = 4x = 36) in contrast with intact diploid plants (2n = 2x = 18). DNA content duplication in induced tetraploid plants was proved through flow cytometry analysis. The highest tetraploidy induction was achieved by applying 0.05% (w/v) colchicine for 24 h with 11.53% efficiency. The tetraploid plants achieved were larger than their diploid intact plants for plant height, leaf length, stem diameter, inflorescence length, peduncle length, and seed length characteristics. The length and width of stomata were increased in induced tetraploid plants, whereas stomata density was decreased, in contrast with initial diploid plants. Gas chromatography mass spectrometry (GC/MS) analysis showed significant increases in thymol content in essential oil of tetraploid plants (69.2%) in contrast with those of diploid plants (49.67%).



Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- FCM:
-
Flow cytometry
- GC/MS:
-
Gas chromatography mass spectrometry
- LSD:
-
Least significant difference
- MS:
-
Murashige and Skoog medium
References
Abdoli M, Moieni A, Badi HN (2013) Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea (L.). Acta Physiol Plant 35(7):2075–2083
Aina O, Quesenberry K, Gallo M (2012) In vitro induction of tetraploids in Arachis paraguariensis. Plant Cell Tissue Organ Cult (PCTOC) 111(2):231–238
Ashraf M, Orooj A (2006) Salt stress effects on growth, ion accumulation and seed oil concentration in an arid zone traditional medicinal plant ajwain (Trachyspermum ammi [L.] Sprague). J Arid Environ 64(2):209–220
Bagheri M, Mansouri H (2015) Effect of induced polyploidy on some biochemical parameters in Cannabis sativa L. Appl Biochem Biotechnol 175(5):2366–2375
Cary NC (2004) SAS Institute. The SAS system for windows. Release 9.1. SAS Inst, North Carolina.
Chattopadhyay D, Sharma AK (1988) A new technique for orcein banding with acid treatment. Stain Technol 63(5):283–287
Dalkani M, Hassani A, Darvishzadeh R (2012) Determination of the genetic variation in Ajowan (Carum Copticum L.) populations using multivariate statistical techniques. Rev Ciênc Agron 43(4):698–705
Das AB, Mallick R (1993) Nuclear DNA and chromosome changes within the tribe Ammineae. Cytobios 74(298–99):197–207
Dhawan OP, Lavania UC (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87(2):81–89
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult (PCTOC) 104(3):359–373
Doležel J, Bartoš JAN (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot 95(1):99–110
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220(4601):1049–1051
Gantait S, Mandal N, Bhattacharyya S, Das PK (2011) Induction and identification of tetraploids using in vitro colchicine treatment of Gerbera jamesonii Bolus cv. Sciella. Plant Cell Tissue Organ Cult (PCTOC) 106(3):485
Gomes SSL, Saldanha CW, Neves CS, Trevizani M, Raposo NRB, Notini MM, de Oliveira Santos M, Campos JMS, Otoni WC, Viccini LF (2014) Karyotype, genome size, and in vitro chromosome doubling of Pfaffia glomerata (Spreng.) Pedersen. Plant Cell, Tissue and Organ Cult (PCTOC) 118(1):45–56
Hannweg K, Visser G, de Jager K, Bertling I (2016) In vitro-induced polyploidy and its effect on horticultural characteristics, essential oil composition and bioactivity of Tetradenia riparia. S Afr J Bot 106:186–191
Joshi SG (2000) Medicinal Plants. Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi, p. 491
Khullar SP, Sharma SS, Verma SC (1988) SOCGI plant chromosome number reports. VI. J Cytol Genet 23:38–52
Lavania UC (2005) Genomic and ploidy manipulation for enhanced production of phyto-pharmaceuticals. Plant Genet Resour Charact Util 3(02):170–177
Levy M (1976) Altered glycoflavone expression in induced autotetraploids of Phlox drummondii. Biochem Syst Ecol 4(4):249–254
Majdi M, Karimzadeh G, Malboobi MA, Omidbaigi R, Mirzaghaderi G (2010) Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): Morphological, physiological, cytological, and phytochemical changes. HortScience 45(1):16–21
Malhotra SK, Vijay OP (2004) Ajowan. In: Peter KV (ed) Handbook of Herbs and Spices, vol 2. Woodhead Publishing Limited, Cambridge, pp 107–116
Mirahmadi SF, Sefidkon F, Aalifar M, Akramian M (2011) Essential oil composition of Tanacetum polycephalum subsp. duderanum (Boiss) Podl., A Plant endemic from Iran. J Essent Oil Bear Plants 14(6):742–745
Moosavi SG, Seghatoleslami MJ, Jouyban Z, Ansarinia E, Moosavi SA (2015) Response Morphological Traits and yield of Ajowan (Carum copticum) to Water deficit stress and Nitrogen Fertilizer. In Biological Forum (Vol. 7, No. 1, p. 293). Research Trend.
Niazian M, Noori SAS, Galuszka P, Tohidfar M, Mortazavian SMM (2017) Genetic stability of regenerated plants via indirect somatic embryogenesis and indirect shoot regeneration of Carum copticum L. Ind Crops Prod 97:330–337
Planchais S, Glab N, Inze´ D, Bergonioux C (2000) Chemical inhibitors: a tool for plant cell cycle studies. FEBS Lett 476:78–83. doi:10.1016/S0014-5793(00)01675-6
Sattler MC, Carvalho CR, Clarindo WR (2016) The polyploidy and its key role in plant breeding. Planta 243(2):281–296
Tavan M, Mirjalili MH and Karimzadeh G (2015) In vitro polyploidy induction: changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult (PCTOC), 122(3):573–583
Urwin NA (2014) Generation and characterisation of colchicine-induced polyploid Lavandula × intermedia. Euphytica 197(3):331–339
Xu C, Huang Z, Liao T, Li Y and Kang X (2016) In vitro tetraploid plants regeneration from leaf explants of multiple genotypes in Populus. Plant Cell Tissue Organ Cult (PCTOC), 125(1):1–9
Acknowledgements
The authors are thankful to Dr M.H Asare, the secretary of science and technological development staff of medicinal plants and traditional medicine of Islamic Republic of Iran for his kindness helps in financial support of this research. Also the authors are thankful to the Research Institute of Forests and Rangelands of Iran for procuring the seeds.
Author contributions
MNI and SASN wrote the manuscript. KS and MNO performed tetraploidy experiments. GK helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical standards
The experiments were performed according to the current laws of Islamic Republic of Iran.
Additional information
Communicated by Maria Antonietta Germanà.
Rights and permissions
About this article
Cite this article
Sadat Noori, S.A., Norouzi, M., Karimzadeh, G. et al. Effect of colchicine-induced polyploidy on morphological characteristics and essential oil composition of ajowan (Trachyspermum ammi L.). Plant Cell Tiss Organ Cult 130, 543–551 (2017). https://doi.org/10.1007/s11240-017-1245-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1245-0