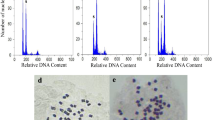
Abstract
This study investigates the capacity of the antimitotic agents colchicine, oryzalin and trifluralin for inducing polyploidisation of Ranunculus asiaticus ‘Alfa’ in vitro shoots. Flow cytometry was used to evaluate the optimal concentration of each antimitotic agent for polyploidisation. Trifluralin at a concentration of 2 μM resulted in the highest percentage of polyploidisation (27.5%), followed by a colchicine treatment of 200 μM, which induced 23.3% of polyploids. For oryzalin the highest percentage was achieved using a concentration of 1 μM. Different exposure periods were tested and turned out to be an important factor. The maximal exposure period tested (10 weeks) resulted in a significant increase in polyploidisation by oryzalin and trifluralin. In contrast, for colchicine (100 μM) exposure times of either 16 or 24 h did not significantly influence polyploidisation. Additionally the effect of the antimitotic agents on the viability was analysed. For colchicine no significant effect on the survival rate was observed, for trifluralin only a concentration of 10 μM affected viability whereas for oryzalin, concentration as well as exposure period were significant parameters. Flow cytometric data were confirmed by counting chromosomes in root tip cells.


Similar content being viewed by others
Abbreviations
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DMSO:
-
Dimethyl sulfoxide
References
Allum JF, Bringloe DH, Roberts AV (2007) Chromosome doubling in a Rosa rugosa Thunb. hybrid by exposure of in vitro nodes to oryzalin: the effects of node length, oryzalin concentration and exposure time. Plant Cell Rep 26:1977–1984. doi:10.1007/s00299-007-0411-y
Baack EJ (2004) Cytotype segregation on regional and microgeographic scales in snow buttercups (Ranunculus adoneus: Ranunculaceae). Am J Bot 91:1783–1788. doi:10.3732/ajb.91.11.1783
Baack EJ (2005) Ecological factors influencing tetraploid establishment in snow buttercup (Ranunculus adoneus, Ranunculaceae): minority cytotype exclusion and barriers to triploid formation. Am J Bot 92:1827–1835. doi:10.3732/ajb.92.11.1827
Bajer AS, Molebajer J (1986) Drugs with colchicine-like effects that specifically disassemble plant but not animal microtubules. Ann N Y Acad Sci 466:767–784. doi:10.1111/j.1749-6632.1986.tb38458.x
Beruto M, Debergh P (2004) Micropropagation of Ranunculus asiaticus: a review and perspectives. Plant Cell Tissue Organ Cult 77:221–230. doi:10.1023/B:TICU.0000018416.38569.7b
Chauvin JE, Label A, Kermarrec MP (2005) In vitro chromosome-doubling in tulip (Tulipa gesneriana L.). J Hortic Sci Biotechnol 80:693–698
Cohen D, Yao JL (1996) In vitro chromosome doubling of nine Zantedeschia cultivars. Plant Cell Tissue Organ Cult 47:43–49. doi:10.1007/BF02318964
Dart S, Kron P, Mable BK (2004) Characterizing polyploidy in Arabidopsis lyrata using chromosome counts and flow cytometry. Can J Bot 82:185–197. doi:10.1139/b03-134
de Nettancourt D (2001) Incompatibility and incongruity in wild and cultivated plants. Springer-Verlag, Berlin
Dhooghe E, Grunewald W, Leus L, Van Labeke MC (2009) In vitro polyploidisation of Helleborus species. Euphytica 165:89–95. doi:10.1007/s10681-008-9763-9
Dunn BL, Lindstrom JT (2007) Oryzalin-induced chromosome doubling in Buddleja to facilitate interspecific hybridization. HortScience 42:1326–1328
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220:1049–1051. doi:10.1126/science.220.4601.1049
Horovitz A (1985) Ranunculus. In: Halevy AH (ed) CRC handbook of flowering, vol IV. CRC Press, Florida, pp 155–161
Hugdahl JD, Morejohn LC (1993) Rapid and reversible high-affinity binding of the dinitroaniline herbicide oryzalin to tubulin from Zea mays L. Plant Physiol 102:725–740
Kermani MJ, Sarasan V, Roberts AV, Yokoya K, Wentworth J, Sieber VK (2003) Oryzalin-induced chromosome doubling in Rosa and its effect on plant morphology and pollen viability. Theor Appl Genet 107:1195–1200. doi:10.1007/s00122-003-1374-1
Khosravi P, Kermani MJ, Nematzadeh GA, Bihamta MR, Yokoya K (2008) Role of mitotic inhibitors and genotype on chromosome doubling of Rosa. Euphytica 160:267–275. doi:10.1007/s10681-007-9571-7
Langlet O (1932) Über Chromosomenverhältnisse und Systematik der Ranunculaceae. Svensk Botanisk Tidskrift 26:381–400
Lu C, Bridgen MP (1997) Chromosome doubling and fertility study of Alstroemeria aurea × A. caryophyllaea. Euphytica 94:75–81. doi:10.1023/A:1002911522748
Mable BK (2004) Polyploidy and self-incompatibility: is there an association? New Phytol 162:803–811. doi:10.1111/j.1469-8137.2004.01055.x
Mears JA (1980) Chemistry of polyploids. In: Lewis WH (ed) Polyploidy: biological relevance, vol 13. Plenum Press, New York, pp 77–102
Meynet J (1993) Ranunculus. In: De Hertogh A, Le Nard M (eds) The physiology of flower bulbs. Elsevier Science Publishers, The Netherlands, pp 603–610
Morejohn LC, Bureau TE, Tocchi LP, Fosket DE (1984) Tubulins from different higher-plant species are immunologically nonidentical and bind colchicine differentially. Proc Natl Acad Sci USA 81:1440–1444. doi:10.1073/pnas.81.5.1440
Morejohn LC, Bureau TE, Molebajer J, Bajer AS, Fosket DE (1987) Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 172:252–264. doi:10.1007/BF00394595
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Ohkawa K (1986) Growth and flowering of Ranunculus asiaticus. Acta Hortic 177:165–177
Otto F (1990) DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In: Darzynkiewicz Z, Crissman HA (eds) Methods in cell biology, vol 33. Academic Press Inc, New York, pp 105–110
Paden DW, Meyer MM, Rayburn AL (1990) Doubling chromosomes with colchicine treatment in vitro as determined by chloroplast number in epidermal guard cells. J Am Rhododendron Soc 44:162–167
Ro KE, Keener CS, McPheron BA (1997) Molecular phylogenetic study of the Ranunculaceae: utility of the nuclear 26S ribosomal DNA in inferring intrafamilial relationships. Mol Phylogenet Evol 8:117–127. doi:10.1006/mpev.1997.0413
Smith JB, Bennett MD (1975) DNA variation in the genus Ranunculus. Heredity 35:231–239. doi:10.1038/hdy.1975.87
Soltis PS, Soltis DE (2000) The role of genetic and genomic attributes in the success of polyploids. Proc Natl Acad Sci USA 97:7051–7057. doi:10.1073/pnas.97.13.7051
Stanton ML, Galen C, Shore J (1997) Population structure along steep environmental gradient: consequences of flowering time and habitat variation in snow buttercup, Ranunculus adoneus. Evolution Int J Org Evolution 51:79–94. doi:10.2307/2410962
Stanys V, Weckman A, Staniene G, Duchovskis P (2006) In vitro induction of polyploidy in Japanese quince (Chaenomeles japonica). Plant Cell Tissue Organ Cult 84:263–268. doi:10.1007/s11240-005-9029-3
Stebbins GL (1971) Chromosome evolution in higher plants. Edward Arnold, London
Takamura T, Miyajima I (1996) Colchicine induced tetraploids in yellow-flowered cyclamens and their characteristics. Sci Hortic (Amsterdam) 65:305–312. doi:10.1016/0304-4238(96)00896-5
Takamura T, Lim KB, Van Tuyl JM (2002) Effect of a new compound on the mitotic polyploidisation of Lilium longiflorum and oriental hybrid lilies. Acta Hortic 572:37–40
Tal M (1980) Physiology of polyploids. In: Lewis WH (ed) Polyploidy: biological relevance, vol 13. Plenum Press, New York, pp 61–76
Tamura M (1993) Ranunculaceae. In: Kubitski K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants: flowering plants—dicotyledons, vol II. Springer-Verlag, Berlin, pp 563–583
Väinölä A (2000) Polyploidization and early screening of Rhododendron hybrids. Euphytica 112:239–244. doi:10.1023/A:1003994800440
Wang ZF, Ren Y (2008) Ovule morphogenesis in Ranunculaceae and its systematic significance. Ann Bot (Lond) 101:447–462. doi:10.1093/aob/mcm298
Wang W, Li RQ, Chen ZD (2005) Systematic position of Asteropyrum (Ranunculaceae) inferred from chloroplast and nuclear sequences. Plant Syst Evol 255:41–54. doi:10.1007/s00606-005-0339-z
Yamaguchi M (1989) Basic studies on the flower color breeding of carnations (Dianthus caryophyllus L.). Bull Fac Hort Minamikyusyu Univ 19:1–79
Zhang Z, Dai H, Xiao M, Liu X (2008) In vitro induction of tetraploids in Phlox subulata L. Euphytica 159:59–65. doi:10.1007/s10681-007-9457-8
Zlesak DC, Thill CA, Anderson NO (2005) Trifluralin-mediated polyploidization of Rosa chinensis minima (Sims) Voss seedlings. Euphytica 141:281–290. doi:10.1007/s10681-005-7512-x
Acknowledgements
The authors thank H. Carlier for the flow cytometric measurements. Special thanks also go to T. Versluys for the technical supports and to IRF (Istituto Regionale per la Floricoltura, Sanremo—Italy) especially to M. Beruto for the supply of the high quality in vitro plant material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhooghe, E., Denis, S., Eeckhaut, T. et al. In vitro induction of tetraploids in ornamental Ranunculus . Euphytica 168, 33–40 (2009). https://doi.org/10.1007/s10681-008-9876-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-008-9876-1