Abstract
Cardiovascular disease (CVD) is the leading cause of death and disease burden worldwide. Nuclear myocardial perfusion imaging with either single-photon emission computed tomography or positron emission tomography has been used extensively to perform diagnosis, monitor therapies, and predict cardiovascular events. Several radiopharmaceutical tracers have recently been developed to evaluate CVD by targeting myocardial perfusion, metabolism, innervation, and inflammation. This article reviews old and newer used in nuclear cardiac imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the leading cause of death and disease burden around the world.1 Advances in single-photon emission computed tomography (SPECT) and positron emission tomography (PET), which allow for non-invasive imaging, are vastly improving the evaluation of myocardial perfusion and function.2,3 Nuclear cardiac imaging is useful to perform diagnosis and risk assessment and to monitor the impact of therapies through serial imaging. Several radiopharmaceutical tracers are used in nuclear cardiology imaging to target perfusion, metabolism, innervation, and inflammatory conditions. Nuclear imaging tests are suitable for almost all patients given the low possibilities of side effects from radiopharmaceutical tracers other than minimal radiation exposure. In this article, we will review SPECT and PET tracers used in assessing CVD.
Tracers Used for Cardiac Imaging (Table 1)
Inorganic Tracers
Inorganic compounds 13N-ammonia (13N-NH3) and 15O-water (15O-H2O) have been used for cardiac perfusion imaging.4 Both tracers are labeled with short-lived positron emitters (13N: 10 minute; 15O: 2 minute), which are therefore produced with an onsite cyclotron. 15O-H2O is freely diffused into cardiomyocytes. In contrast, the uptake mechanism of 13N-NH3 is unclear.5 Almost all ammonia molecules in the blood would be protonated to form NH4 + because of its pKa (9.3 at 25 °C). The ammonium cation would barely penetrate cell membranes to enter cardiomyocytes.
Radiometal Ions
In addition to these inorganic compounds, several radiometal ions have been used as cardiac imaging tracers, especially in myocardial perfusion imaging. Initially, the monovalent cation of potassium-43 (43K+), a γ-emitter, was used for imaging of myocardial perfusion.6 However, the main gamma energy of this radionuclide (0.37 and 0.67 MeV) is somewhat too high for SPECT imaging. Also 43K has a relatively long half-life (22 hours) and emits relatively high-energy β-particles [300 keV (mean)]. K+ is actively transported into the myocyte by the cell membrane via Na+/K+ pumps. Therefore, other monovalent cations that emit γ-rays suitable for SPECT imaging were sought. The ionic radius of the candidate should be comparable to that of K+ (138 pm) to be a substrate of a Na+/K+ pump. The monovalent cation of thallium-201 (201Tl+, ionic radius; 150 pm) fulfills these requirements and has been widely used for diagnosis of coronary artery disease (CAD). Although 201Tl emits γ-rays of 135 and 167 keV, abundantly emitted characteristic x-rays (69 to 80 keV) are used for imaging.
A positron emitter, rubidium-82 (82Rb), has an ionic radius (152 pm) comparable to that of K+ in its monovalent cationic form (82Rb+) and belongs to the same family as K (alkaline metals). The kinetics of Rb+ are similar to those of K+ 7, and therefore, 82Rb+ has been widely used as a perfusion imaging tracer with PET in the United States (USA).8 In addition, the use of a positron-emitting isotope of K, potassium-38, has been also reported.9 Trivalent cations of gallium-67 (67Ga3+), a γ-emitter, have been used to detect inflammatory lesions. Ga3+ binds to ferric iron (Fe3+)-binding proteins such as transferrin and lactoferrin which are accumulated in inflammatory lesions.10 Besides cationic radionuclides, a monovalent anion of fluorine-18 (18F−) that is used for bone imaging has been used for imaging calcification lesions with PET.11
Small Organic Tracers
Tracers of radiolabeled small organic compounds are used for imaging metabolism, synaptic function, and inflammation. In metabolic imaging, radiolabeled biomolecules and their derivatives are used. Biomolecules, acetic acid, and palmitic acid, substrates of oxygen metabolism and fatty acid metabolism, have been labeled with carbon-11 (11C-acetic acid and 11C-palmitic acid) and used for the assessment of respective myocardial metabolism.12 Iodophenylpentadecanoic acid labeled with iodine-123, (123I-IPPA) is also a substrate of fatty acid metabolism. For labeling with 123I, a phenyl group was incorporated into the structure of palmitic acid. In the development of tracers, derivatization of a biomolecule is often performed to obtain a compound that is metabolized by a certain metabolic step without undergoing further metabolism. 2-[18F]fluorodeoxyglucose (18F-FDG) is one such derivative of glucose. β-methyl-p-[123I]iodophenylpentadecanoic acid (123I-BMIPP) and [18F]fluoro-6-thia-heptadecanoic acid (18F-FTHA) introduce a methyl group and thioether in the alkyl chain, respectively, to terminate β-oxidation in the course of fatty acid metabolism.
In presynaptic cardiac imaging, a radiolabeled catecholamine and its derivative are also used as a tracer. 11C-labeled epinephrine and 18F-labeled fluorodopamine (18F-fluorodopamine) have been used to image the presynaptic sympathetic nervous system.13 In addition to biomolecules, xenobiotics including therapeutics are radiolabeled and used as tracers. Derivatives of guanethidine, metaraminol, and vesamicol are used for presynaptic imaging, and neuroreceptor ligands such as prazosin (α-blocker), carazolol (β-blocker) derivative, β-agonists CGP12177 and CGP12388, and quinuclidinyl benzilate (anticholinergic compound) derivatives are used for neuroreceptor imaging (Table 4).13
Radiolabeled receptor ligands for translocator protein 18 kDa (TSPO), peripheral-type benzodiazepine receptors, have also been used to image inflammation. TSPO is highly expressed in activated cells of the mononuclear phagocyte.14
Radiometal Complex Tracers
Some tracers used in nuclear cardiology are radiometal complexes containing copper-64 (64Cu), gallium-68 (68Ga), or technetium-99m (99mTc). They are classified into two groups. One contains those complexes that are used as tracers on their own. 99mTc is used to form a complex with six methoxyisobutylisonitrile (99mTc-sestamibi) and two 1,2-bis(di(2-ethoxyethyl)phosphino) ethane (99mTc-tetrofosmin), which have been used for myocardial perfusion imaging. Their bulky structures contribute to reducing protein binding in the blood through steric hindrance. These tracers are positively charged (monovalent) but lipophilic. Therefore, they can be diffused into myocytes.
The other group includes complexes used as tags for peptides and proteins. Somatostatin analogs and annexin V tagged with 64Cu, 68Ga, or 99mTc have been used for imaging symptomatic carotid atherosclerosis.15 64Cu or 68Ga-tagged somatostatin analogs bind to somatostatin receptor subtype-2, which is upregulated in macrophages. 99mTc-tagged annexin A5 binds to phosphatidylserine, which is externalized in apoptotic cells.
White blood cells enclosing radiometals, which are used for imaging infectious lesions, are prepared using lipophilic radiometal complexes. Indium-111 (111In) complexed with 8-hydroxyquinolines (111In-oxine) and 99mTc complexed with exametazime (99mTc-HMPAO) are diffused into the leucocyte. The subsequent dissociation of ligands results in enclosure of these radiometals in the cell.
Radiotracers Categorized by Use
Perfusion Imaging
Myocardial blood flow (MBF) is supplied by coronary arteries to preserve adequate myocardial oxygen supply. At rest, coronary artery stenosis must exceed 85% to 90% of luminal diameter before there is a significant decrease of MBF. In contrast, maximal coronary flow has been shown to be reduced with stenosis of 45% to 50% under stress condition.16 Myocardial perfusion images during stress and rest are compared to detect the stress-induced ischemic change or myocardial injury (Figure 1).17,18 Several perfusion tracers are used to assess coronary artery disease (CAD) (Table 2, Figure 2).17,19,20,21,22
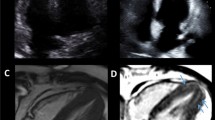
Myocardial perfusion images Perfusion images of short-axis image at stress (A) and rest (B), vertical long-axis image at stress (C) and rest (D) using 99mTc-product, and fused image of stress perfusion and CT coronary angiography (CTCA; E) are displayed. Severe perfusion reduction is detected in the inferior wall at stress (white arrows). Fill-in is seen at rest indicating stress-induced ischemia in the right coronary artery (RCA). CTCA revealed significant stenosis in the RCA (orange arrows)
Schematic representation of tracers for assessing myocardial perfusion 201Tl and 82Rb are potassium analogs and are transported into the myocyte by cell membrane Na+/K+ pumps. Injected uptake of 99mTc-sestamibi, 99mTc-tetrofosmin, and 18F-flurpiridaz in the myocardium is related to the presence of intact mitochondria. The uptake mechanism of 13N-NH3 is unclear. After being taken into the myocyte, 13N-NH3 underwent metabolic trapping with the conversion of NH3 to glutamine, glutamic acid, and carbamoyl phosphate. 15O-H2O is metabolically inert and freely diffusible tracer
SPECT tracers for perfusion imaging
Thallium-201 (201Tl), technetium-99m (99mTc)-sestamibi, and 99mTc-tetrofosmin are available for SPECT myocardial perfusion imaging (MPI).
99mTc-labeled myocardial perfusion tracers. Thallium-201
201Tl, introduced in the 1970 s, was the first SPECT MPI tracer available in a clinical setting.23 In 1975, Wackers et al. reported on the imaging of acute myocardial infarction with 201Tl.24 201Tl is produced in a cyclotron and has a relatively long half-life (73 hours), and therefore requires lower injection doses to minimize radiation exposure. 201Tl is a potassium analog and is transported into the myocyte via cell membrane Na+/K+ pumps during the first transit in proportion to regional MBF.
201Tl emits low-energy photons (71 to 80 keV), therefore requiring longer imaging acquisition times and resulting in limited image quality due to absorption and photon scattering especially in obese patients. Biodistribution of 201Tl is generally proportional to organ blood flow. Injected 201Tl is rapidly cleared from the blood with maximal concentration by normal myocardium (5% to 8% remains in the blood at 5 minutes). The whole-body retention curve can be represented by a biexponential curve. 201Tl is excreted slowly in both feces and urine. Approximately 4% to 8% of the administered dose is excreted in the urine in the first 24 hours.25,26 Lung uptake of 201Tl is generally low. An increased lung uptake is known to be associated with greater segmental myocardial perfusion abnormality, increased severity and extent of CAD, and subsequent adverse cardiac events.27
Whole-body radiation exposure after an injection (2 to 4 mCi) is up to ~ 25 mSv.28,29
201Tl has a higher extraction coefficient than do 99mTc-labeled perfusion tracers (Figure 3). The higher extraction fraction may be an advantage for MBF quantification.30
Extraction fraction of each perfusion tracer The extraction fraction of 15O-H2O is nearly 100% due to its exclusive property of being metabolically inert and freely diffusible. The extraction fraction of 82Rb is lower than that of the other PET tracers. 201Tl has a higher extraction fraction compared to that associated with 99mTc-MIBI
Stress images are acquired 5 to 15 minutes after tracer injection in order to avoid the “upward creep” phenomenon due to rapid respiration if the stress is produced through exercise. Redistribution images are acquired 2 to 4 hours after initial injection. Differential washout rates of normal regions (with faster washout) vs regions with ischemic segments (slower washout) contribute to the redistribution or normalization of the abnormal regions in delayed images.
99mTc-labeled myocardial perfusion tracers
99mTc is a generator-produced agent eluted from molybdenum-99 (99Mo). Despite its initial Food and Drug Administration (FDA) approval, 99mTc-teboroxime is far less commonly used due to the excessive initial uptake in the myocardium and rapid washout.31,32 99mTc-sestamibi and 99mTc-tetrofosmin have had widespread clinical use. The first use of 99mTc-tetrofosmin for humans was reported in 1993 as part of a phase 1 clinical trial.33 Injected 99mTc-labeled perfusion tracer distributes in the myocardium according to regional myocardial perfusion. Its uptake by myocardium is related to the presence of intact mitochondria.34
Because its half-life is 6 hours, the administered dose is relatively larger and the radiation exposure is lower respectively than those associated with 201Tl.29 The peak energy level of γ-rays from 99mTc is about 140 keV, which is suitable for γ-camera imaging and electrocardiographically (ECG) gated myocardial perfusion SPECT.
99mTc-sestamibi is rapidly cleared from blood after intravenous administration. Lung uptake is generally low. However, marked accumulation is present in liver and spleen at resting condition during the first 60 minutes after injection. After an injection with exercise stress, substantially less uptake is observed in the liver and spleen with excellent visualization of heart.35 99mTc-tetrofosmin is rapidly cleared from the blood (< 5% remains in blood by 10 minutes) after intravenous administration. Uptake in myocardium is approximately 1.2% with minimal redistribution, and approximately 1% at 2 hours. Clearance from liver is quick (< 4.5% remains by 60 minutes) and lung uptake is also rapidly reduced.33,36,37 Myocardial uptake of 99mTc-tetrofosmin is higher from 5 to 60 minutes than is that for 99mTc-sestamibi. The biological half-life of 99mTc-tetrofosmin in normal myocardium and liver is significantly shorter than that of 99mTc-sestamibi. Heart-to-lung ratios for 99mTc-tetrofosmin and 99mTc-sestamibi are similar, whereas heart-to-liver ratios for 99mTc-tetrofosmin are significantly higher from 30 to 60 minutes post injection compared to those for 99mTc-sestamibi.37,38
Total whole-body radiation after a typical injection dose (10 to 25 mCi) is ~ 10.6 mSv for 99mTc-tetrofosmin and 12.0 mSv for 99mTc-sestamibi.28
Separate stress and rest injections are required for the detection of stress-induced ischemia due to its slow clearance from myocytes. Both 99mTc-sestamibi and 99mTc-tetrofosmin have lower extraction coefficients than does 201Tl (Figure 3).39 Recent SPECT systems allow the quantification of MBF from dynamic tracer imaging due to the improved sensitivity and temporal resolution.40,41
PET tracers for myocardial perfusion imaging
Several PET tracers can be used to assess myocardial perfusion.18 These include 82Rb, 13N-NH3, and 15O-H2O (Figure 4).19 Both 13N-NH3 and 82Rb are commonly used for both qualitative and quantitative measurements.34,42,43,44 Visual assessment of PET myocardial perfusion imaging provides high diagnostic accuracy in the detection of CAD.17 Dynamic imaging analysis permits quantitative assessment of MBF and coronary flow reserve (CFR), which is defined as the ratio of MBF at peak hyperemia to MBF at rest. CFR measurements provide additional value in the detection of multi-vessel disease and risk stratification of CAD patients.45,46,47,48,49 15O-H2O is an ideal myocardial flow tracer to quantify MBF with a linear relation between first-pass extraction and perfusion, but the perfusion images are not of high quality as they are with the other 2 PET tracers (Figure 3).19,30,50,51
82Rb is the most widely used tracer because it is a strontium-82 (82Sr)/82Rb generator-produced tracer that does not require a cyclotron for its production.52,53 Love et al. initially developed rubidium-86 for myocardial perfusion imaging with a dog.7 Following non-human studies, Selwyn et al. applied 82Rb to a human for the first time in 1982.54. The short physical half-life of 82Rb (76 seconds) enables sequential rest/stress scanning. 82Rb is a potassium analog, and therefore injected 82Rb is actively transported into myocytes through the Na+/K+ adenosine triphosphate (ATP) transport system. This uptake of 82Rb is dependent on MBF and its first-pass retention fraction is approximately 65% at rest. The relatively low lesion contrast with low spatial resolution due to the lower extraction fraction and high positron range is a slight disadvantage of 82Rb.39 In 2000, 13N-NH3 PET was approved by the United States Food and Drug Administration (FDA) to evaluate myocardial perfusion in patients with known or suspected CAD.19 13N-NH3 was also approved by the Japanese Ministry of Health and Welfare in March 2012 (Table 2).55
The uptake mechanism of 13N-NH3 is unclear. After being taken into the myocyte, 13N-NH3 underwent metabolic trapping with the conversion of NH3 to glutamine, glutamic acid, and carbamoyl phosphate.56 13N-NH3 PET is suitable for imaging and measuring of MBF due to its high first-pass extraction fraction and retention in the myocardium with rapid clearance from the blood pool, which also give it high diagnostic accuracy.57 The requirement for a cyclotron limits the clinical use of 13N-NH3. Its relatively longer half-life (9.96 minutes) necessitates a longer interval between rest and stress scans, resulting in low throughput in a clinical setting. These are the main disadvantages of 13N-NH3.39 The FDA has approved 82Rb and 13N-NH3 for clinical use (Table 2). The Japanese Ministry of Health, Labour, and Welfare has approved 13N-NH3 for detecting CAD in cases of CAD unable to be diagnosed with using SPECT MPI.55
15O-H2O is unique in being metabolically inert and freely diffusible, which are considered ideals for measuring MBF due to the linear relationship between first-pass extraction and perfusion.58 The shorter half-life (2.04 minutes) enables consecutive rest/stress protocols, similar to the case with 82Rb.59,60 However, 15O-H2O requires an on-site cyclotron for tracer production and also is suboptimal for visual assessment due to the low signal-to-noise ratios. These conditions lead to its use being limited in clinical settings. 15O-H2O has gained wide popularity in research settings due to its excellent kinetic properties.19,61,62,63 A recent study by Danad et al. examined stress MBF and CFR in 330 patients with CAD,64 possibly indicating that 15O-H2O could move from research to clinical use.
Fluorine-18 (18F)-flurpiridaz, an analog of the insecticide pyridaben, is a novel MPI tracer that can bind to the mitochondrial complex-1 inhibitor.51,65 The positron range of 18F is 1.03 mm, shorter than that of other PET perfusion tracers (Table 2). Injected 18F-flurpiridaz shows very high first-pass extraction and high affinity in myocardial tissue with slow washout from cardiomyocytes (Figure 3). Therefore, accurate quantification of MBF and CFR measurements with high image quality and excellent diagnostic accuracy are expected.66,67,68 Because of the longer half-life of 18F (109.8 minutes), delivery of unit doses from regional cyclotrons may be possible, similar to the case with fluorine-18-labeled fluorodeoxyglucose (18F-FDG). In the meantime, repeated measurements of stress and rest studies would likely be difficult due to the longer half-life, and therefore a separate day protocol or some correction for the residual activity of the first acquisition might be needed. Phase 2 clinical trials showed promise,67 and phase 3 clinical trials demonstrated the diagnostic usefulness for specific subpopulations such as women and obese patients.
Metabolic Imaging
The heart derives its energy from a variety of sources such as free fatty acids (FFA), glucose, lactate, and ketone bodies (Figure 5).69 Glucose metabolism dominates after feeding, and fatty-acid metabolism dominates under long-fasting conditions.69 Carbohydrates taken into cardiomyocytes are metabolized into pyruvic acid using various enzymatic actions. If oxygen supply is sufficient, ATPs are produced from glucose via the glycolysis system in the tricarboxylic acid (TCA) cycle and electron transfer system.70 In the ischemic state, acid metabolism is impaired due to insufficient oxygen supply to the myocardium.71 Alternatively ATP is produced from lactic acid because anaerobic glycolysis with less oxygen consumption becomes predominant. However, anaerobic glycolysis produces less ATP than does aerobic glycolysis. If severe myocardial ischemia continues, myocardial cells become necrotic as ATP production diminishes.72 Several SPECT and PET tracers have been used or tried clinically to assess myocardial metabolism (Table 3, Figure 6).
Schematic representation of cardiac energy metabolism Substrates are transported across the extracellular membrane into the cytosol through GLUT for glucose and FAT for fatty acid. Metabolized intermediates such as pyruvate and acyl-CoA are transported across the inner mitochondrial membrane for oxidation. Then inside the mitochondrion, substrates are oxidized or carboxylated and fed into the TCA cycle and ETC to produce ATP. GLUT, glucose transporter; FAT, fatty acid transporter; G-6-P, glucose-6-phosphate; ATP, adenosine triphosphate; TCA, tricarboxylic acid; ETC, electron transport chain; CA I, carnitine acyltransferase I; CA II, carnitine acyltransferase II
Tracers for assessing cardiac energy metabolism 18F-FDG is a glucose analog in which the oxygen in position C-2 is replaced with 18F. 18F-FDG is actively transported into the cell mediated by GLUT in the same way as glucose. Once inside the cell, glucose and 18F-FDG are phosphorylated by hexokinase. Phosphorylated glucose (G-6-P) continues along the glycolytic pathway for energy production. However, 18F-FDG-6-phosphate cannot enter glycolysis and is trapped intracellularly in a condition known as “metabolic trapping.” GLUT, glucose transporter; G-6-P, glucose-6-phosphate; FDG, 18F-fluorodeoxyglucose; FDG-6-P, 18F-FDG-6-phosphate
SPECT tracers for metabolic imaging
For fatty acid metabolism evaluation, SPECT examination using iodine-123-labeled beta-methyl-p-iodophenylpentadecanoic acid (123I-BMIPP) has been clinically used in Japan.44,73,74 However, 123I-BMIPP was initially developed in the United States and the first human use was in 1986 by Knapp et al.75 After the initial development in the US, the Japanese community took over development of 123I-BMIPP. The first human use in Japan was reported in 1991 in a Japanese article.76 Following this Japanese article, Kurata et al. reported Japanese 123I-BMIPP data in an international journal in 1992.77 123I-BMIPP is an iodinated fatty-acid analog used to assess myocardial fatty acid metabolism.78,79 This tracer, however, is not approved for clinical use in the US despite its successful for clinical use even successive early experience use in that country.80 Iodine-123-labeled iodophenylpentadecanoic acid (123I-IPPA) is a radiolabeled free fatty acid (FFA) analog which is in phase 3 trials in United States but which has not yet been approved.81
Following intravenous injection, 123I-BMIPP and 123I-IPPA are rapidly distributed to various organs, such as liver and heart, and cleared rapidly from the blood.81,82,83,84 Initial uptake of the administered dose of 123I-BMIPP is assumed to be about 6% by the heart and 14% by the liver. The residual 123I-BMIPP is distributed uniformly in other organs and tissues.76,85,86 After initial uptake, only a portion of the 123I-BMIPP and 123I-IPPA is metabolized immediately to water-soluble low-molecular-weight products. Most of the 123I-IPPA undergoes metabolism similar to that of long-chain fatty acids, through rapid mitochondrial beta-oxidation.87,88 The initial and late clearance of 123I-IPPA are thought to reflect β-oxidation and clearance of tracer incorporated into triglyceride pools, respectively.88 123I-IPPA images show minimal background activity and good image quality. The metabolism of 123I-BMIPP is slower than that of 123I-IPPA because 123I-BMIPP is a modified-branched fatty acid analog with a methyl group on the beta-carbon. Both of the end products are excreted in a conjugated form in the urine.76,89,90
123I-BMIPP scintigraphy when combined with perfusion imaging may show preserved perfusion, but fatty acid metabolism is impaired as myocardium shifts from metabolizing fatty acids to metabolizing predominantly glucose following ischemic episodes. Therefore, the region of perfusion-metabolic mismatch (123I-BMIPP defect larger than perfusion defect) indicates the presence of ischemic myocardium (Figure 7). 80,91,92,93 123I-BMIPP has been approved in Japan only for clinical use.44
Ischemic memory imaging Perfusion image of 99mTc product shows slightly reduced perfusion (A, C), whereas moderately reduced 123I-BMIPP uptake is seen in the anterior to septal wall (B, D), which indicates perfusion-metabolic mismatch. Coronary angiogram shows no significant stenosis (E); however, vasospastic angina in the left anterior descending artery due to the spasm is proved through intracoronary injection of acetylcholine (F)
PET tracers for metabolic imaging
18F-FDG is the most frequently used tracer around the world and is employed mainly for the assessment of malignant tumors. For the purposes of nuclear cardiology imaging, 18F-FDG PET was first used to define and identify viable myocardium in CAD in the 1980 s.94 Since 18F-FDG is an analog of glucose, once taken up into the cardiomyocytes via the glucose transporter (GLUT), it is phosphorylated to 18F-FDG-6-phosphate by hexokinase as well as glucose.95 18F-FDG-6-phosphate accumulates intracellularly without being metabolized during glycolysis, a condition referred to as “metabolic trapping” (Figure 6). Therefore, myocardial viability can be evaluated by assessing the accumulation of 18F-FDG in myocardium. To determine myocardial viability, oral glucose loading or an insulin-glucose clamp is applied to enhance 18F-FDG uptake in viable myocardium.96,97 In ischemic myocardium, 18F-FDG accumulation in the myocardium is maintained under a fasting condition due to the dominant anaerobic glucose metabolism. On the other hand, in the infarcted scar tissue, 18F-FDG accumulation is absent due to non-availability of glucose metabolism. In a clinical setting, 18F-FDG PET viability assessment is performed using the myocardial perfusion image obtained by SPECT or PET.94,98 A region with preserved 18F-FDG accumulation but reduced myocardial perfusion indicates viable myocardium. In such a case, functional recovery after coronary revascularization is likely especially with extensive mismatch pattern.
11C-palmitate and fluorine-18-labeled fluoro-6-thia-heptadecanoic acid (18F-FTHA) have been used to evaluate fatty acid metabolism.99,100,101 Similar to the case with to 18F-FDG PET, a shift in myocardial metabolism from fatty acid to glucose can be estimated using these fatty acid analogs.102
Myocardial oxygen metabolism can be non-invasively evaluated by 11C-acetate PET.103,104 11C-acetate taken into myocardium is converted into acetyl-CoA, consecutively metabolized and excreted into 11C-CO2 via the TCA cycle. The 11C-acetate clearance rate is used to assess myocardial oxygen consumption since TCA cycle activity is directly linked with myocardial oxygen consumption which is independent of the concentration of energy substrates for the myocardium.105,106 11C-acetate PET allows for non-invasive observation of regional myocardial oxygen metabolism in the presence of ischemia,107,108 cardiomyopathy,109,110 and heart failure (HF) in a state of deprived energy.111,112 Myocardial oxidative metabolism in the RV can also be estimated using 11C-acetate PET.113,114,115,116 11C-acetate PET permits the evaluation of both blood flow and oxygen metabolism with one examination using some model analysis due to the relatively high extraction fraction.62
Sympathetic Imaging
The heart has extensive innervation, both sympathetic and parasympathetic. The sympathetic nervous system uses norepinephrine (NE), and the parasympathetic nervous system uses acetylcholine (Ach) as the main neurotransmitters. NE is synthesized from the amino acid tyrosine in presynaptic neurons (Figure 8). NE is transported into the presynaptic neuronal terminal vesicles by the vesicular monoamine transporter (VMAT). Exocytosis is led by the activation of voltage-dependent calcium channels and vesicles at the presynaptic neuron. Some of the NE released into the synaptic cleft binds to the adreno-receptors for downstream effects, while much of the NE undergoes reuptake into presynaptic neurons via the terminal transporter (uptake-1).117,118,119
Schema of myocardial adrenergic neuronal terminals Figure A shows the schematic representation of myocardial adrenergic neuronal terminals and Figure B shows the chemical structure of each tracer. MIBG is actively taken up into sympathetic nerves through the uptake-1 mechanism and then stored in the synaptic vesicle in a manner similar to that for norepinephrine (NE). Nerve stimulation releases MIBG and NE into the synaptic cleft through exocytosis. MIBG does not bind to the postsynaptic receptor and is not metabolized by monoamine oxidase (MAO) or catechol-O-methyltransferase (COMT). Most of the released MIBG undergoes reuptake through the uptake-1 mechanism, and the remaining MIBG goes into the blood (spillover). 123 I-MIBG, m-[123I]iodobenzylguanidine; 11 C-HED, 11C-hydroxyephedrine; DAG, diacylglycerol; AR, adrenergic receptor;Gq, phospholipase C-coupled Gq-protein; Gs, phospholipase C-coupled Gs-protein; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; IP 2 , inositol bisphosphate; PIP 2 , phosphatidylinositol biphosphate
The sympathetic nerve is vulnerable to ischemia, and sympathetic nervous function may decline even if myocardial blood flow at rest is maintained.120 In HF, continued stimulation of the β1 receptor due to increased norepinephrine levels results in a decrease of receptor density (down regulation), with corresponding poor reactivity to the stimulation. Moreover, in a persistent state of sympathetic hyperactivity, the ability to retain norepinephrine is also decreased at the nerve terminal end.121 Abnormal neuro-hormonal function is reported in various heart diseases, and worsening of neuronal function is associated with cardiac events and sudden cardiac death.122,123,124
SPECT tracers for sympathetic imaging
Iodine-123-labeled metaiodobenzylguanidine (123I-MIBG) is widely used as a SPECT tracer to evaluate the presynaptic sympathetic innervation of the heart.125,126,127 The first use of 123I-MIBG in humans was in 1981 by a University of Michigan group.128 It is an analog of catecholamine, which is taken up via the uptake-1 mechanism and stored in synaptic vesicles as is NE. Tracers are released into the synaptic cleft from the synaptic vesicle via the exocytosis pathway, but do not lead to any physiological activity without binding to the catecholamine receptor. Since it is not metabolized by monoamine oxidase (MAO) or catechol-O-methyltransferase (COMT), most of the released tracer is reabsorbed at the synapse terminal and again stored in synaptic vesicles. Therefore, information reflecting the process of 123I-MIBG uptake into the synapse terminal, storage in the vesicles, secretion, reabsorption, and release into the blood is obtained from sympathetic imaging.129,130 An early anterior planar image at 15 minutes after injection and a late anterior planar image starting at 3 to 4 hours after injection are acquired to calculate the heart-to-mediastinum ratio (HMR) and the washout ratio (Figure 9). These parameters are considered to be standards. The high liver uptake and relatively high energy of the tracers make the image quality suboptimal. It is difficult to evaluate SPECT images especially in severe HF, which usually has limited myocardial 123I-MIBG radioactivity. Therefore, planar data acquisition is standard for 123I-MIBG imaging.131 Although these images present an easily obtained index, inter-institutional differences of the HMR due to differences in camera-collimator systems being used have hampered multicenter comparisons. Recently, standardization among different collimator types has been achieved using the calibration phantom and could easily be extrapolated to the images of other institutions.132,133 Late HMR provides the relative distribution of cardiac sympathetic nerve terminals, which is related to neuronal function from uptake to release. Washout ratio represents the information of the sympathetic drive. Several studies have presented that patients with chronic HF and a low late HMR and/or an increased washout rate are at increased risk for cardiac death.
Representative case of 123I-MIBG scintigraphy and 11C-hydroxyephedrine PET A male in his 40s suffered from dilated cardiomyopathy, with a left ventricular ejection fraction of approximately 30%. An early anterior planar image at 15 min after injection (A) and a late anterior planar image starting at 4 hours after injection (B) are acquired to calculate the heart-to-mediastinum ratio (HMR) and the washout ratio. Calculated early HMR, delayed HMR, and washout ratio were 1.7, 1.4, and 40.3%, respectively. Whole retention index from 11C-hydroxyephedrine PET was calculated as 0.044. Distribution of sympathetic nerve system was lower especially in the lateral wall
PET tracers for sympathetic imaging
As a PET tracer, carbon-11-labeled hydroxyephedrine (11C-HED) is used mainly to assess presynaptic cardiac sympathetic nerve distribution.134 11C-HED is still the most widely used PET tracer for sympathetic nervous function imaging in mainly research settings.135 Extracardiac uptake is mainly by the liver with very limited lung uptake. In ischemic heart disease, a mismatch region of myocardial blood flow and sympathetic dysfunction is reported as a decision criterion for prediction of fatal arrhythmia and indication for cardioverter-defibrillator implantation (ICD).136,137 The distribution abnormality of cardiac sympathetic denervation has been demonstrated in previous 11C-HED studies, including those involving patients with HF,138,139 cardiac arrhythmias,140,141 myocardial infarction,142,143 cardiac diabetic neuropathy,144,145 and HF with preserved ejection fraction (HFpEF).146
N-[3-bromo-4-(3-18F-fluoro-propoxy)-benzyl]-guanidine (LMI 1195) is a novel 18F-labeled ligand to image the norepinephrine transporter.147 18F-fluorometaraminol,148 11C-phenylephrine,149 18F-fluorodopamine,150 and 11C-epinephrine151 are the other radiotracers for evaluating presynaptic neuronal function. Several tracers such as 18F-fluorocarazolol,152 4-[3-[(1,1-dimethyl)amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-11C-one (11C-CGP12177),153 and (S)-4-(3-(2’-11C-isopropylamino)-2-hydroxypropoxy)-2H-benzimidazol-2-one (11C-CGP12388)154 have been reported for assessing postsynaptic sympathetic neuronal functions through measurement of myocardial β-adrenergic receptor (β-AR) density, which directly regulates LV systolic function.155 There are several reports regarding tracers for imaging the parasympathetic nervous system,156,157 but the clinical role of these has not yet been established (Table 4).
Imaging of Inflammation and Atherosclerosis
Nuclear medicine imaging can be used to view several in vivo pathological processes in inflammation and atherosclerosis. Several novel tracers may have uses for tracking inflammation, hypoxia, or active calcification (Table 5).
SPECT tracers for imaging of inflammation and atherosclerosis
Gallium-67 (67Ga) scintigraphy has been used to detect inflammatory lesions including infection and sarcoidosis.158,159 Several factors influence 67Ga accumulation in inflammatory lesions. These factors include increased delivery and accumulation of transferrin-bound 67Ga due to increased blood flow and vascular membrane permeability. The tendency of 67Ga to bind to lactoferrin and leukocytes also leads to highly concentrated uptake of 67Ga.160 Imaging is performed at 48 to 72 hours after tracer injection. In clinical settings, physicians ideally look to have results immediately following a diagnostic test, and therefore a late imaging protocol is one of the major limitations of 67Ga. 67Ga scanning is useful to differentiate acute myocarditis from acute myocardial infarction.161 67Ga scintigraphy has been a major analytical tool in the diagnosis of cardiac sarcoidosis.162 There is no significant distribution in normal myocardium.163 This is an advantage of 67Ga when applied to cardiac sarcoidosis. However, generally speaking, 67Ga has a limited role in the evaluation and management of sarcoidosis.163
Inflammatory cells such as granulocytes, lymphocytes, and macrophages are migrated into inflammatory lesions, resulting in the activation of a biological defense mechanism. SPECT imaging with indium-111 (111In)-radiolabeled autologous white blood cells (WBC) has proven to be valuable in the detection of endocarditis. 111In-WBC is highly specific for infectious lesions because granulocytes are recruited to the site of inflammatory foci but have limited sensitivity due to a weak signal.164,165, – 166
Apoptosis imaging
Tissue apoptosis is considered to be one of the earlier stages of vascular plaque rupture,167 and therefore detecting apoptotic lesions may precipitate effective treatments to prevent cardiovascular events. Apoptotic cells externalize negatively charged phosphatidylserine (PS).15 Human protein annexin A5 binds to PS. 99mTc-labeled annexin A5 has been shown to have higher uptake in the carotid arteries of vulnerable stroke patients.168 99mTc-tagged annexin A5 specifically accumulates in vascular atherosclerotic lesions, which is a great advantage. In contrast, the signal intensity of 99mTc-labeled annexin A5 is quite a bit lower than that of 18F-FDG.169 99mTc-labeled annexin A5 drew much interest a decade ago but has not had wide clinical application, perhaps due to the lower signal intensity and tracer availability.
PET tracers for imaging of inflammation and atherosclerosis
Glucose is consumed in large quantities in the inflammatory process, and therefore active inflammatory lesions show high 18F-FDG accumulations. It is necessary to suppress physiological myocardial glucose metabolism in order to accurately evaluate myocardial inflammatory lesions using 18F-FDG PET. Among effective approaches to reducing physiological myocardial glucose metabolism, long-period fasting is the most common. Long-period fasting combined with a low-carbohydrate diet and/or high-fat diet and unfractionated heparin intravenous injection are also used. These approaches lead to myocardial free fatty acid metabolism dominance.170 18F-FDG PET is more useful than are perfusion SPECT and delayed enhanced cardiac magnetic resonance (CMR) to not only diagnose but also monitor treatment effects in inflammatory heart disease such as cardiac sarcoidosis (Figure 10).171 Myocardial ischemia (reflecting a shift to glucose metabolism), other cardiomyopathy (reflecting microcirculatory ischemia and inflammation), and cardiac tumors also show 18F-FDG accumulation.172,173,174,175
Representative case of cardiac sarcoidosis Maximum intensity projection (MIP) image of 18F-FDG PET (A), PET/CT coronal image (B), short-axis image of 18F-FDG PET (C), late gadolinium enhancement (LGE)-MRI (D), and fused image of 18F-FDG PET and LGE-MRI (E) at pre-therapy, MIP image of 18F-FDG PET (F) and PET/CT coronal image (G) at post-therapy (steroid 30 mg/1 month) are displayed. 18F-FDG PET detected focal cardiac uptake and multiple lymph node disease in the supraclavicular, mediastinum, hilum, abdominal, and pelvis region at pre-therapy. 18F-FDG uptake is seen at the same site of LGE-MRI abnormal intensity. At post-therapy, 18F-FDG uptakes were markedly lower. 18F-FDG is useful not only for diagnosis but also to confirm the effectiveness of treatments
Incomplete suppression of physiological myocardial 18F-FDG uptake may cause false positives. Therefore, new tracers have been developed to detect inflammatory heart disease and atherosclerotic lesions. These radiopharmaceuticals target tissue apoptosis, tissue calcification, activated macrophages, and tissue hypoxia.
68Ga complexed with [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-1-Nal3-octreotide (68Ga-DOTANOC),176 fluorine-18 fluorothymidine (18F-FLT),177 68Ga complexed with [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-Phe1-Tyr3-octreotide (68Ga-DOTATOC),178 and fluorine-18 fluoromisonidazole (18F-FMISO)179 have been reported to improve specificity with regard to diagnosis of cardiac sarcoidosis.
68Ga-tagged tracers can be prepared using a generator system and have been applied for clinical oncology imaging. Activated macrophages show upregulated G-protein-coupled somatostatin receptor subtype-2 receptors. In an observational study involving oncology patients, uptake of 68Ga complexed with a somatostatin analog, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-d-Phe1-Tyr3-octreotate (68Ga-DOTATATE), in large arteries increased in relation to age.180 A recent study prospectively revealed 68Ga-DOTATATE uptakes in carotid and coronary arteries in patients with unstable CVD.181 Unlike 18F-FDG, 68Ga-DOTATATE does not have physiological myocardial uptake and therefore could potentially play a clinical role in detecting vulnerable plaque.
An alternative to 68Ga, Copper-64 (64Cu) complexed with the somatostatin analog (64Cu-DOTATATE) has been used. 64Cu has a shorter positron range and longer half-life. Thus, 64Cu DOTATATE may have improved spatial resolution over that of 68Ga-DOTATATE. 64Cu DOTATATE also showed positive uptake in carotid atherosclerotic lesions.182 64Cu-labeled DOTATATE uptake was positively linked to the expression of membrane receptor CD163, indicating that 64Cu-labeled DOTATATE uptake was associated with hemorrhagic macrophage migration.
Translocator protein
Translocator protein 18kDa (TSPO), a peripheral-type benzodiazepine receptor, locates in peripheral tissue and the brain.183 TSPO is a protein highly expressed in activated cells of the mononuclear phagocyte lineage.184 Carbon-11 labeled [1-(2-chlorophenyl)-N-methyl-N-1(1-methylpropyl)-3-isoquinolinecarboxamide] (11C-PK11195) is a first specific ligand for TPSO, and its uptake has been revealed in symptomatic carotid atherosclerotic lesions.185 However, 11C-PK11195 has some limitations such as high non-specific binding and high lipophilicity. To overcome these limitations, we developed an 18F-labeled TPSO ligand, N-benzyl-N-methyl-2-[7,8-dihydro-7-(2-[18F]fluoroethyl)-8-oxo-2-phenyl-9H-purin-9-yl] acetamide (18F-FEDAC). 18F-FEDAC showed high in vitro binding affinity for TSPO with high selectivity.186 18F-FEDAC was initially developed as a tracer for imaging brain inflammation, and subsequent study revealed that this tracer could potentially be used for imaging inflammation in peripheral organs.187 Indeed, 18F-FEDAC can be used to visualize lesions in rat liver.14,188 In a rat lung injury model, 18F-FEDAC uptake increased with the progression of lung inflammation (Figure 11).189 The uptake of 18F-FEDAC in the heart of a rat was approximately twice as high as that in the lung.187 With 18F-FEDAC the uptake ratio for heart to lung is higher than that with 13N-NH3. The same is true for the heart-to-liver uptake ratio measured with each of these tracers respectively. However, uptake ratios are similar for heart to lung and heart to liver measured using 18F-FEDAC and 18F-FDG (Figure 12). In this regard, 18F-FEDAC may have potential for detecting cardiac inflammatory lesions or vascular inflammatory lesions.
18F-FEDAC imaging a comparison between 18F-FEDAC imaging and double staining of translocator protein (TSPO) for neutrophils. Arrows indicate examples of cells doubly positive for TSPO (green) and chloroacetate esterase (red spots) staining. Control group showed no positive 18F-FEDAC uptake in either lung (A). No neutrophils were seen in the control. Lung injury model using lipopolysaccharide showed positive 18F-FEDAC uptake in both lungs (B)
Histology showed leukocyte infiltration in the lung injury model. Scale bar: 20 µm. 18F-FEDAC showed higher uptake ratios of heart/lung and heart/liver compared to those with 13N-NH3 and similar to that with 18F-FDG. 18 F-FDG, 18F-fluorodeoxyglucose; 18 F-FEDAC, N-benzyl-N-methyl-2-[7,8-dihydro-7-(2-[18F]-fluoroethyl)-8-oxo-2-phenyl-9H-purin-9-yl] acetamide
Fluorine-18 anion (18F−), which is administered as the sodium salt 18F-NaF, has been used as a bone-imaging agent to detect metastatic bone lesions. Since 18F− accumulates in calcification lesions, it has also been used to evaluate the severity or disease activity of aortic stenosis.190 During the progression of atherosclerosis, calcification may appear in intermediate lesions. In contrast, with inflammation, active calcification may appear during the later stages of disease progression. However, it is still important to detect actively progressing calcification, because this may be one of the signs of plaque rupture.191 Prospective studies with clinical outcomes are ongoing to assess whether coronary 18F uptake represents a future cardiovascular risk.
Summary and Conclusion
Nuclear cardiology using targeted tracers via SPECT and PET allows for diagnosis through non-invasive imaging. Not only myocardial perfusion but also cardiac metabolism, sympathetic nervous system activity, and inflammatory disease are targeted by nuclear cardiology using specific radiopharmaceuticals.
Change history
24 January 2018
Regrettably the original version of the above article contained errors in the three chemical structures presented in the ‘Atherosclerosis imaging’ section of Table 5, namely: 99mTc annexin V, 68Ga DOTATATE, and 64Cu DOTATATE; the chemical structures have been corrected in Table presented here. In addition, the radiopharmaceutical for isotope 67Ga has been corrected to 67Ga citrate, and many of the radiopharmaceuticals presented at the end of the table have been corrected.
Abbreviations
- 11C-CGP12177:
-
(S)-4-(3-((1,1-dimethylethyl)amino)-2-hydroxypropoxy)-[carbonyl-11C]1,3-dihydro-2H-benzimidazol-2-one
- 11C-CGP12388:
-
(S)-4-(3-(2’-[methine-11C]isopropylamino)-2-hydroxypropoxy)-2H-benzimidazol-2-one
- 11C-HED:
-
[11C]hydroxyephedrine
- 11C-PK11195:
-
1-(2-chlorophenyl)-N-[11C]methyl-N-1(1-methylpropyl)-3-isoquinolinecarboxamide
- 123I-BMIPP:
-
β-methyl-p-[123I]iodophenylpentadecanoic acid
- 123I-IPPA:
-
p-/o-[123I]iodophenylpentadecanoic acid
- 123I-MIBG:
-
m-[123I]iodobenzylguanidine
- 13N-NH3 :
-
[13N]ammonia
- 15O-H2O:
-
[15O]water
- 18F-FDG:
-
2-[18F]fluorodeoxyglucose
- 18F-FEDAC:
-
N-benzyl-N-methyl-2-[7,8-dihydro-7-(2-[18F]fluoroethyl)-8-oxo-2-phenyl-9H-purin-9-yl]acetamide
- 18F-FLT:
-
[18F]fluorothymidine
- 18F-FMISO:
-
[18F]fluoromisonidazole
- 18F-FTHA:
-
[18F]fluoro-6-thia-heptadecanoic acid
- 18F-NaF:
-
Sodium [18F]fluoride
- 68Ga-DOTANOC:
-
68Ga-complex with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-1-Nal3-octreotide
- 68Ga-DOTATATE:
-
68Ga-complex with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-d-Phe1-Tyr3-octreotate
- 68Ga-DOTATOC:
-
68Ga-complex with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-d-Phe1-Tyr3-octreotide
- 99mTc-MIBI:
-
99mTc-sestamibi
- acetyl-CoA:
-
Acetyl coenzyme A
- Ach:
-
Acetylcholine
- ATP:
-
Adenosine triphosphate
- CVD:
-
Cardiovascular disease
- CMR:
-
Cardiac magnetic resonance
- COMT:
-
Catechol-O-methyltransferase
- ECG:
-
Electrocardiographically
- FDA:
-
Food and Drug Administration
- FFA:
-
Free fatty acid
- HFpEF:
-
Heart failure with preserved ejection fraction
- HF:
-
Heart failure
- HMR:
-
Heart-to-mediastinum ratio
- HR:
-
Heart rate
- ICD:
-
Indication for cardioverter-defibrillator implantation
- LMI1195:
-
N-(3-bromo-4-(3-[18F]fluoropropoxy)benzyl)-guanidine
- LV:
-
Left ventricular
- MAO:
-
Monoamine oxidase
- MBF:
-
Myocardial blood flow
- MPI:
-
Myocardial perfusion imaging
- NE:
-
Norepinephrine
- PAP:
-
Pulmonary artery pressure
- PET:
-
Positron emission tomography
- PH:
-
Pulmonary hypertension
- PS:
-
Phosphatidylserine
- RV:
-
Right ventricle
- SPECT:
-
Single-photon emission computed tomography
- TCA:
-
Tricarboxylic acid
- TSPO:
-
Translocator protein 18kDa
- VMAT:
-
Vesicular monoamine transporter
- WBC:
-
White blood cell
- β-AR:
-
β-adrenergic receptor
References
Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, Naghavi M, Mensah GA, Murray CJ. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015;372:1333-41.
Bateman TM, Dilsizian V, Beanlands RS, DePuey EG, Heller GV, Wolinsky DA. American Society of Nuclear Cardiology and Society of Nuclear Medicine and Molecular Imaging Joint Position Statement on the Clinical Indications for Myocardial Perfusion PET. J Nucl Med 2016;57:1654-6.
Masuda A, Takeishi Y. Choosing the appropriate examination for diagnosis of stable ischemic heart disease. Ann Nucl Cardiol 2016;2:167-73.
Schindler T, Marashdeh W, Solnes L. Application of myocardial blood flow quantification in CAD patients. Ann Nucl Cardiol 2016;2:84-93.
Rauch B, Helus F, Grunze M, Braunwell E, Mall G, Hasselbach W, Kubler W. Kinetics of 13N-ammonia uptake in myocardial single cells indicating potential limitations in its applicability as a marker of myocardial blood flow. Circulation 1985;71:387-93.
Zaret BL, Strauss HW, Martin ND, Wells HP Jr, Flamm MD Jr. Noninvasive regional myocardial perfusion with radioactive potassium. Study of patients at rest, with exercise and during angina pectoris. N Engl J Med 1973;288:809-12.
Love WD, Romney RB, Burch GE. A comparison of the distribution of potassium and exchangeable rubidium in the organs of the dog, using rubidium. Circ Res 1954;2:112-22.
Bateman T. Current Status of myocardial perfusion PET in the United States. Ann Nucl Cardiol 2017;3:157-62.
Melon PG, Brihaye C, Degueldre C, Guillaume M, Czichosz R, Rigo P, Kulbertus HE, Comar D. Myocardial kinetics of potassium-38 in humans and comparison with copper-62-PTSM. J Nucl Med 1994;35:1116-22.
Tsan MF. Mechanism of gallium-67 accumulation in inflammatory lesions. J Nucl Med 1985;26:88-92.
Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, Marsden M, Pessotto R, Clark JC, Wallace WA, Salter DM, McKillop G, van Beek EJ, Boon NA, Rudd JH, Newby DE. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012;125:76-86.
Tamaki N, Morita K, Kuge Y, Tsukamoto E. The role of fatty acids in cardiac imaging. J Nucl Med 2000;41:1525-34.
Thackeray JT, Bengel FM. PET imaging of the autonomic nervous system. Q J Nucl Med Mol Imaging 2016;60:362-82.
Hatori A, Yui J, Xie L, Yamasaki T, Kumata K, Fujinaga M, Wakizaka H, Ogawa M, Nengaki N, Kawamura K, Zhang MR. Visualization of acute liver damage induced by cycloheximide in rats using PET with [(18)F]FEDAC, a radiotracer for translocator protein (18 kDa). PLoS ONE 2014;9:e86625.
Boersma HH, Kietselaer BL, Stolk LM, Bennaghmouch A, Hofstra L, Narula J, Heidendal GA, Reutelingsperger CP. Past, present, and future of annexin A5: From protein discovery to clinical applications. J Nucl Med 2005;46:2035-50.
Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol 1974;33:87-94.
Yoshinaga K, Manabe O, Tamaki N. Physiological assessment of myocardial perfusion using nuclear cardiology would enhance coronary artery disease patient care: Which imaging modality is best for evaluation of myocardial ischemia? (SPECT-side). Circ J 2011;75:713-22 discussion 731.
Yoshinaga K, Manabe O, Tamaki N. Absolute quantification of myocardial blood flow. J Nucl Cardiol 2016. https://doi.org/10.1007/s12350-016-0591-3.
Manabe O, Naya M, Tamaki N. Feasibility of PET for the management of coronary artery disease: Comparison between CFR and FFR. J Cardiol 2017;2:135-40.
Maddahi J, Czernin J, Lazewatsky J, Huang SC, Dahlbom M, Schelbert H, Sparks R, Ehlgen A, Crane P, Zhu Q, Devine M, Phelps M. Phase I, first-in-human study of BMS747158, a novel 18F-labeled tracer for myocardial perfusion PET: Dosimetry, biodistribution, safety, and imaging characteristics after a single injection at rest. J Nucl Med 2011;52:1490-8.
Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007;116:1290-305.
Maddahi J, Packard RR. Cardiac PET perfusion tracers: Current status and future directions. Semin Nucl Med 2014;44:333-43.
Strauss HW, Harrison K, Langan JK, Lebowitz E, Pitt B. Thallium-201 for myocardial imaging. Relation of thallium-201 to regional myocardial perfusion. Circulation 1975;51:641-5.
Wackers FJ, Schoot JB, Sokole EB, Samson G, Niftrik GJ, Lie KI, Durrer D, Wellens HJ. Noninvasive visualization of acute myocardial infarction in man with thallium-201. Br Heart J 1975;37:741-4.
Atkins HL, Budinger TF, Lebowitz E, Ansari AN, Greene MW, Fairchild RG, Ellis KJ. Thallium-201 for medical use. Part 3: Human distribution and physical imaging properties. J Nucl Med 1977;18:133-40.
Krahwinkel W, Herzog H, Feinendegen LE. Pharmacokinetics of thallium-201 in normal individuals after routine myocardial scintigraphy. J Nucl Med 1988;29:1582-6.
Jain D, Thompson B, Wackers FJ, Zaret BL. Relevance of increased lung thallium uptake on stress imaging in patients with unstable angina and non-Q wave myocardial infarction: Results of the Thrombolysis in Myocardial Infarction (TIMI)-IIIB Study. J Am Coll Cardiol 1997;30:421-9.
Thompson RC, Cullom SJ. Issues regarding radiation dosage of cardiac nuclear and radiography procedures. J Nucl Cardiol 2006;13:19-23.
Otsuka R, Kubo N, Miyazaki Y, Kawahara M, Takaesu J, Fukuchi K. Current status of stress myocardial perfusion imaging pharmaceuticals and radiation exposure in Japan: Results from a nationwide survey. J Nucl Cardiol 2017. https://doi.org/10.1007/s12350-017-0867-2.
Iida H, Maruno H, Koshino K, Shimochi S, Temma T, Hutton B, Matsuo S. Quantitative assessment of regional myocardial blood flow with clinical SPECT. Ann Nucl Cardiol 2016;2:111-21.
Okada RD, Glover DK, Moffett JD, Beju D, Johnson G 3rd. Kinetics of technetium-99m-teboroxime in reperfused nonviable myocardium. J Nucl Med 1997;38:274-9.
Beanlands RS, DeKemp RA, Harmsen E, Veinot JP, Hartman NG, Ruddy TD. Myocardial kinetics of technetium-99m teboroxime in the presence of postischemic injury, necrosis and low flow reperfusion. J Am Coll Cardiol 1996;28:487-94.
Higley B, Smith FW, Smith T, Gemmell HG, Das Gupta P, Gvozdanovic DV, Graham D, Hinge D, Davidson J, Lahiri A. Technetium-99m-1,2-bis[bis(2-ethoxyethyl) phosphino]ethane: human biodistribution, dosimetry and safety of a new myocardial perfusion imaging agent. J Nucl Med 1993;34:30-8.
Masuda A, Yoshinaga K, Naya M, Manabe O, Yamada S, Iwano H, Okada T, Katoh C, Takeishi Y, Tsutsui H, Tamaki N. Accelerated (99m)Tc-sestamibi clearance associated with mitochondrial dysfunction and regional left ventricular dysfunction in reperfused myocardium in patients with acute coronary syndrome. EJNMMI Res 2016;6:41.
Wackers FJ, Berman DS, Maddahi J, Watson DD, Beller GA, Strauss HW, Boucher CA, Picard M, Holman BL, Fridrich R, et al. Technetium-99m hexakis 2-methoxyisobutyl isonitrile: Human biodistribution, dosimetry, safety, and preliminary comparison to thallium-201 for myocardial perfusion imaging. J Nucl Med 1989;30:301-11.
Zaret BL, Rigo P, Wackers FJ, Hendel RC, Braat SH, Iskandrian AS, Sridhara BS, Jain D, Itti R, Serafini AN, et al. Myocardial perfusion imaging with 99mTc tetrofosmin. Comparison to 201Tl imaging and coronary angiography in a phase III multicenter trial. Tetrofosmin International Trial Study Group. Circulation 1995;91:313-9.
Flamen P, Bossuyt A, Franken PR. Technetium-99m-tetrofosmin in dipyridamole-stress myocardial SPECT imaging: Intraindividual comparison with technetium-99m-sestamibi. J Nucl Med 1995;36:2009-15.
Munch G, Neverve J, Matsunari I, Schroter G, Schwaiger M. Myocardial technetium-99m-tetrofosmin and technetium-99m-sestamibi kinetics in normal subjects and patients with coronary artery disease. J Nucl Med 1997;38:428-32.
Yoshinaga K, Tomiyama Y, Suzuki E, Tamaki N. Myocardial blood flow quantification using positron-emission tomography: Analysis and practice in the clinical setting. Circ J 2013;77:1662-71.
Wang L, Wu D, Yang Y, Chen IJ, Lin CY, Hsu B, Fang W, Tang YD. Avoiding full corrections in dynamic SPECT images impacts the performance of SPECT myocardial blood flow quantitation. J Nucl Cardiol 2016. https://doi.org/10.1007/s12350-016-0513-4.
Wells RG, Timmins R, Klein R, Lockwood J, Marvin B, deKemp RA, Wei L, Ruddy TD. Dynamic SPECT measurement of absolute myocardial blood flow in a porcine model. J Nucl Med 2014;55:1685-91.
Yoshinaga K, Katoh C, Manabe O, Klein R, Naya M, Sakakibara M, Yamada S, Dekemp RA, Tsutsui H, Tamaki N. Incremental diagnostic value of regional myocardial blood flow quantification over relative perfusion imaging with generator-produced rubidium-82 PET. Circ J 2011;75:2628-34.
Giubbini R, Peli A, Milan E, Sciagra R, Camoni L, Albano D, Bertoli M, Bonacina M, Motta F, Statuto M, Rodella CA, De Agostini A, Calabretta R, Bertagna F, Italian Nuclear Cardiology G. Comparison between the summed difference score and myocardial blood flow measured by 13N-ammonia. J Nucl Cardiol 2017. https://doi.org/10.1007/s12350-017-0789-z.
Yoshinaga K, Tamaki N. Current status of nuclear cardiology in Japan: Ongoing efforts to improve clinical standards and to establish evidence. J Nucl Cardiol 2015;22:690-9.
Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215-24.
Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015;131:19-27.
Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, Dorbala S, Hainer J, Blankstein R, Resnic F, Di Carli MF. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med 2014;55:248-55.
Naya M, Tamaki N, Tsutsui H. Coronary flow reserve estimated by positron emission tomography to diagnose significant coronary artery disease and predict cardiac events. Circ J 2015;79:15-23.
Ziadi MC, Dekemp RA, Williams K, Guo A, Renaud JM, Chow BJ, Klein R, Ruddy TD, Aung M, Garrard L, Beanlands RS. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol 2012;19:670-80.
Klein R, Beanlands RS, deKemp RA. Quantification of myocardial blood flow and flow reserve: Technical aspects. J Nucl Cardiol 2010;17:555-70.
Packard RR, Huang SC, Dahlbom M, Czernin J, Maddahi J. Absolute quantitation of myocardial blood flow in human subjects with or without myocardial ischemia using dynamic flurpiridaz F 18 PET. J Nucl Med 2014;55:1438-44.
Yoshinaga K, Klein R, Tamaki N. Generator-produced rubidium-82 positron emission tomography myocardial perfusion imaging-From basic aspects to clinical applications. J Cardiol 2010;55:163-73.
Gould KL. Clinical cardiac PET using generator-produced Rb-82: A review. Cardiovasc Interv Radiol 1989;12:245-51.
Selwyn AP, Allan RM, L’Abbate A, Horlock P, Camici P, Clark J, O’Brien HA, Grant PM. Relation between regional myocardial uptake of rubidium-82 and perfusion: Absolute reduction of cation uptake in ischemia. Am J Cardiol 1982;50:112-21.
Yoshinaga K. Current clinical practice of nuclear cardiology in Japan. Ann Nucl Cardiol 2016;2:50-2.
Krivokapich J, Huang SC, Phelps ME, MacDonald NS, Shine KI. Dependence of 13NH3 myocardial extraction and clearance on flow and metabolism. Am J Physiol 1982;242:H536-42.
Suda M, Onoguchi M, Tomiyama T, Ishihara K, Takahashi N, Sakurai M, Matsumoto K, Kumita S. The reproducibility of time-of-flight PET and conventional PET for the quantification of myocardial blood flow and coronary flow reserve with (13)N-ammonia. J Nucl Cardiol 2016;23:457-72.
Katoh C, Morita K, Shiga T, Kubo N, Nakada K, Tamaki N. Improvement of algorithm for quantification of regional myocardial blood flow using 15O-water with PET. J Nucl Med 2004;45:1908-16.
Maruo A, Manabe O, Yoshinaga K, Naya M, Tomiyama Y, Oyama-Manabe N, Hirata K, Magota K, Tsutsui H, Katoh C, Tamaki N. Feasibility of quantifying myocardial blood flow with a shorter acquisition time using 15O-H2O PET. Ann Nucl Cardiol 2016;2:30-7.
Yoshinaga K, Manabe O, Katoh C, Chen L, Klein R, Naya M, deKemp RA, Williams K, Beanlands RS, Tamaki N. Quantitative analysis of coronary endothelial function with generator-produced 82Rb PET: Comparison with 15O-labelled water PET. Eur J Nucl Med Mol Imaging 2010;37:2233-41.
Tomiyama Y, Manabe O, Oyama-Manabe N, Naya M, Sugimori H, Hirata K, Mori Y, Tsutsui H, Kudo K, Tamaki N, Katoh C. Quantification of myocardial blood flow with dynamic perfusion 3.0 Tesla MRI: Validation with (15) O-water PET. J Magn Reson Imaging 2015;42:754-62.
Mori Y, Manabe O, Naya M, Tomiyama Y, Yoshinaga K, Magota K, Oyama-Manabe N, Hirata K, Tsutsui H, Tamaki N, Katoh C. Improved spillover correction model to quantify myocardial blood flow by 11C-acetate PET: Comparison with 15O-H 2O PET. Ann Nucl Med 2015;29:15-20.
Naya M, Morita K, Yoshinaga K, Manabe O, Goto D, Hirata K, Katoh C, Tamaki N, Tsutsui H. Long-term smoking causes more advanced coronary endothelial dysfunction in middle-aged smokers compared to young smokers. Eur J Nucl Med Mol Imaging 2011;38:491-8.
Danad I, Uusitalo V, Kero T, Saraste A, Raijmakers PG, Lammertsma AA, Heymans MW, Kajander SA, Pietila M, James S, Sorensen J, Knaapen P, Knuuti J. Quantitative assessment of myocardial perfusion in the detection of significant coronary artery disease: Cutoff values and diagnostic accuracy of quantitative [(15)O]H2O PET imaging. J Am Coll Cardiol 2014;64:1464-75.
Yu M, Nekolla SG, Schwaiger M, Robinson SP. The next generation of cardiac positron emission tomography imaging agents: Discovery of flurpiridaz F-18 for detection of coronary disease. Semin Nucl Med 2011;41:305-13.
Huisman MC, Higuchi T, Reder S, Nekolla SG, Poethko T, Wester HJ, Ziegler SI, Casebier DS, Robinson SP, Schwaiger M. Initial characterization of an 18F-labeled myocardial perfusion tracer. J Nucl Med 2008;49:630-6.
Berman DS, Maddahi J, Tamarappoo BK, Czernin J, Taillefer R, Udelson JE, Gibson CM, Devine M, Lazewatsky J, Bhat G, Washburn D. Phase II safety and clinical comparison with single-photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease: Flurpiridaz F 18 positron emission tomography. J Am Coll Cardiol 2013;61:469-77.
Nekolla SG, Reder S, Saraste A, Higuchi T, Dzewas G, Preissel A, Huisman M, Poethko T, Schuster T, Yu M, Robinson S, Casebier D, Henke J, Wester HJ, Schwaiger M. Evaluation of the novel myocardial perfusion positron-emission tomography tracer 18F-BMS-747158-02: Comparison to 13N-ammonia and validation with microspheres in a pig model. Circulation 2009;119:2333-42.
Manabe O, Yoshinaga K, Ohira H, Masuda A, Sato T, Tsujino I, Yamada A, Oyama-Manabe N, Hirata K, Nishimura M, Tamaki N. The effects of 18-h fasting with low-carbohydrate diet preparation on suppressed physiological myocardial (18)F-fluorodeoxyglucose (FDG) uptake and possible minimal effects of unfractionated heparin use in patients with suspected cardiac involvement sarcoidosis. J Nucl Cardiol 2016;23:244-52.
Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 2005;85:1093-129.
Rosano GM, Fini M, Caminiti G, Barbaro G. Cardiac metabolism in myocardial ischemia. Curr Pharm Des 2008;14:2551-62.
Neubauer S. The failing heart-an engine out of fuel. N Engl J Med 2007;356:1140-51.
Matsumoto N, Hirayama A. Current Japanese Ministry of Health, Labor, and Welfare Approval of cardiac single photon emission computed tomography. Ann Nucl Cardiol 2015;1:108-9.
Higashi MIY, Miyauchi H, Zaima N, Suzuki A, Li M, Kobayashi K, Naito H, Hirano K. Imaging modalities for Triglyceride deposit cardiomyovasculopathy. Ann Nucl Cardiol 2017;3:94-102.
Knapp FF Jr, Goodman MM, Callahan AP, Kirsch G. Radioiodinated 15-(p-iodophenyl)-3,3-dimethylpentadecanoic acid: A useful new agent to evaluate myocardial fatty acid uptake. J Nucl Med 1986;27:521-31.
Torizuka K, Yonekura Y, Nishimura T, Tamaki N, Uehara T, Ikekubo K, Hino M. A phase 1 study of beta-methyl-p-(123I)-iodophenyl-pentadecanoic acid (123I-BMIPP). Kaku igaku Jpn J Nucl Med 1991;28:681-90.
Kurata C, Tawarahara K, Taguchi T, Aoshima S, Kobayashi A, Yamazaki N, Kawai H, Kaneko M. Myocardial emission computed tomography with iodine-123-labeled beta-methyl-branched fatty acid in patients with hypertrophic cardiomyopathy. J Nucl Med 1992;33:6-13.
Tamaki N, Kawamoto M, Yonekura Y, Fujibayashi Y, Takahashi N, Konishi J, Nohara R, Kambara H, Kawai C, Ikekubo K, et al. Regional metabolic abnormality in relation to perfusion and wall motion in patients with myocardial infarction: Assessment with emission tomography using an iodinated branched fatty acid analog. J Nucl Med 1992;33:659-67.
Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, Umemura T, Nakamura S, Yoshida M, Sato H. Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. J Am Coll Cardiol 2003;41:743-8.
Dilsizian V, Bateman TM, Bergmann SR, Des Prez R, Magram MY, Goodbody AE, Babich JW, Udelson JE. Metabolic imaging with beta-methyl-p-[(123)I]-iodophenyl-pentadecanoic acid identifies ischemic memory after demand ischemia. Circulation 2005;112:2169-74.
Knapp FF Jr, Kropp J. Iodine-123-labelled fatty acids for myocardial single-photon emission tomography: Current status and future perspectives. Eur J Nucl Med 1995;22:361-81.
Yoshizumi T, Nozaki S, Fukuchi K, Yamasaki K, Fukuchi T, Maruyama T, Tomiyama Y, Yamashita S, Nishimura T, Matsuzawa Y. Pharmacokinetics and metabolism of 123I-BMIPP fatty acid analog in healthy and CD36-deficient subjects. J Nucl Med 2000;41:1134-8.
Caveliers V, Franken PR, Lin Q, Mokler FT, Luo H, Knapp FF Jr. Intra-individual comparison of 3(R)-BMIPP and 3(S)-BMIPP isomers in humans. J Nucl Med 1998;39:1672-5.
Fujibayashi Y, Yonekura Y, Takemura Y, Wada K, Matsumoto K, Tamaki N, Yamamoto K, Konishi J, Yokoyama A. Myocardial accumulation of iodinated beta-methyl-branched fatty acid analogue, iodine-125-15-(p-iodophenyl)-3-(R, S)methylpentadecanoic acid (BMIPP), in relation to ATP concentration. J Nucl Med 1990;31:1818-22.
Dudczak R, Schmoliner R, Angelberger P, Knapp FF, Goodman MM. Structurally modified fatty acids: Clinical potential as tracers of metabolism. Eur J Nucl Med 1986;12:S45-8.
Gunnarsson M, Stenstrom K, Leide-Svegborn S, Faarinen M, Magnusson CE, Aberg M, Skog G, Hellborg R, Mattsson S. Biokinetics and radiation dosimetry for patients undergoing a glycerol tri[1-14C]oleate fat malabsorption breath test. Appl Radiat Isotopes 2003;58:517-26.
Reske SN, Sauer W, Machulla HJ, Knust J, Winkler C. Metabolism of 15 (p 123I iodophenyl-)pentadecanoic acid in heart muscle and noncardiac tissues. Eur J Nucl Med 1985;10:228-34.
Reske SN, Sauer W, Machulla HJ, Winkler C. 15(p-[123I]Iodophenyl)pentadecanoic acid as tracer of lipid metabolism: Comparison with [1-14C]palmitic acid in murine tissues. J Nucl Med 1984;25:1335-42.
Torizuka K, Yonekura Y, Nishimura T, Tamaki N, Uehara T. Phase 2 study of beta-methyl-p-(123I)-iodophenyl-pentadecanoic acid, a myocardial imaging agent for evaluating myocardial fatty acid metabolism. Kaku igaku Jpn J Nucl Med 1992;29:305-17.
De Geeter F, Franken PR, Knapp FF Jr, Bossuyt A. Relationship between blood flow and fatty acid metabolism in subacute myocardial infarction: A study by means of 99mTc-Sestamibi and 123I-beta-methyl-iodo-phenyl pentadecanoic acid. Eur J Nucl Med 1994;21:283-91.
Kawai Y, Tsukamoto E, Nozaki Y, Morita K, Sakurai M, Tamaki N. Significance of reduced uptake of iodinated fatty acid analogue for the evaluation of patients with acute chest pain. J Am Coll Cardiol 2001;38:1888-94.
Yoshinaga K, Naya M, Shiga T, Suzuki E, Tamaki N. Ischaemic memory imaging using metabolic radiopharmaceuticals: Overview of clinical settings and ongoing investigations. Eur J Nucl Med Mol Imaging 2014;41:384-93.
Fukuzawa S, Ozawa S, Shimada K, Sugioka J, Inagaki M. Prognostic values of perfusion-metabolic mismatch in Tl-201 and BMIPP scintigraphic imaging in patients with chronic coronary artery disease and left ventricular dysfunction undergoing revascularization. Ann Nucl Med 2002;16:109-15.
Tillisch J, Brunken R, Marshall R, Schwaiger M, Mandelkern M, Phelps M, Schelbert H. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med 1986;314:884-8.
Landau BR, Spring-Robinson CL, Muzic RF Jr, Rachdaoui N, Rubin D, Berridge MS, Schumann WC, Chandramouli V, Kern TS, Ismail-Beigi F. 6-Fluoro-6-deoxy-D-glucose as a tracer of glucose transport. Am J Physiol Endocrinol Metab 2007;293:E237-45.
Hasegawa S, Kusuoka H, Uehara T, Yamaguchi H, Hori M, Nishimura T. Glucose tolerance and myocardial F-18 fluorodeoxyglucose uptake in normal regions in coronary heart disease patients. Ann Nucl Med 1998;12:363-8.
Vitale GD, deKemp RA, Ruddy TD, Williams K, Beanlands RS. Myocardial glucose utilization and optimization of (18)F-FDG PET imaging in patients with non-insulin-dependent diabetes mellitus, coronary artery disease, and left ventricular dysfunction. J Nucl Med 2001;42:1730-6.
Tamaki N, Yonekura Y, Yamashita K, Saji H, Magata Y, Senda M, Konishi Y, Hirata K, Ban T, Konishi J. Positron emission tomography using fluorine-18 deoxyglucose in evaluation of coronary artery bypass grafting. Am J Cardiol 1989;64:860-5.
Kisrieva-Ware Z, Coggan AR, Sharp TL, Dence CS, Gropler RJ, Herrero P. Assessment of myocardial triglyceride oxidation with PET and 11C-palmitate. J Nucl Cardiol 2009;16:411-21.
Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in Patients with Congestive Heart Failure. J Nucl Med 2001;42:55-62.
Maki MT, Haaparanta M, Nuutila P, Oikonen V, Luotolahti M, Eskola O, Knuuti JM. Free fatty acid uptake in the myocardium and skeletal muscle using fluorine-18-fluoro-6-thia-heptadecanoic acid. J Nucl Med 1998;39:1320-7.
Ohira H, deKemp R, Pena E, Davies RA, Stewart DJ, Chandy G, Contreras-Dominguez V, Dennie C, Mc Ardle B, Mc Klein R, Renaud JM, DaSilva JN, Pugliese C, Dunne R, Beanlands R, Mielniczuk LM. Shifts in myocardial fatty acid and glucose metabolism in pulmonary arterial hypertension: A potential mechanism for a maladaptive right ventricular response. Eur Heart J Cardiovasc Imaging 2016;17:1424-31.
Nesterov SV, Turta O, Han C, Maki M, Lisinen I, Tuunanen H, Knuuti J. C-11 acetate has excellent reproducibility for quantification of myocardial oxidative metabolism. Eur Heart J Cardiovasc Imaging 2015;16:500-6.
Tamaki N, Magata Y, Takahashi N, Kawamoto M, Torizuka T, Yonekura Y, Nishizawa S, Sadato N, Tadamura E, Ono S, et al. Myocardial oxidative metabolism in normal subjects in fasting, glucose loading and dobutamine infusion states. Ann Nucl Med 1992;6:221-8.
Brown M, Marshall DR, Sobel BE, Bergmann SR. Delineation of myocardial oxygen utilization with carbon-11-labeled acetate. Circulation 1987;76:687-96.
Buxton DB, Schwaiger M, Nguyen A, Phelps ME, Schelbert HR. Radiolabeled acetate as a tracer of myocardial tricarboxylic acid cycle flux. Circ Res 1988;63:628-34.
Walsh MN, Geltman EM, Brown MA, Henes CG, Weinheimer CJ, Sobel BE, Bergmann SR. Noninvasive estimation of regional myocardial oxygen consumption by positron emission tomography with carbon-11 acetate in patients with myocardial infarction. J Nucl Med 1989;30:1798-808.
Kalff V, Hicks RJ, Hutchins G, Topol E, Schwaiger M. Use of carbon-11 acetate and dynamic positron emission tomography to assess regional myocardial oxygen consumption in patients with acute myocardial infarction receiving thrombolysis or coronary angioplasty. Am J Cardiol 1993;71:529-35.
Wu YW, Naya M, Tsukamoto T, Komatsu H, Morita K, Yoshinaga K, Kuge Y, Tsutsui H, Tamaki N. Heterogeneous reduction of myocardial oxidative metabolism in patients with ischemic and dilated cardiomyopathy using C-11 acetate PET. Circ J 2008;72:786-92.
Tadamura E, Tamaki N, Matsumori A, Magata Y, Yonekura Y, Nohara R, Sasayama S, Yoshibayashi M, Kamiya T, Konishi J. Myocardial metabolic changes in hypertrophic cardiomyopathy. J Nucl Med 1996;37:572-7.
Beanlands RS, Nahmias C, Gordon E, Coates G, deKemp R, Firnau G, Fallen E. The effects of beta(1)-blockade on oxidative metabolism and the metabolic cost of ventricular work in patients with left ventricular dysfunction: A double-blind, placebo-controlled, positron-emission tomography study. Circulation 2000;102:2070-5.
Hall AB, Ziadi MC, Leech JA, Chen SY, Burwash IG, Renaud J, deKemp RA, Haddad H, Mielniczuk LM, Yoshinaga K, Guo A, Chen L, Walter O, Garrard L, DaSilva JN, Floras JS, Beanlands RS. Effects of short-term continuous positive airway pressure on myocardial sympathetic nerve function and energetics in patients with heart failure and obstructive sleep apnea: A randomized study. Circulation 2014;130:892-901.
Wong YY, Raijmakers P, van Campen J, van der Laarse WJ, Knaapen P, Lubberink M, Ruiter G, Vonk Noordegraaf A, Lammertsma AA. 11C-Acetate clearance as an index of oxygen consumption of the right myocardium in idiopathic pulmonary arterial hypertension: A validation study using 15O-labeled tracers and PET. J Nucl Med 2013;54:1258-62.
Stolen KQ, Kemppainen J, Ukkonen H, Kalliokoski KK, Luotolahti M, Lehikoinen P, Hamalainen H, Salo T, Airaksinen KE, Nuutila P, Knuuti J. Exercise training improves biventricular oxidative metabolism and left ventricular efficiency in patients with dilated cardiomyopathy. J Am Coll Cardiol 2003;41:460-7.
Hicks RJ, Kalff V, Savas V, Starling MR, Schwaiger M. Assessment of right ventricular oxidative metabolism by positron emission tomography with C-11 acetate in aortic valve disease. Am J Cardiol 1991;67:753-7.
Yoshinaga K, Ohira H, Tsujino I, Oyama-Manabe N, Mielniczuk L, Beanlands RS, Katoh C, Kasai K, Manabe O, Sato T, Fujii S, Ito YM, Tomiyama Y, Nishimura M, Tamaki N. Attenuated right ventricular energetics evaluated using (1)(1)C-acetate PET in patients with pulmonary hypertension. Eur J Nucl Med Mol Imaging 2014;41:1240-50.
Francis GS. Modulation of peripheral sympathetic nerve transmission. J Am Coll Cardiol 1988;12:250-4.
Melon PG, Nguyen N, DeGrado TR, Mangner TJ, Wieland DM, Schwaiger M. Imaging of cardiac neuronal function after cocaine exposure using carbon-11 hydroxyephedrine and positron emission tomography. J Am Coll Cardiol 1994;23:1693-9.
Nomura Y, Matsunari I, Takamatsu H, Murakami Y, Matsuya T, Taki J, Nakajima K, Nekolla SG, Chen WP, Kajinami K. Quantitation of cardiac sympathetic innervation in rabbits using 11C-hydroxyephedrine PET: Relation to 123I-MIBG uptake. Eur J Nucl Med Mol Imaging 2006;33:871-8.
Mitrani RD, Klein LS, Miles WM, Hackett FK, Burt RW, Wellman HN, Zipes DP. Regional cardiac sympathetic denervation in patients with ventricular tachycardia in the absence of coronary artery disease. J Am Coll Cardiol 1993;22:1344-53.
Tsukamoto T, Morita K, Naya M, Inubushi M, Katoh C, Nishijima K, Kuge Y, Okamoto H, Tsutsui H, Tamaki N. Decreased myocardial beta-adrenergic receptor density in relation to increased sympathetic tone in patients with nonischemic cardiomyopathy. J Nucl Med 2007;48:1777-82.
Brunner-La Rocca HP, Esler MD, Jennings GL, Kaye DM. Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur Heart J 2001;22:1136-43.
Paul M, Schafers M, Kies P, Acil T, Schafers K, Breithardt G, Schober O, Wichter T. Impact of sympathetic innervation on recurrent life-threatening arrhythmias in the follow-up of patients with idiopathic ventricular fibrillation. Eur J Nucl Med Mol Imaging 2006;33:866-70.
Nakata T, Miyamoto K, Doi A, Sasao H, Wakabayashi T, Kobayashi H, Tsuchihashi K, Shimamoto K. Cardiac death prediction and impaired cardiac sympathetic innervation assessed by MIBG in patients with failing and nonfailing hearts. J Nucl Cardiol 1998;5:579-90.
Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, Matsui T, Matsuo S, Travin MI, Jacobson AF. A pooled analysis of multicenter cohort studies of (123)I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging 2013;6:772-84.
Nakajima K, Scholte A, Nakata T, Dimitriu-Leen AC, Chikamori T, Vitola JV, Yoshinaga K. Cardiac sympathetic nervous system imaging with 123I-meta-iodobenzylguanidine: Perspectives from Japan and Europe. J Nucl Cardiol 2017;24:952-60.
Nakajima K, Nakata T. Cardiac 123I-MIBG imaging for clinical decision making: 22-year Experience in Japan. J Nucl Med 2015;56:11S-9S.
Kline RC, Swanson DP, Wieland DM, Thrall JH, Gross MD, Pitt B, Beierwaltes WH. Myocardial imaging in man with I-123 meta-iodobenzylguanidine. J Nucl Med 1981;22:129-32.
Bengel FM. Imaging targets of the sympathetic nervous system of the heart: Translational considerations. J Nucl Med 2011;52:1167-70.
Lautamaki R, Tipre D, Bengel FM. Cardiac sympathetic neuronal imaging using PET. Eur J Nucl Med Mol Imaging 2007;34:S74-85.
Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016;23:606-39.
Nakajima K, Okuda K, Matsuo S, Yoshita M, Taki J, Yamada M, Kinuya S. Standardization of metaiodobenzylguanidine heart to mediastinum ratio using a calibration phantom: Effects of correction on normal databases and a multicentre study. Eur J Nucl Med Mol Imaging 2012;39:113-9.
Nakajima K, Scholte JHAA, Nakata T, Dimitriu-Leen AC, Chikamori T, Yoshinaga K. Cardiac sympathetic nervous system imaging with 123I-meta-iodobenzylguanidine: Perspectives from Japan and Europe. Ann Nucl Cardiol 2017;24:952-60.
Thackeray JT, Bengel FM. Assessment of cardiac autonomic neuronal function using PET imaging. J Nucl Cardiol 2013;20:150-65.
Fallavollita JA, Heavey BM, Luisi AJ Jr, Michalek SM, Baldwa S, Mashtare TL Jr, Hutson AD, Dekemp RA, Haka MS, Sajjad M, Cimato TR, Curtis AB, Cain ME, Canty JM Jr. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol 2014;63:141-9.
Bulow HP, Stahl F, Lauer B, Nekolla SG, Schuler G, Schwaiger M, Bengel FM. Alterations of myocardial presynaptic sympathetic innervation in patients with multi-vessel coronary artery disease but without history of myocardial infarction. Nucl Med Commun 2003;24:233-9.
Harms HJ, Lubberink M, de Haan S, Knaapen P, Huisman MC, Schuit RC, Windhorst AD, Allaart CP, Lammertsma AA. Use of a single 11C-Meta-hydroxyephedrine scan for assessing flow-innervation mismatches in patients with ischemic cardiomyopathy. J Nucl Med 2015;56:1706-11.
Pietila M, Malminiemi K, Ukkonen H, Saraste M, Nagren K, Lehikoinen P, Voipio-Pulkki LM. Reduced myocardial carbon-11 hydroxyephedrine retention is associated with poor prognosis in chronic heart failure. Eur J Nucl Med 2001;28:373-6.
Bengel FM, Permanetter B, Ungerer M, Nekolla SG, Schwaiger M. Alterations of the sympathetic nervous system and metabolic performance of the cardiomyopathic heart. Eur J Nucl Med Mol Imaging 2002;29:198-202.
Mazzadi AN, Andre-Fouet X, Duisit J, Gebuhrer V, Costes N, Chevalier P, Rodriguez C, Schott JJ, Le Marec H, Guicheney P, Le Bars D, Janier M. Cardiac retention of [11C]HED in genotyped long QT patients: a potential amplifier role for severity of the disease. Am J Physiol Heart Circ Physiol 2003;285:H1286-93.
Olgin JE, Sih HJ, Hanish S, Jayachandran JV, Wu J, Zheng QH, Winkle W, Mulholland GK, Zipes DP, Hutchins G. Heterogeneous atrial denervation creates substrate for sustained atrial fibrillation. Circulation 1998;98:2608-14.
Allman KC, Wieland DM, Muzik O, Degrado TR, Wolfe ER Jr, Schwaiger M. Carbon-11 hydroxyephedrine with positron emission tomography for serial assessment of cardiac adrenergic neuronal function after acute myocardial infarction in humans. J Am Coll Cardiol 1993;22:368-75.
Aoki H, Matsunari I, Nomura Y, Fujita W, Komatsu R, Miyazaki Y, Nekolla SG, Kajinami K. Myocardial sympathetic innervation, function, and oxidative metabolism in non-infarcted myocardium in patients with prior myocardial infarction. Ann Nucl Med 2013;27:523-31.
Allman KC, Stevens MJ, Wieland DM, Hutchins GD, Wolfe ER Jr, Greene DA, Schwaiger M. Noninvasive assessment of cardiac diabetic neuropathy by carbon-11 hydroxyephedrine and positron emission tomography. J Am Coll Cardiol 1993;22:1425-32.
Stevens MJ, Raffel DM, Allman KC, Schwaiger M, Wieland DM. Regression and progression of cardiac sympathetic dysinnervation complicating diabetes: An assessment by C-11 hydroxyephedrine and positron emission tomography. Metabolism 1999;48:92-101.
Aikawa T, Naya M, Obara M, Manabe O, Tomiyama Y, Magota K, Yamada S, Katoh C, Tamaki N, Tsutsui H. Impaired myocardial sympathetic innervation is associated with diastolic dysfunction in heart failure with preserved ejection fraction: 11C-Hydroxyephedrine PET study. J Nucl Med 2017;58:784-90.
Sinusas AJ, Lazewatsky J, Brunetti J, Heller G, Srivastava A, Liu YH, Sparks R, Puretskiy A, Lin SF, Crane P, Carson RE, Lee LV. Biodistribution and radiation dosimetry of LMI1195: First-in-human study of a novel 18F-labeled tracer for imaging myocardial innervation. J Nucl Med 2014;55:1445-51.
Eskola O, Gronroos T, Bergman J, Haaparanta M, Marjamaki P, Lehikoinen P, Forsback S, Langer O, Hinnen F, Dolle F, Halldin C, Solin O. A novel electrophilic synthesis and evaluation of medium specific radioactivity (1R,2S)-4-[18F]fluorometaraminol, a tracer for the assessment of cardiac sympathetic nerve integrity with PET. Nucl Med Biol 2004;31:103-10.
Raffel DM, Corbett JR, del Rosario RB, Mukhopadhyay SK, Gildersleeve DL, Rose P, Wieland DM. Sensitivity of [11C]phenylephrine kinetics to monoamine oxidase activity in normal human heart. J Nucl Med 1999;40:232-8.
Goldstein DS, Eisenhofer G, Dunn BB, Armando I, Lenders J, Grossman E, Holmes C, Kirk KL, Bacharach S, Adams R, et al. Positron emission tomographic imaging of cardiac sympathetic innervation using 6-[18F]fluorodopamine: Initial findings in humans. J Am Coll Cardiol 1993;22:1961-71.
Munch G, Nguyen NT, Nekolla S, Ziegler S, Muzik O, Chakraborty P, Wieland DM, Schwaiger M. Evaluation of sympathetic nerve terminals with [(11)C]epinephrine and [(11)C]hydroxyephedrine and positron emission tomography. Circulation 2000;101:516-23.
Salinas C, Muzic RF Jr, Berridge M, Ernsberger P. PET imaging of myocardial beta-adrenergic receptors with fluorocarazolol: Lack of interference by endogenous catecholamines. J Cardiovasc Pharmacol 2005;46:222-31.
Naya M, Tsukamoto T, Morita K, Katoh C, Nishijima K, Komatsu H, Yamada S, Kuge Y, Tamaki N, Tsutsui H. Myocardial beta-adrenergic receptor density assessed by 11C-CGP12177 PET predicts improvement of cardiac function after carvedilol treatment in patients with idiopathic dilated cardiomyopathy. J Nucl Med 2009;50:220-5.
de Jong RM, Willemsen AT, Slart RH, Blanksma PK, van Waarde A, Cornel JH, Vaalburg W, van Veldhuisen DJ, Elsinga PH. Myocardial beta-adrenoceptor downregulation in idiopathic dilated cardiomyopathy measured in vivo with PET using the new radioligand (S)-[11C]CGP12388. Eur J Nucl Med Mol Imaging 2005;32:443-7.
Bengel FM, Higuchi T, Javadi MS, Lautamaki R. Cardiac positron emission tomography. J Am Coll Cardiol 2009;54:1-15.
Petrou M, Frey KA, Kilbourn MR, Scott PJ, Raffel DM, Bohnen NI, Muller ML, Albin RL, Koeppe RA. In vivo imaging of human cholinergic nerve terminals with (-)-5-(18)F-fluoroethoxybenzovesamicol: Biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med 2014;55:396-404.
Mulholland GK, Otto CA, Jewett DM, Kilbourn MR, Koeppe RA, Sherman PS, Petry NA, Carey JE, Atkinson ER, Archer S, et al. Synthesis, rodent biodistribution, dosimetry, metabolism, and monkey images of carbon-11-labeled (+)-2 alpha-tropanyl benzilate: A central muscarinic receptor imaging agent. J Nucl Med 1992;33:423-30.
Futamatsu H, Suzuki J, Adachi S, Okada H, Otomo K, Ohara T, Hashimoto Y, Kakuta T, Iesaka Y, Yamaguchi H, Sakurada H, Sato A, Obayashi T, Niwa A, Hirao K, Isobe M. Utility of gallium-67 scintigraphy for evaluation of cardiac sarcoidosis with ventricular tachycardia. Int J Cardiovasc Imaging 2006;22:443-8.
Palestro CJ. The current role of gallium imaging in infection. Semin Nucl Med 1994;24:128-41.
Love C, Palestro CJ. Radionuclide imaging of infection. J Nucl Med Technol 2004;32:47-57.
Hung MY, Hung MJ, Cheng CW. Use of gallium 67 scintigraphy to differentiate acute myocarditis from acute myocardial infarction. Tex Heart Inst J 2007;34:305-9.
Terasaki F, Yoshinaga K. New guidelines for diagnosis of cardiac sarcoidosis in Japan. Ann Nucl Cardiol 2017;3:42-5.
Ishimaru S, Tsujino I, Takei T, Tsukamoto E, Sakaue S, Kamigaki M, Ito N, Ohira H, Ikeda D, Tamaki N, Nishimura M. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005;26:1538-43.
Cerqueira MD, Jacobson AF. Indium-111 leukocyte scintigraphic detection of myocardial abscess formation in patients with endocarditis. J Nucl Med 1989;30:703-6.
Erba PA, Sollini M, Conti U, Bandera F, Tascini C, De Tommasi SM, Zucchelli G, Doria R, Menichetti F, Bongiorni MG, Lazzeri E, Mariani G. Radiolabeled WBC scintigraphy in the diagnostic workup of patients with suspected device-related infections. JACC Cardiovasc Imaging 2013;6:1075-86.
Rouzet F, Chequer R, Benali K, Lepage L, Ghodbane W, Duval X, Iung B, Vahanian A, Le Guludec D, Hyafil F. Respective performance of 18F-FDG PET and radiolabeled leukocyte scintigraphy for the diagnosis of prosthetic valve endocarditis. J Nucl Med 2014;55:1980-5.
Libby P, Nahrendorf M, Swirski FK. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease: An expanded “cardiovascular continuum”. J Am Coll Cardiol 2016;67:1091-103.
Kietselaer BL, Reutelingsperger CP, Heidendal GA, Daemen MJ, Mess WH, Hofstra L, Narula J. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med 2004;350:1472-3.
Yamashita A, Zhao Y, Zhao S, Matsuura Y, Sugita C, Iwakiri T, Okuyama N, Ohe K, Koshimoto C, Kawai K, Tamaki N, Kuge Y, Asada Y. Arterial (18)F-fluorodeoxyglucose uptake reflects balloon catheter-induced thrombus formation and tissue factor expression via nuclear factor-kappaB in rabbit atherosclerotic lesions. Circ J 2013;77:2626-35.
Ohira H, Tsujino I, Yoshinaga K. (1)(8)F-Fluoro-2-deoxyglucose positron emission tomography in cardiac sarcoidosis. Eur J Nucl Med Mol Imaging 2011;38:1773-83.
Manabe O, Oyama-Manabe N, Ohira H, Tsutsui H, Tamaki N. Multimodality evaluation of cardiac sarcoidosis. J Nucl Cardiol 2012;19:621-4.
Kong EJ, Lee SH, Cho IH. Myocardial fibrosis in hypertrophic cardiomyopathy demonstrated by integrated cardiac F-18 FDG PET/MR. Nucl Med Mol Imaging 2013;47:196-200.
Masuda A, Manabe O, Oyama-Manabe N, Naya M, Obara M, Sakakibara M, Hirata K, Yamada S, Naka T, Tsutsui H, Tamaki N. Cardiac fibroma with high 18F-FDG uptake mimicking malignant tumor. J Nucl Cardiol 2017;24:323-4.
Kikuchi Y, Oyama-Manabe N, Manabe O, Naya M, Ito YM, Hatanaka KC, Tsutsui H, Terae S, Tamaki N, Shirato H. Imaging characteristics of cardiac dominant diffuse large B-cell lymphoma demonstrated with MDCT and PET/CT. Eur J Nucl Med Mol Imaging 2013;40:1337-44.
Aikawa T, Naya M, Manabe O, Obara M, Matsushima S, Tamaki N, Tsutsui H. Incidental focal myocardial (18)F-FDG uptake indicating asymptomatic coronary artery disease. J Nucl Cardiol 2016;23:596-8.
Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer (68)Ga-DOTANOC PET/CT and (18)F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res 2016;6:52.
Norikane T, Yamamoto Y, Maeda Y, Noma T, Nishiyama Y. 18F-FLT PET imaging in a patient with sarcoidosis with cardiac involvement. Clin Nucl Med 2015;40:433-4.
Lapa C, Reiter T, Kircher M, Schirbel A, Werner RA, Pelzer T, Pizarro C, Skowasch D, Thomas L, Schlesinger-Irsch U, Thomas D, Bundschuh RA, Bauer WR, Gartner FC. Somatostatin receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: An initial comparison to cardiac MRI. Oncotarget 2016;7:77807-14.
Manabe O, Hirata K, Shozo O, Shiga T, Uchiyama Y, Kobayashi K, Watanabe S, Toyonaga T, Kikuchi H, Oyama-Manabe N, Tamaki N. 18F-fluoromisonidazole (FMISO) PET may have the potential to detect cardiac sarcoidosis. J Nucl Cardiol 2017;24:329-31.
Schatka I, Wollenweber T, Haense C, Brunz F, Gratz KF, Bengel FM. Peptide receptor-targeted radionuclide therapy alters inflammation in atherosclerotic plaques. J Am Coll Cardiol 2013;62:2344-5.
Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV, Starks LT, Martin-Garrido A, Manavaki R, Yu E, Kuc RE, Grassi L, Kreuzhuber R, Kostadima MA, Frontini M, Kirkpatrick PJ, Coughlin PA, Gopalan D, Fryer TD, Buscombe JR, Groves AM, Ouwehand WH, Bennett MR, Warburton EA, Davenport AP, Rudd JH. Detection of Atherosclerotic Inflammation by 68Ga-DOTATATE PET Compared to [18F]FDG PET Imaging. J Am Coll Cardiol 2017;69:1774-91.
Pedersen SF, Sandholt BV, Keller SH, Hansen AE, Clemmensen AE, Sillesen H, Hojgaard L, Ripa RS, Kjaer A. 64Cu-DOTATATE PET/MRI for detection of activated macrophages in carotid atherosclerotic plaques: Studies in patients undergoing endarterectomy. Arterioscler Thromb Vasc Biol 2015;35:1696-703.
Yui J, Maeda J, Kumata K, Kawamura K, Yanamoto K, Hatori A, Yamasaki T, Nengaki N, Higuchi M, Zhang MR. 18F-FEAC and 18F-FEDAC: PET of the monkey brain and imaging of translocator protein (18 kDa) in the infarcted rat brain. J Nucl Med 2010;51:1301-9.
Veenman L, Gavish M. The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharmacol Ther 2006;110:503-24.
Gaemperli O, Shalhoub J, Owen DR, Lamare F, Johansson S, Fouladi N, Davies AH, Rimoldi OE, Camici PG. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J 2012;33:1902-10.
Yanamoto K, Kumata K, Yamasaki T, Odawara C, Kawamura K, Yui J, Hatori A, Suzuki K, Zhang MR. [18F]FEAC and [18F]FEDAC: Two novel positron emission tomography ligands for peripheral-type benzodiazepine receptor in the brain. Bioorg Med Chem Lett 2009;19:1707-10.
Yanamoto K, Kumata K, Fujinaga M, Nengaki N, Takei M, Wakizaka H, Hosoi R, Momosaki S, Yamasaki T, Yui J, Kawamura K, Hatori A, Inoue O, Zhang MR. In vivo imaging and quantitative analysis of TSPO in rat peripheral tissues using small-animal PET with [18F]FEDAC. Nucl Med Biol 2010;37:853-60.
Hatori A, Yui J, Xie L, Kumata K, Yamasaki T, Fujinaga M, Wakizaka H, Ogawa M, Nengaki N, Kawamura K, Wang F, Zhang MR. Utility of translocator protein (18 kDa) as a molecular imaging biomarker to monitor the progression of liver fibrosis. Sci Rep 2015;5:17327.
Hatori A, Yui J, Yamasaki T, Xie L, Kumata K, Fujinaga M, Yoshida Y, Ogawa M, Nengaki N, Kawamura K, Fukumura T, Zhang MR. PET imaging of lung inflammation with [18F]FEDAC, a radioligand for translocator protein (18 kDa). PLoS ONE 2012;7:e45065.
Krishnan S, Otaki Y, Doris M, Slipczuk L, Arnson Y, Rubeaux M, Dey D, Slomka P, Berman DS, Tamarappoo B. Molecular imaging of vulnerable coronary plaque: A pathophysiologic perspective. J Nucl Med 2017;58:359-64.
Cocker MS, Mc Ardle B, Spence JD, Lum C, Hammond RR, Ongaro DC, McDonald MA, Dekemp RA, Tardif JC, Beanlands RS. Imaging atherosclerosis with hybrid [18F]fluorodeoxyglucose positron emission tomography/computed tomography imaging: What Leonardo da Vinci could not see. J Nucl Cardiol 2012;19:1211-25.
Acknowledgments
The authors thank Ms. Mariko Yamasaki and Natuse Ito for their administrative assistance. This manuscript has been reviewed by a North American English-language professional editor, Ms. Holly Beanlands. The authors also thank Ms. Holly Beanlands for critical reading of the manuscript.
Disclosures
O. Manabe, T. Kikuchi, M. El Mahdiui, R. Nishii, M.-R. Zhang, E. Suzuki and K. Yoshinaga have nothing to disclose. A.J.H.A. Scholte is a Consultant for GE Healthcare and Toshiba Medical Systems.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Manabe, O., Kikuchi, T., Scholte, A.J.H.A. et al. Radiopharmaceutical tracers for cardiac imaging. J. Nucl. Cardiol. 25, 1204–1236 (2018). https://doi.org/10.1007/s12350-017-1131-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-017-1131-5