Abstract
Prodigious efforts and landmark discoveries have led toward significant advances in our understanding of atherosclerosis. Despite significant efforts, atherosclerosis continues globally to be a leading cause of mortality and reduced quality of life. With surges in the prevalence of obesity and diabetes, atherosclerosis is expected to have an even more pronounced impact upon the global burden of disease. It is imperative to develop strategies for the early detection of disease. Positron emission tomography (PET) imaging utilizing [18F]fluorodeoxyglucose (FDG) may provide a non-invasive means of characterizing inflammatory activity within atherosclerotic plaque, thus serving as a surrogate biomarker for detecting vulnerable plaque. The aim of this review is to explore the rationale for performing FDG imaging, provide an overview into the mechanism of action, and summarize findings from the early application of FDG PET imaging in the clinical setting to evaluate vascular disease. Alternative imaging biomarkers and approaches are briefly discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Vessels in the elderly restrict the transit of blood through thickening of the tunics.
Leonardo da Vinci, 1452–1519.
Introduction
Leonardo da Vinci presented perhaps one of the first known descriptions of atherosclerosis. In his collection of notes from post-mortem observations of human anatomy and poorly understood pathology, da Vinci merged what were the distinct disciplines of art and science to describe and depict human development and physiology.1 Five-hundred years later, significant advances in technology enable non-invasive visualization of atherosclerosis using multiple imaging modalities, moving beyond anatomical characterization toward directly imaging disease processes and pathophysiology. The current challenge for imaging scientists lies in identifying high-risk atherosclerotic lesions that could lead to coronary or cerebrovascular sequelae prior to adverse vascular events such as myocardial infarction and stroke, and thereby enable personalized therapy while mitigating risk.
Herein, we present a general introduction to advanced non-invasive imaging of atherosclerosis with particular emphasis upon [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET). We will discuss the role of FDG PET for the identification of the high-risk “vulnerable plaque” across the arterial tree and its potential for monitoring disease progression and response to therapy.
Atherosclerosis
Atherosclerosis is the most common underlying pathology responsible for adverse cardiovascular outcomes including angina, myocardial infarction, transient ischemic attacks, and stroke. With an aging population, obesity and diabetes pandemics, cardiovascular disease is projected to become a leading source of global disease burden.2-5
Atherosclerosis is a chronic disease process that begins with the disruption and inflammation of vascular endothelium, leading to the formation of lipid-rich fatty streaks that may arise as early as infancy. 6 Inflammatory activity within these lesions increases as lipids and macrophages progressively accumulate, resulting in complex remodeling of fibrofatty plaques.7,8 A typical plaque consists of a central lipid-rich core bound by a fibrous cap. The fibrous cap can suddenly rupture or erode away, exposing the core of the plaque to clotting factors within blood. Activation of these factors results in a cascade that can completely occlude the vessel and induce severe ischemic injury or tissue necrosis.9,10
Plaque that is thrombosis-prone and likely to progress rapidly, resulting in vessel occlusion is referred to as vulnerable plaque.11 Proposed major criteria for defining vulnerable plaque include the presence of active inflammation, particularly increased activated macrophage content.11 Evidence suggests that the impending risk to a patient posed by the presence of vulnerable plaque cannot be sufficiently determined by assessing for the anatomic presence of plaque at a specific site or vessel within the arterial tree. Rather, global plaque burden across the entire arterial bed may be a stronger correlate to determine patient risk, thus allowing for risk stratification.12 In this regard, non-invasive cardiovascular imaging may be useful as it could visualize disease across the entire arterial bed, and may also be utilized to monitor disease progression or even regression with novel therapies.
Non-invasive Imaging of Atherosclerosis
Contrast-Enhanced Ultrasound
Contrast-enhanced ultrasound takes advantage of portability, bedside imaging and wide availability of carotid ultrasound. Injection of intravascular micro-bubbles permits the visualization of micro- and macro-vasculature, but more importantly, intraplaque neovascularization.13 Neovascularization occurs during the early stages of atherosclerosis where undeveloped leaky micro-vessels are formed within the plaque.14 The risk associated with these micro-vessels is the development of an intraplaque hemorrhage that potentiates inflammation and contributes toward plaque instability.14,15 Therefore, neovascularization detected by contrast-enhanced ultrasound may serve as a marker of a high-risk lesion or a vulnerable plaque.16 Furthermore, the intima-media is hypoechoic while the adventitia is echogenic, resulting in contrast that can delineate vessel lumen and plaque ulcerations or irregularities on the plaque surface.17,18 Additionally, intima-media thickness (IMT) can also be measured by contrast-enhanced ultrasound and is a marker of premature atherosclerosis.
3-Dimensional Ultrasound
3D ultrasound imaging builds further upon the principles of ultrasound imaging. With 3D ultrasound, it is feasible to visualize carotid plaque and accurately quantify plaque and vessel volume. Plaque volume assessed with 3D ultrasound is a more robust parameter than IMT and thus a highly sensitive means to detect plaque progression, as much as two orders of magnitude better than IMT.19-22 Plaque grows and extends longitudinally at a faster rate than it thickens. Using 3D ultrasound it is possible to utilize significantly reduced sample sizes to evaluate the impact of an intervention upon plaque progression.21 Whether 3D ultrasound can define other high-risk plaque parameters such as ulceration and other aspects of plaque morphology is being evaluated in collaboration with the Canadian Atherosclerosis Imaging Network (clinicaltrials.gov NCT01456403).23,24
Computed Tomography (CT)
Computed tomography (CT) enables accurate identification of stenosis within arterial vessels with a high degree of special resolution.25 In addition, CT offers the unique ability to characterize calcification within plaque26 and stage lesions according to their developmental phase to determine the risk of plaque rupture.27 A non-calcified lipid-rich plaque reflects plaque in the early stages of atherosclerosis.28 With remodeling, a non-calcified lesion transitions into a mixed plaque that contains calcium deposits and a lipid-rich core.28 Progressive accumulation of calcium deposits within plaque leads to the formation of a dense mature calcified plaque. Mixed and non-calcified plaques are at greatest risk for plaque erosion or rupture.29 Indeed the presence of spotty calcification in lesions evaluated with multi-slice computed tomography has been associated with acute coronary syndrome.30
Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI) offers good spatial resolution for the delineation of vascular lumen and offers advantages in demonstrating plaque structure and composition.31 MRI can accurately assess mean wall thickness and vessel wall area.32 In addition, MRI can lend insight into plaque composition including the lipid-rich core, intraplaque hemorrhage and fibrous cap.33-35 Compared to CT, MRI does not use ionizing radiation. For these reasons, MRI has been utilized as an endpoint in clinical trials to monitor disease progression and the impact of therapy.36 Novel contrast agents that may enable targeted MRI-based molecular imaging of plaque progression are under currently evaluation.37-41
Positron Emission Tomography (PET)
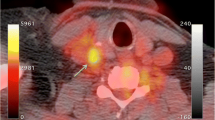
PET imaging utilizes radiolabeled ligands and tracers that may directly bind to specific targeted molecules or accumulate within specific tissue beds, thereby providing insight into active biologic metabolic processes.42 This is an important advantage as it is feasible to probe directly the in vivo expression of molecular and metabolic activity within plaque. In comparison to the anatomic imaging modalities that mainly characterize plaque structure, composition and morphology, PET can evaluate dynamic intraplaque activity such as inflammation, active plaque calcification, and other biologic processes (Figure 1).
Detection of inflamed plaque in a symptomatic patient a significantly stenotic left internal carotid artery. In transverse and coronal contrast-enhanced CT images (top row), there is evidence for significant obliteration of the lumen with little calcification on CT. Hybrid PET/CT images provide evidence for increased [18F]fluorodeoxyglucose at the site of the symptomatic lesion (bottom row). 24 (Reproduced with permission of Informa UK, Ltd.)
Hybrid PET Imaging
The spatial resolution of clinical PET imaging ranges between 3 and 5 mm.43 With such resolution, it is challenging to assess the uptake of radiotracer in small structures such as the carotid and coronary vasculature. To circumvent this limitation, hybrid imaging is performed where PET images are co-registered with either CT or more recently MRI44,45 (Figure 1). Thus, hybrid imaging may contribute toward greater sensitivity for the anatomical detection of plaque, as well as potentially increased specificity for active disease detection.
Imaging with [18F]FDG PET
“The prime cause of cancer is the replacement of the respiration of oxygen in normal body cells by a fermentation of sugar” (The Prime Cause and Prevention of Cancer, Lecture by Otto Warburg, Annual Meeting of Nobelists, Lindau, Germany, 1966). This pivotal discovery by Nobel Laureate Dr. Otto Heinrich Warburg in 1920s paved the way for future clinical application of [18F]FDG imaging. By the early 1980s, FDG was being used to distinguish between benign and malignant lesions, define metabolic activity of cancer cells and myocardium.46-48 Subsequently, one of the initial applications for imaging vascular inflammation was in the setting of Takayasu Arteritis.49 Rudd et al50 further expanded the use of FDG for evaluating atherosclerotic plaque within carotid vasculature and subsequently demonstrated that this is a highly reproducible technique (Figure 2).51
Reproducible carotid and aortic [18F]FDG uptake imaged with hybrid PET/CT over 2 weeks. A reflects carotid CT, PET, and hybrid PET/CT images demonstrating reproducible FDG uptake in the right coronary artery (arrows). Similarly, B is indicative of reproducible FDG uptake at the aortic arch and descending aorta of a patient (arrows) (Reprinted from Ref51with permission from Elsevier)
The Rationale for [18F]FDG PET Imaging of Atherosclerosis
Active inflammation within plaque has been proposed as a major criterion to identify high-risk vulnerable plaque.12 Furthermore, the inflammatory burden within ruptured plaque, as reflected by activated macrophages, is significantly increased.52 Macrophages potentiate localized inflammatory responses and are pivotal mediators of atherosclerosis. Macrophages themselves have high metabolic rates and require an equally abundant energy supply.53 In fact, in comparison to smooth muscle cells, foam cells consume significantly greater amounts of oxygen.54 This is partly due to phagocytic activity consisting of oxidative or respiratory bursts that yield superoxides and hydrogen peroxide to degrade engulfed material.55
Radiolabelled FDG is a glucose analog that is taken up by active cells to fuel in vivo metabolic processes.56 Therefore, FDG uptake may serve as a marker of metabolic activity within a specific tissue. Importantly, given that activated macrophages have high metabolic rates, localized uptake of FDG within plaque may serve as a surrogate marker of an inflamed high-risk lesion. Indeed in patients with a history of transient ischemic attacks arising from a specific carotid artery distribution, as well as significantly stenosed internal carotid artery, FDG was found to localize to macrophage-rich regions50 (Figure 3). The question that arises is whether the observed FDG activity is actually reflective of FDG taken up by macrophages, as opposed to surrounding inflammatory cells.
Tritriated deoxyglucose autoradiography of an excised plaque from a symptomatic patient establishes that silver grains accumulate between the lipid core and fibrous cap within macrophages (inset) (magnification: ×10 and ×20) (Reprinted from Ref50 with permission from Wolters Kluwer Health)
Kubota et al57have demonstrated that FDG and 2-deoxy-d-[3H]glucose (3H-DG) uptake is greatest in macrophage-rich regions assessed by macro- and micro-autoradiography in an experimental tumor-induced murine model. Moreover, cellular uptake studies also demonstrate that FDG uptake is almost three-fold greater in macrophages, as compared to tumor cells.58 These findings suggest that FDG uptake is a marker of macrophage activity and further research reveals that FDG may also be a marker of early foam cell development.59
Finally, pioneering work by Rudd et al,50 Tawakol et al,60 and others61-66 supports an association between FDG uptake quantified in human carotid plaque specimens with macrophage-specific CD68 immunohistology staining (Figure 4).
Mean within-patient [18F]fluorodeoxyglucose uptake (expressed as a target-to-background ratio) is significantly correlated with inflammation (r = 0.85; P < .001). Inflammation was defined as the percent of CD68 macrophage staining with immunohistology (Reprinted from Ref60 with permission from Elsevier)
Insights into Atherosclerotic Disease Across Vascular Beds Derived from [18F]FDG PET
Carotid Vasculature
The relationship between carotid plaque composition and FDG uptake has been evaluated in several studies.67 In terms of calcification, an inverse relationship between calcium content within carotid plaque and FDG uptake is noted.61 Lipid-rich necrotic plaque has been shown to have greater FDG uptake than collagenous or calcified plaque.68 Additionally, FDG uptake has also been related to high-risk morphological features of carotid plaque such as positive remodeling, low attenuation profile (suggestive of a lipid-rich core), and luminal irregularity (marker of plaque ulceration), as characterized with hybrid PET/CT imaging,62 inferring that FDG uptake may identify patients at higher risk of vascular events. Indeed FDG uptake within carotid vasculature has been related to cerebral micro-embolism69 and risk of stroke.70
FDG uptake in carotid vasculature has been shown to correlate with serum levels of C-reactive protein, a marker of systemic inflammation.67,71 This supports the concept of defining a “vulnerable patient” whereby the presence of inflamed plaque within one node of the arterial tree may increase the likelihood for the presence of a vulnerable lesions within other vascular beds.71 Furthermore, other pre-existing co-morbidities may contribute toward the burden of risk in certain patient populations. Patients presenting with impaired glucose tolerance and type-2 diabetes mellitus have increased carotid FDG uptake that correlates with Framingham risk score.72 Likewise, patients presenting with metabolic syndrome or with features suggestive of metabolic syndrome also have increased carotid FDG uptake.73,74
There is also evidence that suggests that symptomatic carotid lesions tend to have greater FDG uptake when compared to asymptomatic lesions, although the extent of vascular stenosis is an important determinant.50 In general, while the degree of vascular stenosis evaluated with angiography is related to FDG uptake, 25% of non-stenotic lesions detected in a vascular territory compatible with patient presentation using high-resolution MRI angiography has significantly inflamed plaques that can be imaged with FDG.75 Therefore, FDG imaging of atherosclerotic lesions may be of incremental benefit when performed in conjunction with angiography (e.g., CTA or MRA) to identify culprit lesions at high risk of rupture.75
Large Arterial Vasculature
Possibly, the first clinical experience of imaging FDG uptake in large arterial vasculature was reported by Yun et al.76 From a cohort of 156 patients referred for various clinical indications, Yun et al77 reported that 51% of subjects had evidence for FDG uptake at the level of the abdominal aorta, 51% at the iliac, and 63% at proximal femoral arteries. Along all vascular beds, age and hypercholesterolemia were correlated with the degree of FDG uptake.77 Of further interest, a variation existed among the atherosclerotic risk factors associated with FDG uptake across vascular beds.77 These risk factors included age and hypercholesterolemia (abdominal aorta and iliac arteries), hypertension (iliac arteries), and diabetes (femoral arteries).77 These findings suggest that each atherogenic risk factor may have non-uniform potency in contributing to disease progression in different vascular beds. In addition to inflammation, aging has been associated with increased aortic wall and calcification volume, as well as metabolically active inflamed lesions78-80 Female patients, patients with cardiovascular disease and those with cardiovascular risk factors have also been noted to present with increased FDG uptake or highly inflamed plaques, while diabetic patients may have more pronounced aortic calcification.81
Coronary Vasculature
Imaging of the coronary vasculature has proven more challenging due to the small size of these vessels. Confounders such as cardiac motion, myocardial FDG uptake and the spatial resolution of PET have impeded progress in imaging FDG uptake in coronary vessels. Despite such challenges, Dunphy et al82 presented what may be the first clinical experience of imaging coronary vasculature using FDG. They evaluated calcification and FDG uptake within the coronary arteries of 78 patients referred for oncology imaging82 and found that there was a significant correlation between coronary FDG uptake and abnormal myocardial perfusion in 32 patients.82 FDG uptake was also associated with cardiac risk factors, although no cardiac events were reported during a follow-up period of 7 months.82 However, 34 patients were excluded from the analysis due to severe motion artifacts. Similarly, in a larger cohort assessed by Saam et al,83 FDG uptake at the left anterior descending artery would only be assessed 55% of patients. Nonetheless, uptake was related to hypertension, coronary heart disease, body mass index, calcified plaque burden, and pericardial fat volume.
In order to suppress myocardial FDG uptake, Wykrzykowska et al84 instructed patients to consume a low-carbohydrate, high-fat meal the night prior to imaging and drink a vegetable oil-based drink on the morning of the imaging study. In 32 patients with a history of treated malignancy and who underwent both FDG PET/CT and cardiac catherization, good cardiac (muscle) FDG suppression was achieved in 63% of patients.84 Coronary FDG uptake was identified in 15 patients, and when compared to angiography results, there was a trend toward an association between anatomic disease and metabolic FDG uptake.84 Furthermore, FDG uptake in the left main coronary artery has been shown to be higher in patients presenting with acute coronary syndrome when compared to those with stable angina.85
Multi-vascular Disease
The interrelationship of atherosclerotic disease across arterial beds has also been evaluated using FDG imaging. Studies suggest that among different vascular beds, there is evidence for variable FDG uptake,82,86 while there is a positive relationship between uptake in adjacent territories and along paired left and right arterial beds.87 Multi-vascular evaluation of disease has been proposed, given that high levels of FDG uptake across major vascular beds including the aorta, iliac, and carotid arteries has been shown to be predictive of future cardiovascular events.88
In terms of identifying patients at greatest risk, older patients presenting with more cardiovascular risk factors tend to have more inflamed active and calcified inactive plaques.89 Although quantification of FDG uptake is a highly reproducible measure, it does vary over time suggesting that inflammation may be a transient feature of atherosclerosis which waxes and wanes as disease progresses.51,90-92
The Emergence of [18F]FDG in Clinical Trials
Serial FDG PET scanning has the potential to evaluate changes in inflammatory activity. Lifestyle modification induces a detectable reduction in FDG uptake within aortic and iliac vasculature.93 Likewise, reduced FDG uptake in the aorta and carotid vessels has been observed in association with Simvastatin lipid-lowering therapy94 (Figure 5). Furthermore, in patients randomized to low- and high-dose Atorvastatin therapy and followed for 6 months, high-dose therapy was associated with significantly reduced FDG uptake at the femoral artery and aorta.95 A similar reduction in inflammation has also been observed following treatment with Atorvastatin for 12 weeks.96
Reduced [18F]FDG following simvastatin therapy. Representative images of a patient on dietary management alone (top row). Three-months of dietary management alone had no impact upon FDG uptake in aortic and carotid vasculature (arrows). However, FDG uptake is visibly reduced in the carotid arteries and aortic arch following 3 months of therapy with simvastatin (middle row). Hybrid FDG PET/CT images demonstrate that following 3 months of therapy with simvastatin, there is no evidence for visible FDG uptake (bottom row) (Reprinted from Ref94with permission Elsevier)
More recently, 130 patients were randomized to either dalcetrapib (a novel HDL raising drug) or placebo to determine whether dalcetrapib modulates plaque progression and inflammation.36 Treated patients had reduced FDG uptake at the most diseased segments of carotid artery.36 Upon dividing patients into three tertiles according to total change in vessel area at 24 months, patients in the two lowest tertiles had reduced vascular inflammation observed after 6 months (P = .01).36 Thus, FDG PET may be a sensitive and specific marker to non-invasively monitor disease progression and response to therapy. Ongoing trials such as the Canadian Atherosclerosis Imaging Network (CAIN) will contribute toward validating and establishing FDG PET against advanced immunohistology, as well as develop other imaging-derived biomarkers using a multimodality approach to characterize aspects of plaque biology, disease burden, and identifying high-risk lesions.
Limitations of PET/CT Imaging
Given that FDG imaging of vasculature is a relatively new area of science and investigation, it suffers from several limitations. Although FDG uptake is related to macrophage expression, direct evidence demonstrating that FDG is taken up directly into macrophage cells is still lacking. This may be achievable in the future with higher resolution imaging and amplified immunohistology. Imaging coronary vasculature with FDG continues to be hampered by myocardial motion and myocardial FDG uptake. Acquiring respiratory- and cardiac-gated images may reduce the impact of coronary vessel motion. Furthermore, as described, patient preparation and diets may lower myocardial FDG uptake.
Serial imaging is required to accurately stage atherosclerosis as it matures, waxes, and wanes. PET and CT imaging requires exposure to radiation which currently precludes the feasibility of frequent serial imaging. Algorithms to reduce patient radiation exposure are under development and will eventually contribute toward enabling serial imaging. Hybrid PET/MRI and PET/CT imaging also require accurate image co-registration between both modalities, especially when evaluating small structures such as the internal carotid vasculature. Slight patient motion between image acquisitions can result in misalignment that can bias attenuation correction, precluding accurate co-localization, and image interpretation. The use of neck-braces and hybrid scanning systems can significantly reduce this.
Emerging PET/CT Imaging Probes for Human Atherosclerosis
With the limitations of FDG and the complexity of atherosclerotic process, other surrogate makers of inflammation have also been used in human including: [11C]PK11195, [11C]choline and 68Ga-[1,4,7,10-tetraazacyclododecane-N,N′,N″,N9′′′-tetraacetic acid]-D-Phe1, Tyr3-octreotate (DOTATATE) (Table 1). [11C]PK11195 is a selective ligand of a translocator protein that is highly expressed by macrophages.97-99 Similarly, [68Ga]DOTATATE binds to somatostatin receptors subtype 2 that are expressed by macrophages.100 The uptake of [11C]PK11195 and [68Ga]DOTATATE are considered to be indicative of macrophage density within plaque. [11C]choline differs in that it is taken up by inflammatory cells—primarily macrophages, following which it undergoes phosphorylation and is metabolized into forming phosphatidylcholine that is eventually incorporated into the cellular membrane.101 Other tracers such as Annexin-V that are currently being utilized to detect apoptosis may be more specific markers of phagocytosing macrophages are being evaluated as single-photon emission computed tomography radiotracers but can also be labeled with 18F.102-104
In addition to inflammation, calcification of plaque may also contribute toward potentiating plaque vulnerability.105-107 Hydroxyapatite is expressed in regions with active calcium deposition. [18F]sodium fluoride (NaF) binds to hydroxyapatite molecules by replacing hydroxyl groups, and could, therefore, serve as a surrogate marker of active calcification within plaque108 (Figure 6). Early findings suggest that NaF uptake imaged with PET may identify regions of active calcium deposition.109 Recently, NaF has been applied in coronary vasculature where compared to FDG, there is very little competing radiotracer uptake by myocardium resulting in high target-to-background signal.109
NaF PET/CT imaging of left and right internal carotid arteries of active calcification in a 72-year-old symptomatic patient evaluated at the University of Ottawa Heart Institute. Upper row evidence of NaF uptake with a small foci of calcification on CT in the left internal carotid symptomatic culprit vessel. There is a mismatch between the region of NaF uptake and calcification on CT. Lower row Evidence of calcium nodules with matched NaF uptake at the right internal carotid artery
These tracers are still very early in development and application. Further studies are required to establish and validate them prior to wider application in clinical research and potential future clinical application.
Integration of PET/CT Imaging Biomarkers to Understand Plaque Progression
Utilizing PET/CT imaging biomarkers, it may be possible to non-invasively stage lesions and assess different aspects of plaque progression. Among a cohort of 45 oncology patients, plaque was characterized for inflammation (FDG uptake), active mineral deposition (NaF uptake) and calcification (CT).109 Of 105 lesions that had NaF uptake, 81 (77.1%) had evidence of calcification on CT, while 18 (14.5%) had evidence of FDG uptake.109 Therefore, one could hypothesize that imaging macrophages with FDG, active calcification imaging with NaF and calcium deposition with computed tomography could be markers of independent processes, and that these markers could be utilized to stage atheroma formation, disease progression and potentially plaque and patient vulnerability.
One may speculate that in the early stages of advanced atherosclerosis, only FDG uptake would be detected as inflammation is predominant process present (Figure 7). Active calcification progressively initiates given that the inflammatory cascade contributes to calcium deposition. As inflammation peaks, early calcium deposits would also be present. From imaging, this phase of atheroma progression would be reflected by uptake of both FDG and NaF. Once the density of calcium deposits exceeds a certain threshold, these would also be visible on CT—possibly as early speckled calcification (a presumed marker of risk on CT angiography studies).30 Eventually, calcification and mineralization processes would exceed the inflammatory activity present within plaque. At this stage, there would be evidence of NaF uptake in the absence of FDG as well as calcium deposits on CT. Ongoing calcification may finally lead to development of an end-stage longstanding atheroma that is densely calcified with very little active calcium turnover. At this final stage, there would only be evidence for calcium on CT.
A proposed schematic staging inflammatory and calcification activity within atherosclerotic lesions with FDG and NaF as imaging biomarkers. During early stages of atherosclerosis, inflammation is the predominant mechanism active within plaque. During these stages, [18F]FDG may be taken up by the lesion. As inflammation peaks, the risk of plaque rupture may increase. Inflammation also contributes toward initiating calcium metabolism within lesions that results in the formation of early calcium deposits. This would be reflected by uptake of both FDG and hydroxyapatite-specific [18F]sodium fluoride (NaF). Once the density of calcium deposits exceeds a certain threshold, it becomes visible with CT. During active calcification, plaque may still be vulnerable. Eventually, the calcification and mineralization processes exceed the inflammatory activity present within plaque, which might be demarcated by only NaF uptake (in the absence of FDG), as well as calcium deposits on CT. Ongoing calcification eventually leads to forming an end-stage stable atheroma that is densely calcified with only evidence for calcium on CT. Model of plaque progression (top bar) is adapted from Koenig and Khuseyinova115
Conclusion
Considerable advancement has occurred in imaging atherosclerosis, with FDG PET/CT at the forefront for characterizing actively inflamed plaque. FDG uptake within plaque has transitioned from an incidental finding to a potential biomarker of vulnerable plaque that has even been implemented to evaluate the efficacy of vascular risk reduction therapy. Despite the proliferation of imaging research, significant progress is yet to be achieved in fully understanding the molecular biology of plaque progression and rupture. Recent pre-clinical developments may help in understanding how FDG and other novel tracers track inflammation in plaque.42,110,111
While FDG imaging has enhanced our understanding of the inflammatory processes that underlie atherosclerosis, further studies are needed to validate FDG uptake. It also remains to be determined whether FDG uptake within plaque could be a specific marker of activated, polarized M1 (pro-inflammatory) or M2 (anti-inflammatory) macrophages,112 as validated by advanced immunohistology. Furthermore, whether FDG uptake is truly predictive of future cardiovascular events and outcomes remains to be determined. Also, while reductions in FDG uptake have been observed following therapies that modify vascular risk, to date, a reduction in subsequent downstream vascular events has only been demonstrated in small observational studies. Prospective trials will further yield insight into the role of FDG to detect inflamed carotid plaque, as validated by advanced immunohistochemistry in patients with high-risk carotid artery disease36,113 and its role in imaging other vascular beds as in the BIOIMAGE study.114 Furthermore, although FDG has advantages in light of its widespread availability and is currently the PET/CT imaging biomarker of choice for large trials evaluating plaque inflammation and progression, whether it will be the optimal PET/CT plaque imaging biomarker remains to determined.
While we have moved far beyond da Vinci’s initial gross observations 500 years ago to visualizing inherent processes within plaque, we still need to bridge the gap between translating imaging of atherosclerosis to accurately predicting life-threatening vascular events. At that point, we may begin to realize imaging-guided personalized care for patients with atherosclerosis.
References
Keele KD. Leonardo da Vinci’s views on arteriosclerosis. Med Hist 1973;17:304-8.
Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997;349:1498-504.
Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011;123:933-44.
Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431-7.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4-14.
Ross R. Atherosclerosis is an inflammatory disease. Am Heart J 1999;138:S419-20.
Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 1986;6:131-8.
Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med 2011;17:1410-22.
Falk E. Coronary thrombosis: Pathogenesis and clinical manifestations. Am J Cardiol 1991;68:28B-35B.
Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol 2010;30:1282-92.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003;108:1664-72.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation 2003;108:1772-8.
Coli S, Magnoni M, Sangiorgi G, Marrocco-Trischitta MM, Melisurgo G, Mauriello A, et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: Correlation with histology and plaque echogenicity. J Am Coll Cardiol 2008;52:223-30.
Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation 2006;113:2245-52.
Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316-25.
Staub D, Patel MB, Tibrewala A, Ludden D, Johnson M, Espinosa P, et al. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke 2010;41:41-7.
Sirlin CB, Lee YZ, Girard MS, Peterson TM, Steinbach GC, Baker KG, et al. Contrast-enhanced B-mode US angiography in the assessment of experimental in vivo and in vitro atherosclerotic disease. Acad Radiol 2001;8:162-72.
Kono Y, Pinnell SP, Sirlin CB, Sparks SR, Georgy B, Wong W, et al. Carotid arteries: Contrast-enhanced US angiography—preliminary clinical experience. Radiology 2004;230:561-8.
Spence JD. Ultrasound measurement of carotid plaque as a surrogate outcome for coronary artery disease. Am J Cardiol 2002;89:10B-5B. (discussion 5B-6B).
Spence JD. Carotid plaque measurement is superior to IMT Invited editorial comment on: Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis—Yoichi Inaba, M.D., Jennifer A. Chen M.D., Steven R. Bergmann M.D., Ph.D. Atherosclerosis 2012;220:34-5.
Ainsworth CD, Blake CC, Tamayo A, Beletsky V, Fenster A, Spence JD. 3D ultrasound measurement of change in carotid plaque volume: A tool for rapid evaluation of new therapies. Stroke 2005;36:1904-9.
Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: A tool for targeting and evaluating vascular preventive therapy. Stroke 2002;33:2916-22.
Tardif JC, Lesage F, Harel F, Romeo P, Pressacco J. Imaging biomarkers in atherosclerosis trials. Circ Cardiovasc Imaging 2011;4:319-33.
Bogiatzi C, Cocker MS, Beanlands RS, Spence JD. Identifying high-risk asymptomatic carotid stenosis. Exp Opin Med Diagn 2012;6:139-51.
Leber AW, Becker A, Knez A, von Ziegler F, Sirol M, Nikolaou K, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: A comparative study using intravascular ultrasound. J Am Coll Cardiol 2006;47:672-7.
Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography). Circulation 2007;115:402-26.
Schuijf JD, Beck T, Burgstahler C, Jukema JW, Dirksen MS, de Roos A, et al. Differences in plaque composition and distribution in stable coronary artery disease versus acute coronary syndromes; non-invasive evaluation with multi-slice computed tomography. Acute Card Care 2007;9:48-53.
Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995;92:1355-74.
Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: The pathology of unstable coronary lesions. J Interv Cardiol 2002;15:439-46.
Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319-26.
Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: Comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005;112:3437-44.
Yuan C, Beach KW, Smith LH Jr, Hatsukami TS. Measurement of atherosclerotic carotid plaque size in vivo using high resolution magnetic resonance imaging. Circulation 1998;98:2666-71.
Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol 2005;25:234-9.
Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000;102:959-64.
Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001;104:2051-6.
Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): A randomised clinical trial. Lancet 2011;378:1547-59.
Flacke S, Fischer S, Scott MJ, Fuhrhop RJ, Allen JS, McLean M, et al. Novel MRI contrast agent for molecular imaging of fibrin: Implications for detecting vulnerable plaques. Circulation 2001;104:1280-5.
Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant HDL-like nanoparticles: A specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc 2004;126:16316-7.
Metz S, Beer AJ, Settles M, Pelisek J, Botnar RM, Rummeny EJ, et al. Characterization of carotid artery plaques with USPIO-enhanced MRI: Assessment of inflammation and vascularity as in vivo imaging biomarkers for plaque vulnerability. Int J Cardiovasc Imaging 2011;27:901-12.
Trivedi RA, Mallawarachi C. JM UK-I, Graves MJ, Horsley J, Goddard MJ et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol 2006;26:1601-6.
Kanwar RK, Chaudhary R, Tsuzuki T, Kanwar JR. Emerging engineered magnetic nanoparticulate probes for targeted MRI of atherosclerotic plaque macrophages. Nanomedicine (Lond) 2012;7:735-49.
Small GR, Ruddy TD. PET imaging of aortic atherosclerosis: Is combined imaging of plaque anatomy and function an amaranthine quest or conceivable reality? J Nucl Cardiol 2011;18:717-28.
Saha GB (2010) Basics of PET imaging: physics, chemistry, and regulations, 2nd edn. Springer, New York.
Di Carli MF, Hachamovitch R. Hybrid PET/CT is greater than the sum of its parts. J Nucl Cardiol 2008;15:118-22.
Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, et al. Simultaneous PET-MRI: A new approach for functional and morphological imaging. Nat Med 2008;14:459-65.
Yonekura Y, Benua RS, Brill AB, Som P, Yeh SD, Kemeny NE, et al. Increased accumulation of 2-deoxy-2-[18F]fluoro-d-glucose in liver metastases from colon carcinoma. J Nucl Med 1982;23:1133-7.
Di Chiro G, DeLaPaz RL, Brooks RA, Sokoloff L, Kornblith PL, Smith BH, et al. Glucose utilization of cerebral gliomas measured by [18F] fluorodeoxyglucose and positron emission tomography. Neurology 1982;32:1323-9.
Marshall RC, Tillisch JH, Phelps ME, Huang SC, Carson R, Henze E, et al. Identification and differentiation of resting myocardial ischemia and infarction in man with positron computed tomography, 18F-labeled fluorodeoxyglucose and N-13 ammonia. Circulation 1983;67:766-78.
Theron J, Tyler JL. Takayasu’s arteritis of the aortic arch: Endovascular treatment and correlation with positron emission tomography. AJNR Am J Neuroradiol 1987;8:621-6.
Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708-11.
Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: Implications for atherosclerosis therapy trials. J Am Coll Cardiol 2007;50:892-6.
Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005;25:2054-61.
Newsholme P, Gordon S, Newsholme EA. Rates of utilization and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages. Biochem J 1987;242:631-6.
Bjornheden T, Bondjers G. Oxygen consumption in aortic tissue from rabbits with diet-induced atherosclerosis. Arteriosclerosis 1987;7:238-47.
Babior BM. The respiratory burst of phagocytes. J Clin Invest 1984;73:599-601.
Phelps ME, Hoffman EJ, Selin C, Huang SC, Robinson G, MacDonald N, et al. Investigation of [18F]2-fluoro-2-deoxyglucose for the measure of myocardial glucose metabolism. J Nucl Med 1978;19:1311-9.
Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: High accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992;33:1972-80.
Kim SL, Kim EM, Cheong SJ, Lee CM, Kim DW, Jeong HJ, et al. The effect of PPAR-gamma agonist on (18)F-FDG uptake in tumor and macrophages and tumor cells. Nucl Med Biol 2009;36:427-33.
Ogawa M, Nakamura S, Saito Y, Kosugi M, Magata Y. What can be seen by 18F-FDG PET in atherosclerosis imaging? The effect of foam cell formation on 18F-FDG uptake to macrophages in vitro. J Nucl Med 2012;53:55-8.
Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818-24.
Menezes LJ, Kotze CW, Agu O, Richards T, Brookes J, Goh VJ, et al. Investigating vulnerable atheroma using combined (18)F-FDG PET/CT angiography of carotid plaque with immunohistochemical validation. J Nucl Med 2011;52:1698-703.
Figueroa AL, Subramanian SS, Cury RC, Truong QA, Gardecki JA, Tearney GJ, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: A comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging 2012;5:69-77.
Masteling MG, Zeebregts CJ, Tio RA, Breek JC, Tietge UJ, de Boer JF, et al. High-resolution imaging of human atherosclerotic carotid plaques with micro 18F-FDG PET scanning exploring plaque vulnerability. J Nucl Cardiol 2011;18:1066-75.
Pedersen SF, Graebe M, Fisker Hag AM, Hojgaard L, Sillesen H, Kjaer A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun 2010;31:423-9.
Graebe M, Pedersen SF, Borgwardt L, Hojgaard L, Sillesen H, Kjaer A. Molecular pathology in vulnerable carotid plaques: Correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET). Eur J Vasc Endovasc Surg 2009;37:714-21.
Cocker MS, Hammond R, Spence JD, Mc Ardle B, Dekemp R, Brennan J, et al. Abstract 17612: Is glucose corrected [18f]-fluorodeoxyglucose uptake in human carotid plaque associated with the extent of inflammation on immunohistology? A sub-study of the Canadian atherosclerosis imaging network (CAIN). Circulation 2012 (in press).
Choi YS, Youn HJ, Chung WB, Hwang HJ, Lee DH, Park CS, et al. Uptake of F-18 FDG and ultrasound analysis of carotid plaque. J Nucl Cardiol 2011;18:267-72.
Silvera SS, Aidi HE, Rudd JH, Mani V, Yang L, Farkouh M, et al. Multimodality imaging of atherosclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis 2009;207:139-43.
Moustafa RR, Izquierdo-Garcia D, Fryer TD, Graves MJ, Rudd JH, Gillard JH, et al. Carotid plaque inflammation is associated with cerebral microembolism in patients with recent transient ischemic attack or stroke: A pilot study. Circ Cardiovasc Imaging 2010;3:536-41.
Grandpierre S, Desandes E, Meneroux B, Djaballah W, Mandry D, Netter F, et al. Arterial foci of F-18 fluorodeoxyglucose are associated with an enhanced risk of subsequent ischemic stroke in cancer patients: A case-control pilot study. Clin Nucl Med 2011;36:85-90.
Yang SJ, Kim S, Choi HY, Kim TN, Yoo HJ, Seo JA, et al. High-sensitivity C-reactive protein in the low- and intermediate-Framingham risk score groups: Analysis with (18)F-fluorodeoxyglucose positron emission tomography. Int J Cardiol 2011 (in press).
Kim TN, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, et al. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: Analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging 2010;3:142-8.
Tahara N, Kai H, Nakaura H, Mizoguchi M, Ishibashi M, Kaida H, et al. The prevalence of inflammation in carotid atherosclerosis: Analysis with fluorodeoxyglucose-positron emission tomography. Eur Heart J 2007;28:2243-8.
Tahara N, Kai H, Yamagishi S, Mizoguchi M, Nakaura H, Ishibashi M, et al. Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol 2007;49:1533-9.
Davies JR, Rudd JH, Fryer TD, Graves MJ, Clark JC, Kirkpatrick PJ, et al. Identification of culprit lesions after transient ischemic attack by combined 18F fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke 2005;36:2642-7.
Yun M, Yeh D, Araujo LI, Jang S, Newberg A, Alavi A. F-18 FDG uptake in the large arteries: A new observation. Clin Nucl Med 2001;26:314-9.
Yun M, Jang S, Cucchiara A, Newberg AB, Alavi A. 18F FDG uptake in the large arteries: A correlation study with the atherogenic risk factors. Semin Nucl Med 2002;32:70-6.
Bural GG, Torigian DA, Botvinick E, Houseni M, Basu S, Chen W, et al. A pilot study of changes in (18)F-FDG uptake, calcification and global metabolic activity of the aorta with aging. Hell J Nucl Med 2009;12:123-8.
Bural GG, Torigian DA, Chamroonrat W, Alkhawaldeh K, Houseni M, El-Haddad G, et al. Quantitative assessment of the atherosclerotic burden of the aorta by combined FDG-PET and CT image analysis: A new concept. Nucl Med Biol 2006;33:1037-43.
Stout RW. Ageing and atherosclerosis. Age Ageing 1987;16:65-72.
Tatsumi M, Cohade C, Nakamoto Y, Wahl RL. Fluorodeoxyglucose uptake in the aortic wall at PET/CT: Possible finding for active atherosclerosis. Radiology 2003;229:831-7.
Dunphy MP, Freiman A, Larson SM, Strauss HW. Association of vascular 18F-FDG uptake with vascular calcification. J Nucl Med 2005;46:1278-84.
Saam T, Rominger A, Wolpers S, Nikolaou K, Rist C, Greif M, et al. Association of inflammation of the left anterior descending coronary artery with cardiovascular risk factors, plaque burden and pericardial fat volume: A PET/CT study. Eur J Nucl Med Mol Imaging 2010;37:1203-12.
Wykrzykowska J, Lehman S, Williams G, Parker JA, Palmer MR, Varkey S, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med 2009;50:563-8.
Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, et al. Feasibility of FDG imaging of the coronary arteries: Comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging 2010;3:388-97.
Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: Carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med 2008;49:871-8.
Rudd JH, Myers KS, Bansilal S, Machac J, Woodward M, Fuster V, et al. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: A prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging 2009;2:107-15.
Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med 2009;50:1611-20.
Wasselius J, Larsson S, Sundin A, Jacobsson H. Assessment of inactive, active and mixed atherosclerotic plaques by 18F-FDG-PET; an age group-based correlation with cardiovascular risk factors. Int J Cardiovasc Imaging 2009;25:133-40.
Ben-Haim S, Kupzov E, Tamir A, Frenkel A, Israel O. Changing patterns of abnormal vascular wall F-18 fluorodeoxyglucose uptake on follow-up PET/CT studies. J Nucl Cardiol 2006;13:791-800.
Menezes LJ, Kayani I, Ben-Haim S, Hutton B, Ell PJ, Groves AM. What is the natural history of 18F-FDG uptake in arterial atheroma on PET/CT? Implications for imaging the vulnerable plaque. Atherosclerosis 2010;211:136-40.
Wasselius J, Larsson S, Jacobsson H. Time-to-time correlation of high-risk atherosclerotic lesions identified with [(18)F]-FDG-PET/CT. Ann Nucl Med 2009;23:59-64.
Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med 2008;49:1277-82.
Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: Evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006;48:1825-31.
Ishii H, Nishio M, Takahashi H, Aoyama T, Tanaka M, Toriyama T, et al. Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: A randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin Ther 2010;32:2337-47.
Wu YW, Kao HL, Huang CL, Chen MF, Lin LY, Wang YC, et al. The effects of 3-month atorvastatin therapy on arterial inflammation, calcification, abdominal adipose tissue and circulating biomarkers. Eur J Nucl Med Mol Imaging 2012;39:399-407.
Fujimura Y, Hwang PM, Trout Iii H, Kozloff L, Imaizumi M, Innis RB. Increased peripheral benzodiazepine receptors in arterial plaque of patients with atherosclerosis: An autoradiographic study with [(3)H]PK 11195. Atherosclerosis 2008;201:108-11.
Gaemperli O, Shalhoub J, Owen DR, Lamare F, Johansson S, Fouladi N, et al. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J 2012;33:1902-10.
Lamare F, Hinz R, Gaemperli O, Pugliese F, Mason JC, Spinks T, et al. Detection and quantification of large-vessel inflammation with 11C-(R)-PK11195 PET/CT. J Nucl Med 2011;52:33-9.
Rominger A, Saam T, Vogl E, Ubleis C, la Fougere C, Forster S, et al. In vivo imaging of macrophage activity in the coronary arteries using 68 Ga-DOTATATE PET/CT: Correlation with coronary calcium burden and risk factors. J Nucl Med 2010;51:193-7.
Kato K, Schober O, Ikeda M, Schafers M, Ishigaki T, Kies P, et al. Evaluation and comparison of 11C-choline uptake and calcification in aortic and common carotid arterial walls with combined PET/CT. Eur J Nucl Med Mol Imaging 2009;36:1622-8.
Laufer EM, Winkens HM, Corsten MF, Reutelingsperger CP, Narula J, Hofstra L. PET and SPECT imaging of apoptosis in vulnerable atherosclerotic plaques with radiolabeled Annexin A5. Q J Nucl Med Mol Imaging 2009;53:26-34.
Kietselaer BL, Reutelingsperger CP, Heidendal GA, Daemen MJ, Mess WH, Hofstra L, et al. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med 2004;350:1472-3.
Jaffer FA, Libby P, Weissleder R. Molecular and cellular imaging of atherosclerosis: Emerging applications. J Am Coll Cardiol 2006;47:1328-38.
Hoshino T, Chow LA, Hsu JJ, Perlowski AA, Abedin M, Tobis J, et al. Mechanical stress analysis of a rigid inclusion in distensible material: A model of atherosclerotic calcification and plaque vulnerability. Am J Physiol Heart Circ Physiol 2009;297:H802-10.
Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 1996;93:1354-63.
Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: An intravascular ultrasound study. Circulation 2004;110:3424-9.
Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, Sajdak R, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med 2010;51:1813-20.
Derlin T, Toth Z, Papp L, Wisotzki C, Apostolova I, Habermann CR, et al. Correlation of inflammation assessed by 18F-FDG PET, active mineral deposition assessed by 18F-fluoride PET, and vascular calcification in atherosclerotic plaque: A dual-tracer PET/CT study. J Nucl Med 2011;52:1020-7.
Silvola JM, Saraste A, Laitinen I, Savisto N, Laine VJ, Heinonen SE, et al. Effects of age, diet, and type 2 diabetes on the development and FDG uptake of atherosclerotic plaques. JACC Cardiovasc Imaging 2011;4:1294-301.
Davies JR, Rudd JH, Weissberg PL, Narula J. Radionuclide imaging for the detection of inflammation in vulnerable plaques. J Am Coll Cardiol 2006;47:C57-68.
Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: A question of balance. Arterioscler Thromb Vasc Biol 2009;29:1419-23.
Tardif JC, Spence JD, Heinonen TM, Moody A, Pressacco J, Frayne R et al. Atherosclerosis imaging and the Canadian Atherosclerosis Imaging Network (CAIN). Can J Cardiol 2012 (in press).
Muntendam P, McCall C, Sanz J, Falk E, Fuster V. The BioImage Study: Novel approaches to risk assessment in the primary prevention of atherosclerotic cardiovascular disease—study design and objectives. Am Heart J 2010;160:e1.
Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol 2007;27:15-26.
Derlin T, Habermann CR, Lengyel Z, Busch JD, Wisotzki C, Mester J, et al. Feasibility of 11C-acetate PET/CT for imaging of fatty acid synthesis in the atherosclerotic vessel wall. J Nucl Med 2011;52:1848-54.
Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012;125:76-86.
Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J, et al. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med 2010;51:862-5.
Derlin T, Wisotzki C, Richter U, Apostolova I, Bannas P, Weber C, et al. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: Correlation with atherogenic risk factors. J Nucl Med 2011;52:362-8.
Acknowledgments
This work was supported in part by the Canadian Atherosclerosis Imaging Network (CAIN) (CIHR Grant #CRI-88057)113 and the Molecular Function and Imaging (MFI) Program [Program Grant from the Heart and Stroke Foundation of Ontario (HSFO Grant #PRG6242)]. M.S.C. is a Research Fellow of the Heart and Stoke Foundation of Canada. R.S.B. is a Career Investigator supported by the HSFO and a tier 1 University of Ottawa Chair in Cardiovascular Research. B.M. is supported in part by the MFI HSFO Program Grant (#PRG6242).
Conflict of interest
RSB and RdK are consultants with Jubilant DRAXImage and have received Grant funding from a government/industry program (partners: GE Healthcare, Nordion, Lantheus Medical Imaging, DRAXImage). RSB is a consultant for Lantheus Medical Imaging.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cocker, M.S., Mc Ardle, B., Spence, J.D. et al. Imaging atherosclerosis with hybrid [18F]fluorodeoxyglucose positron emission tomography/computed tomography imaging: What Leonardo da Vinci could not see. J. Nucl. Cardiol. 19, 1211–1225 (2012). https://doi.org/10.1007/s12350-012-9631-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-012-9631-9