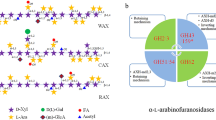
Abstract
Two α-arabinofuranosidases from the fungus Chrysoporthe cubensis COAD 3356 were partially purified, identified, characterized, and applied to the sugarcane bagasse saccharification to evaluate the potential of these enzymes to increase the sugar production from lignocellulosic biomass. The α-arabinofuranosidases were classified on GH51 (α-Ara1) and GH54/CBM42 (α-Ara2) families. After sugarcane bagasse saccharification, using the commercial cellulase-rich cocktail supplemented with α-Ara2 (15 U/g), there was an increase of 1.6, 3.9, and 6.1 times in the release of glucose, xylose, and arabinose, respectively. On the other hand, there was no increase in sugar release with α-Ara1 supplementation under the same saccharification conditions. The enzymes presented maximum activity at pH 4.0, and 60 °C. Both α-Ara1 and α-Ara2 were thermostable at 50 °C, presenting half-life values of 68 and 77 h, respectively. The enzyme α-Ara2 presented higher KMapp for synthetic substrate ρNP-α-arabinofuranoside (1.38 mmol/L) and wheat arabinoxylan (1.28 mmol/L) when compared with α-Ara1. A new fungal α-arabinofuranosidase structure, still little described in the GH51 family, was predicted. Furthermore, the results indicated that α-Ara2 is a promising molecule to be used to supplement cocktails for lignocellulose degradation.
Graphical Abstract




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Brosa M, Yelle DJ, Serwańska K (2022) Bioethanol production from lignocellulosic biomass — challenges and solutions. Molecules 27(24):8717. https://doi.org/10.3390/molecules27248717
de Albuquerque MFG, Leal TF, Ázar RISL, Milagres AMF, Guimarães VM, de Rezende ST (2021) Hydrothermal pretreatment as a strategy for the improvement of sugarcane bagasse saccharification by fungal enzyme blend. Braz Arch Biol Technol 64:e21200422. https://doi.org/10.1590/1678-4324-2021200422
De Albuquerque MFG, Guimarães VM, De Rezende ST (2021) Use of sugar beet flour and wheat bran as carbon source improves the efficiency of Chrysoporthe cubensis enzymes in sugarcane bagasse saccharification. Bioenergy Res 14:1147–1160. https://doi.org/10.1007/s12155-020-10224-6
Dutra TR, Guimarães VM, Varela EM (2017) A Chrysoporthe cubensis enzyme cocktail produced from a low-cost carbon source with high biomass hydrolysis efficiency. Sci Rep 1–9. https://doi.org/10.1038/s41598-017-04262-y
Patel H, Chapla D, Divecha J, Shah A (2015) Improved yield of α-L-arabinofuranosidase by newly isolated Aspergillus niger ADH-11 and synergistic effect of crude enzyme on saccharification of maize stover. Bioresour Bioprocess 2:1–14. https://doi.org/10.1186/s40643-015-0039-7
Xin D, Chen X, Wen P, Zhang J (2019) Insight into the role of α - arabinofuranosidase in biomass hydrolysis : cellulose digestibility and inhibition by xylooligomers. Biotechnol Biofuels 12:1–11. https://doi.org/10.1186/s13068-019-1412-0
Li X, Dilokpimol A, Kabel MA (2022) De Vries, RP (2022) Fungal xylanolytic enzymes: diversity and applications. Bioresour Technol 344:126–290. https://doi.org/10.1016/j.biortech.2021.126290
Poria V, Saini JK, Singh S, Nain L, Kuhad RC (2020) Arabinofuranosidases: characteristics, microbial production, and potential in waste valorization and industrial applications. Bioresour Technol 304:123019. https://doi.org/10.1016/j.biortech.2020.123019
Temer B, Terrasan CRF, Carmona EC (2014) a-L-Arabinofuranosidase from Penicillium janczewskii: Production with brewers spent grain and orange waste. Afri J Biotechnol 13:1796–1806. https://doi.org/10.5897/AJB2013.13361
Numan MT, Bhosle NB (2006) α-L-arabinofuranosidases: the potential applications in biotechnology. J Ind Microbiol Biotechnol 33:247–260. https://doi.org/10.1007/s10295-005-0072-1
Falkoski DL, Guimarães VM, de Almeida MN, Alfenas ACA, Colodette JL, de Rezende ST (2013) Chrysoporthe cubensis: a new source of cellulases and hemicellulases to application in biomass saccharification processes. Bioresour Technol 130:296–305. https://doi.org/10.1016/j.biortech.2012.11.140
Maitan-Alfenas GP, Michael E, Ferreira R, Ris B, Nogueira G, Galvão G et al (2015) The influence of pretreatment methods on saccharification of sugarcane bagasse by an enzyme extract from Chrysoporthe cubensis and commercial cocktails : a comparative study. Bioresour Technol 192:670–676. https://doi.org/10.1016/j.biortech.2015.05.109
Gomes KS, Maitan-Alfenas GP, de Andrade LGA, Falkoski DL, Guimarães VM, Alfenas AC et al (2017) Purification and characterization of xylanases from the fungus Chrysoporthe cubensis for production of xylooligosaccharides and fermentable sugars. Appl Biochem Biotechnol 182:818–830. https://doi.org/10.1007/s12010-016-2364-5
de Andrade LGA, Maitan-Alfenas GP, Morgan T, Gomes KS, Falkoski DL, Alfenas RF et al (2017) Sugarcane bagasse saccharification by purified β-glucosidases from Chrysoporthe cubensis. Biocatal Agric Biotechnol 12:199–205. https://doi.org/10.1016/j.bcab.2017.10.007
Tavares MP, Morgan T, Gomes RF, Mendes JPR, Castro-Borges W, Maitan-Alfenas GP, Guimarães VM (2024) Comparative analysis of Chrysoporthe cubensis exoproteomes and their specificity for saccharification of sugarcane bagasse. Enzyme Microb Technol 173–110365. https://doi.org/10.1016/j.enzmictec.2023.110365
Tavares MP, Morgan T, Gomes RF, Rodrigues MQRB, Castro-borges W, de Rezende ST, Mendes TAO, Guimarães VM (2021) Secretomic insight into the biomass hydrolysis potential of the phytopathogenic fungus Chrysoporthe cubensis. J Proteomics 236:1–14. https://doi.org/10.1016/j.jprot.2021.104121
Canilha L, Chandel AK, Milessi TSDS et al (2012) Bioconversion of sugarcane biomass into ethanol: an overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J Biomed Biotechnol 2012:1–15. https://doi.org/10.1155/2012/989572
Thite VS, Nerurkar AS (2019) Valorization of sugarcane bagasse by chemical pretreatment and enzyme mediated deconstruction. Sci Rep 9:15904. https://doi.org/10.1038/s41598-019-52347-7
Kellock M, Rahikainen J, Marjamaa K, Kruus K (2017) Ligninderived inhibition of monocomponent cellulases and a xylanase in the hydrolysis of lignocellulosics. Bioresour Technol 232:183–191. https://doi.org/10.1016/j.biortech.2017.01.072
Cardona CA, Quintero JA, Paz IC (2010) Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour Technol 101:4754–4766. https://doi.org/10.1016/j.biortech.2009.10.097
Alvira P, Negro MJ, Ballesteros M (2019) Effect of endoxylanase and alpha-L-arabinofuranosidase supplementation on the enzymatic hydrolysis of steam exploded wheat straw. Bioresour Technol 102(6):4552–4558. https://doi.org/10.1016/j.biortech.2010.12.112
Cintra LC, Costa IC, Oliveira ICM, Fernandes AG, Faria SP, Jesuíno RSA, Ravanal MC, Eyzaguirre J, Ramos LP, Faria FP, Ulhoa CJ (2020) The boosting effect of recombinant hemicellulases on the enzymatic hydrolysis of steam-treated sugarcane bagasse. Enzyme Microb Technol 133. https://doi.org/10.1016/j.enzmictec.2019.109447
Gao D, Uppugundla N, Chundawat SPS, Yu X, Hermanson S, Gowda K et al (2011) Hemicellulases and auxiliary enzymes for improved conversion of lignocellulosic biomass to monosaccharides. Biotechnol Biofuels 4(1):5. https://doi.org/10.1186/1754-6834-4-5
Zhang J, Siika-aho M, Tenkanen M, Viikari L (2011) The role of acetyl xylan esterase in the solubilization of xylan and enzymatic hydrolysis of wheat straw and giant reed. Biotechnol Biofuels 4:1–60. https://doi.org/10.1186/1754-6834-4-60
Selig MJ, Thygesen LG, Felby C, Master ER (2015) Debranching of soluble wheat arabinoxylan dramatically enhances recalcitrant binding to cellulose. Biotechnol Lett 37:633–641. https://doi.org/10.1007/s10529-014-1705-0
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85. https://doi.org/10.1016/0003-2697(85)90442-7
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Miller GL (1959) Use of dinitrosalicyclic reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Bischoff KM, de Rezende ST, Larson TM, Liu S, Hughes SR, Rich JO (2011) Purification and characterization of arabinofuranosidase from the corn endophyte Acremonium zeae. Biotechnol Lett 33:2013–2018. https://doi.org/10.1007/s10529-011-0658-9
Kaneko S, Arimoto M, Ohba M, Kobayashi H, Ishii T, Kusakabe I (1998) Purification and substrate specificities of two α-l-arabinofuranosidases from Aspergillus awamori IFO 4033. Appl Environ Microbiol 64:4021–7. https://doi.org/10.1128/2Faem.64.10.4021-4027.1998
Terrone CC, Nascimento JMF, Terrasan CRF, Brienzo M, Carmona EC (2020) Salt-tolerant α-arabinofuranosidase from a new specie Aspergillus hortai CRM1919: production in acid conditions, purification, characterization and application on xylan hydrolysis. Biocatal Agric Biotechnol 23. https://doi.org/10.1016/j.bcab.2019.101460
Lavarack BP, Griffin GJ, Rodman D (2002) The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenergy 23(5):367–380. https://doi.org/10.1016/S0961-9534(02)00066-1
Rezende CA, De LMA, Maziero P, Ribeiro E (2011) Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels 4:54. https://doi.org/10.1186/1754-6834-4-54
Faria SP, Ramos G, Melo D, Cardoso L, Pereira L, Santos R et al (2020) Production of cellulases and xylanases by Humicola grisea var. thermoidea and application in sugarcane bagasse arabinoxylan hydrolysis. Ind Crops Prod 158:1–11. https://doi.org/10.1016/j.indcrop.2020.112968
Lagaert S, Pollet A, Courtin CM, Volckaert G (2014) β-xylosidases and α-L-arabinofuranosidases: accessory enzymes for arabinoxylan degradation. Biotechnol Adv 32(2):316–332. https://doi.org/10.1016/j.biotechadv.2013.11.005
Tu T, Li X, Meng K, Bai Y, Wang Y, Wang Z et al (2019) A GH51 α-l-arabinofuranosidase from Talaromyces leycettanus strain JCM12802 that selectively drives synergistic lignocellulose hydrolysis. Microb Cell Fact 18:3–10. https://doi.org/10.1186/s12934-019-1192-z
Long L, Sun L, Lin Q, Ding S, St John FJ (2020) Characterization and functional analysis of two novel thermotolerant α-l-arabinofuranosidases belonging to glycoside hydrolase family 51 from Thielavia terrestris and family 62 from Eupenicillium parvum. Appl Microbiol Biotechnol 104:8719–8733. https://doi.org/10.1007/s00253-020-10867-7
Motta MLL, Filho JAF, de Melo RR et al (2021) A novel fungal metal-dependent α-L-arabinofuranosidase of family 54 glycoside hydrolase shows expanded substrate specificity. Sci Rep 11:10961. https://doi.org/10.1038/s41598-021-90490-2
Gilead S, Shoham Y (1995) Purification and characterization of alpha-L-arabinofuranosidase from Bacillus stearothermophilus T-6. Appl Environ Microbiol 61(1):170–174. https://doi.org/10.1128/aem.61.1.170-174.1995
Miyanaga A, Koseki T, Matsuzawa H, Wakagi T, Shoun H, Fushinobu S (2004) Crystal structure of a family 54 a-L-arabinofuranosidase reveals a novel carbohydrate-binding module that can bind arabinose. J Biol Chem 279:44907–44914. https://doi.org/10.1074/jbc.M405390200
Carli S, Meleiro LP, Salgado JCS, Ward RJ (2022) Synthetic carbohydrate - binding module - endogalacturonase chimeras increase catalytic efficiency and saccharification of lignocellulose residues. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02716-6
Gonçalves TA, Damásio ARL, Segato F, Alvarez TM, Bragatto J, Brenelli LB et al (2012) Functional characterization and synergic action of fungal xylanase and arabinofuranosidase for production of xylooligosaccharides. Bioresource Technol 119:293–299. https://doi.org/10.1016/j.biortech.2012.05.062
Coconi Linares N, Li X, Dilokpimol A, de Vries RP (2022) Comparative characterization of nine novel GH51, GH54 and GH62 α-l-arabinofuranosidases from Penicillium subrubescens. FEBS Lett 596:360–368. https://doi.org/10.1002/1873-3468.14278
Wu X, Zhang S, Zhao S, Dai L, Huang S, Liu X et al (2022) Functional specificity of three α-arabinofuranosidases from different glycoside hydrolase families in Aspergillus niger An76. J Agric Food Chem 70(16):5039–5048. https://doi.org/10.1021/acs.jafc.1c08388
Qiao J, Cui H, Wang M, Fu X, Wang X, Li X et al (2022) Integrated biorefinery approaches for the industrialization of cellulosic ethanol fuel. Bioresource Technol 360:127516. https://doi.org/10.1016/j.biortech.2022.127516
Karnchanatat A, Petsom A, Sangvanich P, Piapukiew J, Whalley AJS, Reynolds CD et al (2008) A novel thermostable endoglucanase from the wood-decaying fungus Daldinia eschscholzii (Ehrenb.:Fr.) Rehm. Enzyme Microb Technol 42:404–413. https://doi.org/10.1016/j.enzmictec.2007.11.009
Ribeiro T, Santos-Silva T, Alves VD, Dias FMV, Luís AS, Prates JAM et al (2010) Family 42 carbohydrate-binding modules display multiple arabinoxylan-binding interfaces presenting different ligand affinities. Biochim Biophys Acta - Proteins Proteom 1804:2054–2062. https://doi.org/10.1016/j.bbapap.2010.07.006
De Wet BJM, Matthew MKA, Storbeck KH, Van Zyl WH, Prior BA (2008) Characterization of a family 54 α-L-arabinofuranosidase from Aureobasidium pullulans. Appl Microbiol Biotechnol 77:975–983. https://doi.org/10.1007/s00253-007-1235-y
Sakamoto T, Ogura A, Inui M, Tokuda S, Hosokawa S, Ihara H et al (2011) Identification of a GH62 α-l-arabinofuranosidase specific for arabinoxylan produced by Penicillium chrysogenum. Appl Microbiol Biotechnol 90:137–146. https://doi.org/10.1007/s00253-010-2988-2
Fujimoto Z (2013) Structure and function of carbohydrate-binding module families 13 and 42 of glycoside hydrolases, Comprising a β-Trefoil Fold. Biosci Biotechnol Biochem 77:1363–1371. https://doi.org/10.1271/bbb.130183
Acknowledgements
We acknowledge the Brazilian institutions Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship granted to the first author, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the resources provided to complete this experiment. We would also like to thank Felipe Bini for reviewing the English-language version of the manuscript.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Mariana Furtado Granato de Albuquerque, Murillo Peterlini Tavares, Lílian da Silva Fialho, and Rafaela Inês de Souza Ladeira Ázar. The first draft of the manuscript was written by Mariana Furtado Granato de Albuquerque, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Albuquerque, M.F.G., de Almeida, M.N., Tavares, M.P. et al. Two α-Arabinofuranosidases from Chrysoporthe cubensis and Their Effects on Sugarcane Bagasse Saccharification. Bioenerg. Res. (2024). https://doi.org/10.1007/s12155-024-10721-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12155-024-10721-y