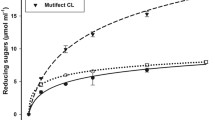
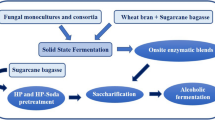
Abstract
High cost and low efficiency of lignocellulolytic enzymes are the main challenges that must be overcome to make second-generation ethanol more competitive in the fuel market. The cultivation of microorganisms using different agro-industrial wastes as substrates is one of the alternatives to reduce the process costs and obtain more efficient enzyme cocktails in the biomass hydrolysis. Since the fungus Chrysoporthe cubensis has proved to be a promising source of lignocellulolytic enzymes from wheat bran, this study aimed to evaluate the effect of different lignocellulosic materials in its enzyme production and the effectiveness of a new enzymatic cocktail on sugarcane bagasse saccharification. Primarily, this fungus was grown under solid-state fermentation, using wheat bran, elephant grass, or sugarcane bagasse as carbon sources. Afterwards, the wheat bran was combined with sugar beet flour in different ratios, and both were used as carbon sources. The enzymatic profiles were investigated and the most promising enzyme extract was applied to pretreated sugarcane bagasse saccharification. A new cocktail obtained from the combination of wheat bran and sugar beet flour in ratio 1:1 showed the highest activity for almost all enzymes tested and was more efficient than extract obtained with only wheat bran, especially in saccharification of alkaline pretreated sugarcane bagasse, releasing 18 g.L−1 of glucose and 14.8 g.L−1 of xylose, which correspond to 38.5% of cellulose and 61.6% of hemicellulose, respectively. Therefore, the combination of both substrates is an effective strategy to induce Chrysoporthe cubensis to produce a complex and efficient enzymatic cocktail in the biomass hydrolysis.



Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
de Almeida MN, Guimarães VM, Falkoski DL, Visser EM, Siqueira GA, Milagres AMF, de Rezende ST (2013) Direct ethanol production from glucose, xylose and sugarcane bagasse by the corn endophytic fungi Fusarium verticillioides and Acremonium zeae. J Biotechnol 168:71–77. https://doi.org/10.1016/j.jbiotec.2013.07.032
Cardona CA, Quintero JA, Paz IC (2010) Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour Technol 101:4754–4766. https://doi.org/10.1016/j.biortech.2009.10.097
Visser EM, Ferreira TF, De Almeida MN, Guimarães VM (2015) Increased enzymatic hydrolysis of sugarcane bagasse from enzyme recycling. Biotechnol Biofuels 8:1–9. https://doi.org/10.1186/s13068-014-0185-8
Maitan-Alfenas GP, Visser EM, Alfenas RF, Nogueira BRG, de Campos GG, Milagres AF, de Vries RP, Guimarães VM (2015) The influence of pretreatment methods on saccharification of sugarcane bagasse by an enzyme extract from Chrysoporthe cubensis and commercial cocktails: a comparative study. Bioresour Technol 192:670–676. https://doi.org/10.1016/j.biortech.2015.05.109
Banerjee S, Mudliar S, Sen R, Giri B, Satpute D, Chakrabarti T, Pandey RA (2010) Commercializing lignocellulosic bioethanol: technology bottlenecks and possible remedies. Biofuels Bioprod Biorefin 4:77–93. https://doi.org/10.1002/bbb.188
Maitan-Alfenas GP, Visser EM, Guimarães VM (2015) Enzymatic hydrolysis of lignocellulosic biomass: converting food waste in valuable products. Curr Opin Food Sci 1:44–49. https://doi.org/10.1016/j.cofs.2014.10.001
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37:19–27. https://doi.org/10.1016/j.renene.2011.06.045
Kapoor M, Panwar D, Kaira GS (2016) Bioprocesses for enzyme production using agro-industrial wastes: technical challenges and commercialization potential. Agro-industrial wastes as feedstock for enzyme production. Academic Press, In, pp 61–93
Menegol D, Scholl AL, Dillon AJP, Camassola M (2017) Use of elephant grass (Pennisetum purpureum) as substrate for cellulase and xylanase production in solid-state cultivation by Penicillium echinulatum. Braz J Chem Eng 34:691–700. https://doi.org/10.1590/0104-6632.20170343s20150822
Fontoura CF, Brandão LE, Gomes LL (2015) Elephant grass biorefineries: towards a cleaner Brazilian energy matrix? J Clean Prod 96:85–93. https://doi.org/10.1016/j.jclepro.2014.02.062
Basanta R, Delgado MAG, Martínez JEC, Vázquez HM, Vázquez GB (2007) Sustainable recycling of waste from sugarcane agroindustry: a review. Cienc Tecnol Aliment 5:293–305. https://doi.org/10.1080/11358120709487704
Veana F, Martínez-Hernández JL, Aguilar CN, Rodríguez-Herrera R, Michelena G (2014) Utilization of molasses and sugar cane bagasse for production of fungal invertase in solid state fermentation using Aspergillus niger GH1. Braz J Microbiol 45:373–377. https://doi.org/10.1590/S1517-83822014000200002
Dutra TR, Guimarães VM, Varela EM, Fialho LS, Milagres AMF, Falkoski DL, Zanuncio JC, Rezende ST (2017) A Chrysoporthe cubensis enzyme cocktail produced from a low-cost carbon source with high biomass hydrolysis efficiency. Sci Rep 7:1–9. https://doi.org/10.1038/s41598-017-04262-y
Duraisam R, Salelgn K, Berekete AK (2017) Production of beet sugar and bio-ethanol from sugar beet and it bagasse: a review. Int J Eng Trends Technol 43:222–233. https://doi.org/10.14445/22315381/IJETT-V43P237
Chamy R, Illanes A, Aroca G, Nuñez L (1994) Acid hydrolysis of sugar beet pulp as pretreatment for fermentation. Bioresour Technol 50:149–152. https://doi.org/10.1016/0960-8524(94)90067-1
Sharma R, Oberoi HS, Dhillon GS (2016) Fruit and vegetable processing waste. Agro-industrial wastes as feedstock for enzyme production. Elsevier, In, pp 23–59
de Almeida MN, Falkoski DL, Guimarães VM, Ramos HJO, Visser EM, Maitan-Alfenas GP, de Rezende ST (2013) Characteristics of free endoglucanase and glycosidases multienzyme complex from Fusarium verticillioides. Bioresour Technol 143:413–422. https://doi.org/10.1016/j.biortech.2013.06.021
Amore A, Giacobbe S, Faraco V (2013) Regulation of cellulase and hemicellulase gene expression in fungi. Curr Genomics 14:230–249. https://doi.org/10.2174/1389202911314040002
Kikot GE, Hours RA, Alconada TM (2009) Contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum: a review. J Basic Microbiol 49:231–241. https://doi.org/10.1002/jobm.200800231
Falkoski DL, Guimarães VM, de Almeida MN, Alfenas AC, Colodette JL, de Rezende ST (2013) Chrysoporthe cubensis: a new source of cellulases and hemicellulases to application in biomass saccharification processes. Bioresour Technol 130:296–305. https://doi.org/10.1016/j.biortech.2012.11.140
Chen SF, Gryzenhout M, Roux J, Xie YJ, Wingfield MJ, Zhou XD (2010) Identification and pathogenicity of Chrysoporthe cubensis on Eucalyptus and Syzygium spp. in South China. Plant Dis 94:1143–1150. https://doi.org/10.1094/PDIS-94-9-1143
Gryzenhout M, Rodas CA, Mena Portales J et al (2006) Novel hosts of the Eucalyptus canker pathogen Chrysoporthe cubensis and a new Chrysoporthe species from Colombia. Mycol Res 110:833–845. https://doi.org/10.1016/j.mycres.2006.02.010
Gryzenhout M, Myburg H, Rodas CA, Wingfield BD, Wingfield MJ (2006) Aurapex penicillata gen. sp. nov. from native Miconia theaezans and Tibouchina spp. in Colombia. Mycologia 98:105–115. https://doi.org/10.1080/15572536.2006.11832716
de Andrade LGA, Maitan-Alfenas GP, Morgan T, Gomes KS, Falkoski DL, Alfenas RF, Guimarães VM (2017) Sugarcane bagasse saccharification by purified β-glucosidases from Chrysoporthe cubensis. Biocatal Agric Biotechnol 12:199–205. https://doi.org/10.1016/j.bcab.2017.10.007
de Sousa GK, Maitan-Alfenas GP, de Andrade LGA et al (2017) Purification and characterization of xylanases from the fungus Chrysoporthe cubensis for production of xylooligosaccharides and fermentable sugars. Appl Biochem Biotechnol 182:818–830. https://doi.org/10.1007/s12010-016-2364-5
Visser EM, Falkoski DL, de Almeida MN, Maitan-Alfenas GP, Guimarães VM (2013) Production and application of an enzyme blend from Chrysoporthe cubensis and Penicillium pinophilum with potential for hydrolysis of sugarcane bagasse. Bioresour Technol 144:587–594. https://doi.org/10.1016/j.biortech.2013.07.015
Ladeira Ázar RIS, Morgan T, dos Santos ACF, de Aquino Ximenes E, Ladisch MR, Guimarães VM (2018) Deactivation and activation of lignocellulose degrading enzymes in the presence of laccase. Enzym Microb Technol 109:25–30. https://doi.org/10.1016/j.enzmictec.2017.09.007
Ladeira-Ázar RIS, Morgan T, Maitan-Alfenas GP, Guimarães VM (2019) Inhibitors compounds on sugarcane bagasse saccharification: effects of pretreatment methods and alternatives to decrease inhibition. Appl Biochem Biotechnol 188:29–42. https://doi.org/10.1007/s12010-018-2900-6
Landhäusser SM, Chow PS, Dickman LT, Furze ME, Kuhlman I, Schmid S, Wiesenbauer J, Wild B, Gleixner G, Hartmann H, Hoch G, McDowell NG, Richardson AD, Richter A, Adams HD (2018) Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiol 38:1764–1778. https://doi.org/10.1093/treephys/tpy118
Miller GL (1959) Use of dinitrosalicyclic reagent for determination of reducing sugar. Anal Chem 31:426–428
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Albersheim P (1966) [107] Pectin lyase from fungi. Methods Enzymol 8:628–631. https://doi.org/10.1016/0076-6879(66)08113-8
Braga FR, Araújo JV, Soares FEF, Araujo JM, Genier HLA, Silva AR, Carvalho RO, Queiroz JH, Ferreira SR (2011) Optimizing protease production from an isolate of the nematophagous fungus Duddingtonia flagrans using response surface methodology and its larvicidal activity on horse cyathostomins. J Helminthol 85:164–170. https://doi.org/10.1017/S0022149X10000416
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein– dye binding. Anal Biochem 72:248–254
TAPPI - Technical Association of the Pulp and Paper Industry (1999) Tappi standard methods (T-222 om-98). Atlanta
TAPPI - Technical Association of the Pulp and Paper Industry (1991) Tappi useful methods (UM-250). Norcross
Gervais P, Molin P (2003) The role of water in solid-state fermentation. Biochem Eng J 13:85–101. https://doi.org/10.1016/S1369-703X(02)00122-5
Raimbault M (1998) General and microbiological aspects of solid substrate fermentation. Electron J Biotechnol 1:1–15. https://doi.org/10.2225/vol1-issue3-fulltext-9
Sun X, Liu Z, Qu Y, Li X (2008) The effects of wheat bran composition on the production of biomass-hydrolyzing enzymes by Penicillium decumbens. Appl Biochem Biotechnol 146:119–128. https://doi.org/10.1007/s12010-007-8049-3
Canilha L, Chandel AK, Milessi TSDS et al (2012) Bioconversion of sugarcane biomass into ethanol: an overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J Biomed Biotechnol 2012:1–15. https://doi.org/10.1155/2012/989572
Santos CC, de Souza W, Sant Anna C, Brienzo M (2018) Elephant grass leaves have lower recalcitrance to acid pretreatment than stems, with higher potential for ethanol production. Ind Crop Prod 111:193–200. https://doi.org/10.1016/j.indcrop.2017.10.013
Singhania RR, Sukumaran RK, Pandey A (2007) Improved cellulase production by Trichoderma reesei RUT C30 under SSF through process optimization. Appl Biochem Biotechnol 142:60–70. https://doi.org/10.1007/s12010-007-0019-2
Shallom D, Shoham Y (2003) Microbial hemicellulases. Curr Opin Microbiol 6:219–228
Juturu V, Wu JC (2012) Microbial xylanases: engineering, production and industrial applications. Biotechnol Adv 30:1219–1227. https://doi.org/10.1016/J.BIOTECHADV.2011.11.006
Sukumaran RK, Singhania RR, Mathew GM, Pandey A (2009) Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renew Energy 34:421–424. https://doi.org/10.1016/j.renene.2008.05.008
Oberoi HS, Chavan Y, Bansal S, Dhillon GS (2010) Production of cellulases through solid state fermentation using kinnow pulp as a major substrate. Food Bioprocess Technol 3:528–536. https://doi.org/10.1007/s11947-008-0092-8
Brijwani K, Oberoi HS, Vadlani PV (2010) Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochem 45:120–128. https://doi.org/10.1016/j.procbio.2009.08.015
Jacob N (2009) Pectinolytic enzymes. In: P. Singh nee’ Nigam AP (ed) Biotechnology for agro-industrial residues utilisation. pp 383–396
Azzaz H, Murad H, Kholif A et al (2013) Pectinase production optimization and its application in banana fiber degradation. Egypt J Nutr Feed 16:117–125
De Ioannes P, Peirano A, Steiner J, Eyzaguirre J (2000) An a-L-arabinofuranosidase from Penicillium purpurogenum: production, purification and properties. J Biotechnol 76:253–258
Gomes J, Gomes I, Terler K, Gubala N, Ditzelmüller G, Steiner W (2000) Optimisation of culture medium and conditions for a-L-arabinofuranosidase production by the extreme thermophilic eubacterium Rhodothermus marinus. Enzym Microb Technol 27:414–422
Heerd D, Diercks-Horn S, Fernández-Lahore M (2014) Efficient polygalacturonase production from agricultural and agro-industrial residues by solid-state culture of Aspergillus sojae under optimized conditions. Springerplus 3:1–14. https://doi.org/10.1186/2193-1801-3-742
de Vries RP, Visser J (2001) Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev 65:497–522. https://doi.org/10.1128/MMBR.65.4.497-522.2001
Ruijter GJG, Vanhanen SA, Gielkens MMC, van de Vondervoort P, Visser J (1997) Isolation of Aspergillus niger creA mutants and effects of the mutations on expression of arabinases and L-arabinose catabolic enzymes. Microbiology 143:2991–2998. https://doi.org/10.1099/00221287-143-9-2991
Bajpai P (1999) Application of enzymes in the pulp and paper industry. Biotechnol Prog 15:147–157. https://doi.org/10.1021/bp990013k
Mateos-Espejel E, Savulescu L, Maréchal F, Paris J (2011) Unified methodology for thermal energy efficiency improvement: application to Kraft process. Chem Eng Sci 66:135–151. https://doi.org/10.1016/j.ces.2010.09.032
Nagar S, Jain RK, Thakur VV, Gupta VK (2013) Biobleaching application of cellulase poor and alkali stable xylanase from Bacillus pumilus SV-85S. 3 Biotech 3:277–285. https://doi.org/10.1007/s13205-012-0096-y
Bischoff KM, Wicklow DT, Jordan DB, de Rezende ST, Liu S, Hughes SR, Rich JO (2009) Extracellular hemicellulolytic enzymes from the maize endophyte Acremonium zeae. Curr Microbiol 58:499–503. https://doi.org/10.1007/s00284-008-9353-z
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29:675–685. https://doi.org/10.1016/j.biotechadv.2011.05.005
Badiei M, Asim N, Jahim JM, Sopian K (2014) Comparison of chemical pretreatment methods for cellulosic biomass. APCBEE Procedia 9:170–174. https://doi.org/10.1016/j.apcbee.2014.01.030
Jørgensen H, Kristensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod Biorefin 1:119–134. https://doi.org/10.1002/bbb.4
Kellock M, Rahikainen J, Marjamaa K, Kruus K (2017) Lignin-derived inhibition of monocomponent cellulases and a xylanase in the hydrolysis of lignocellulosics. Bioresour Technol 232:183–191. https://doi.org/10.1016/j.biortech.2017.01.072
Berlin A, Maximenko V, Gilkes N, Saddler J (2007) Optimization of enzyme complexes for lignocellulose hydrolysis. Biotechnol Bioeng 97:287–296. https://doi.org/10.1002/bit.21238
Jiang X, Geng A, He N, Li Q (2011) New isolate of Trichoderma viride strain for enhanced cellulolytic enzyme complex production. J Biosci Bioeng 111:121–127. https://doi.org/10.1016/j.jbiosc.2010.09.004
Prajapati BP, Jana UK, Suryawanshi RK, Kango N (2020) Sugarcane bagasse saccharification using Aspergillus tubingensis enzymatic cocktail for 2G bio-ethanol production. Renew Energy 152:653–663. https://doi.org/10.1016/j.renene.2020.01.063
Xu X, Lin M, Zang Q, Shi S (2018) Solid state bioconversion of lignocellulosic residues by Inonotus obliquus for production of cellulolytic enzymes and saccharification. Bioresour Technol 247:88–95. https://doi.org/10.1016/j.biortech.2017.08.192
Ajijolakewu KA, Leh CP, Lee CK, Wan Nadiah WA (2017) Characterization of novel Trichoderma hemicellulase and its use to enhance downstream processing of lignocellulosic biomass to simple fermentable sugars. Biocatal Agric Biotechnol 11:166–175. https://doi.org/10.1016/j.bcab.2017.06.005
Acknowledgments
We acknowledge the Brazilian institutions Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) for the scholarship granted to the first author, Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the resources provided to complete this experiment.
Funding
CAPES, FAPEMIG, and CNPq.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Albuquerque, M.F.G., Guimarães, V.M. & de Rezende, S.T. Use of Sugar Beet Flour and Wheat Bran as Carbon Source Improves the Efficiency of Chrysoporthe cubensis Enzymes in Sugarcane Bagasse Saccharification. Bioenerg. Res. 14, 1147–1160 (2021). https://doi.org/10.1007/s12155-020-10224-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10224-6