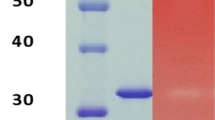
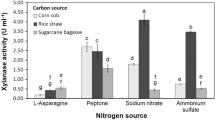
Abstract
Xylanases from the pathogen fungus Chrysoporthe cubensis were produced under solid state fermentation (SSF) using wheat bran as carbon source. The enzymatic extracts were submitted to ion exchange (Q Sepharose) and gel filtration chromatography methods (Sephadex S-200) for purification. The xylanases were divided into three groups: P1 showed better performance at 60 °C and pH 4.0, P2 at 55 °C and pH 3.0, and P3 at 80 °C and pH 3.0. Oat spelt xylan was the best substrate hydrolyzed by P1 and P3, while beechwood xylan was better degraded by P2. Carboxymethyl cellulose (CMC) and p-nitrophenyl-β-d-xylopyranoside (p-NPβXyl) were not hydrolyzed by any of the xylanases. The K M ’ or K M values, using oat spelt xylan as substrate, were 2.65 mg/mL for P1, 1.81 mg/mL for P2, and 1.18 mg/mL for P3. Xylobiose and xylotriose were the main xylooligosaccharides of oat spelt xylan degradation, indicating that the xylanases act as endo-β-1,4-xylanases. Xylanases also proved to be efficient for hydrolysis of sugarcane bagasse when used as supplement of a commercial cocktail due to the increase of the reducing sugar release.







Similar content being viewed by others
References
Bon, E. P. S., Ferrara, M. A., & Corvo, M. L. (2008). Enzimas em biotecnologia: produção, aplicações e mercado. Rio de Janeiro: Interciência: UFRJ: CAPES: FAPERJ: FCT (Portugal).
Sangkharak, K., Vangsirikul, P., & Janthachat, S. (2011). Isolation of novel cellulase from agricultural soil and application for ethanol production. International Journal of Advanced Biotechnology and Research, 2, 230–239.
Zhang, J. H., et al. (2011). Comparison of the synergistic action of two thermostable xylanases from GH families 10 and 11 with thermostable cellulases in lignocellulose hydrolysis. Bioresource Technology, 102, 9090–9095.
Motta, F., Andrade, C., Santana, M. (2013). A review of xylanase production by the fermentation of xylan: classification, characterization and applications. In: Chandel, A. K. E Silva, S. S. (Eds). Sustainable degradation of lignocellulosic biomass—techniques, applications and commercialization: InTech, cap. 10.
Polizeli, M. L. T. M., Rizzatti, A. C. S., Montir, R., Terenzi, H. F., Jorge, J. A., & Amorim, D. S. (2005). Xylanases from fungi: properties and industrial applications. Applied Microbiology and Biotechnology, 67(5), 577–591.
Biely, P., Vrsanska, M., Tenkanen, M., & Kluepfel, D. (1997). Endo-b-1,4-xylanase families: differences in catalytic properties. Journal of Biotechnology, 151–166.
Kulkarni, N., Shendye, A., & Rao, M. (1999). Molecular and biotechnological aspects of xylanases. FEMS Microbiology Reviews, 23, 411–456.
Subramaniyan, S., & Prema, P. (2002). Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Critical Reviews in Biotechnology, 22, 33–64.
Dodd, D., & Cann, I. K. (2009). Enzymatic deconstruction of xylan for biofuel production. Global Change Biology Bioenergy, 18, 2–17.
Butt, M. S., Tahir-Nadeem, M., Ahmad, Z., & Sultan, M. T. (2008). Xylanases in baking industry. Food Technology and Biotechnology, 46(1), 22–31.
Huang, J., Chen, D., Wei, Y., Wang, Q., Li, Z., Chen, Y., Huang, R. (2014). Direct ethanol production from lignocellulosic sugars and sugarcane bagasse by a recombinant Trichoderma reesei strain HJ48. The Scientific World Journal, ID. 798683.
Buckeridge, M. S., et al. (2012). Ethanol from sugarcane in Brazil: a ‘midway’ strategy for increasing ethanol production while maximizing environmental benefits. Global Change Biology Bioenergy, 4, 119–126.
Lafond, M., Tauzin, A., Desseaux, V., Bonnin, E., Ajandouz, H., & Giardina, T. (2011). GH10 xylanase D from Penicillium funiculosum: biochemical studies and xylooligosaccharide production. Microbial Cell Factories, 10, 20.
Fitzpatrick, M., Champagne, P., Cunningham, M. F., & Whitney, R. E. (2010). A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value-added products. Bioresource Technology, 101, 8915–8923.
Van Den Brink, J., Maitan-Alfenas, G. P., Zou, G., Wang, C., Zhou, Z., Guimarães, V. M., & de Vries, R. P. (2014). Synergistic effect of Aspergillus niger and Trichoderma reesei enzyme sets on the saccharification of wheat straw and sugarcane bagasse. Biotechnology Journal, 9, 1329–1338.
Falkoski, D. L., Guimarães, V. M., de Almeida, M. N., Alfenas, A. C., Colodette, J. L., & de Rezende, S. T. (2013). Chrysoporthe cubensis: a new source of cellulases and hemicellulases to application in biomass saccharification processes. Bioresource Technology, 130, 296–305.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Miller, G. L. (1959). Use of dinitrosalicycilic acid reagent for determianiton of reducing sugars. Analytica Chemistry, 31, 426–430.
Mcllvaine, T. C. (1921). A buffer solution for colorimetric comparison. Journal of Biological Biochemistry, 49, 183–186.
Monti, R., Cardello, L., Custódio, M. F., Goulart, A. J., Sayama, A. H., & Contiero, J. (2003). Production and purification of an endo-1,4-b-xylanase from Humicola grisea var. thermoidea by electroelution. Brazilian Journal of Microbiology, 34(2), 124–128.
Querido, A. L. S., Coelho, J. L. C., Araujo, E. F., & Chaves-Alves, V. M. (2006). Partial purification and characterization of xylanase produced by Penicillium expansum. Brazilian Archives of Biology and Technology, 49, 474–480.
Kamble, R. D., Jadhav, A. R. (2012). Isolation, purification, and characterization of xylanase produced by a new species of Bacillus in solid state fermentation. International Journal of Microbiology, ID 683193.
Chi, W. J., Park, D. Y., Chang, Y. K., & Hong, S. K. (2012). A novel alkaliphilic xylanase from the newly isolated mesophilic Bacillus sp. mx47: production, purification, and characterization. Applied Biochemistry and Biotechnology, 168(4), 899–909.
Knob, A., Beitel, S.M., Fortkamp, D., Terrasan, C.R.F., de Almeida, A.F. (2013). Production, purification, and characterization of a major Penicillium glabrum xylanase using brewer’s spent grain as substrate. BioMed Research International, ID 728735.
Zheng, H. C., Sun, M. Z., Meng, L. C., Pei, H. S., Zhang, X. Q., Yan, Z., Zeng, W. H., Zhang, J. S., Hu, J. R., Lu, F. P., & Sun, J. S. (2014). Purification and characterization of a thermostable xylanase from Paenibacillus sp. NF1 and its application in xylooligosaccharides production. Journal of Microbiology and Biotechnology, 24(4), 489–496.
Shin, K., Jeya, M., Lee, J., & Kim, Y. (2010). Purification and characterization of a thermostable xylanase from Fomitopsis pinicola. Journal of Microbiology and Biotechnology, 20(10), 1415–1423.
Taibi, Z., Saoudi, B., Boudelaa, M., Trigui, H., Belghith, H., Gargouri, A., & Ladjama, A. (2012). Purification and biochemical characterization of a highly thermostable xylanase from Actinomadura sp. strain cpt20 isolated from poultry compost. Applied Biochemistry and Biotechnology, 166(3), 663–679.
Vikramathithan, J., Ravikumar, S., Muthuraman, P., Nirmalkumar, G., Shayamala, S., & Srikumar, K. (2012). Purification and biochemical characterization of two major and thermophilic xylanase isoforms (T70 and T90) from xerophytic Opuntia vulgaris plant spp. Cellulose, 19, 1373–1383.
Gonçalves, T. A., Damásio, A. R., Segato, F., Alvarez, T. M., Bragatto, J., Brenelli, L. B., Citadini, A. P., Murakami, M. T., Ruller, R., Paes Leme, A. F., Prade, R. A., & Squina, F. M. (2012). Functional characterization and synergic action of fungal xylanase and arabinofuranosidase for production of xylooligosaccharides. Bioresource Technology, 119, 293–299.
Wongwisansri, S., Promdonkoy, P., Matetaviparee, P., Roongsawang, N., Eurwilaichitr, L., & Tanapongpipat, S. (2013). High-level production of thermotolerant β-xylosidase of Aspergillus sp. BCC125 in Pichia pastoris: characterization and its application in ethanol production. Bioresource Technology, 132, 410–413.
Visser, E. M., Falkoski, D. L., de Almeida, M. N., Maitan-Alfenas, G. P., & Guimarães, V. M. (2013). Production and application of an enzyme blend from Chrysoporthe cubensis and Penicillium pinophilum with potential for hydrolysis of sugarcane bagasse. Bioresource Technology, 144, 587–594.
Verma, D., Anand, A., & Satyanarayana, T. (2013). Thermostable and alkalistable endoxylanase of the extremely thermophilic bacterium Geobacillus thermodenitrificans TSAA1: cloning, expression, characteristics and its applicability in generating xylooligosaccharides and fermentable sugars. Applied Biochemistry and Biotechnology, 170, 119–130.
Wu, Q., Li, Y., Li, Y., Gao, S., Wang, M., Zhang, T., & Chen, J. (2013). Identification of a novel fungus, Leptosphaerulina chartarum SJTU 59 and characterization of its xylanolytic enzymes. PloS One, 8(9), e73729.
Goldemberg, J. (2007). Ethanol for a sustainable energy future. Scienc, 315, 808–810.
Ladisch, M., Mosier, N. S., Kim, Y., Ximenes, E., & Hogsett, D. (2010). Converting cellulose to biofuels. Biofuels, 106(3), 56–63.
Acknowledgments
We thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing scholarships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Sousa Gomes, K., Maitan-Alfenas, G.P., de Andrade, L.G.A. et al. Purification and Characterization of Xylanases from the Fungus Chrysoporthe cubensis for Production of Xylooligosaccharides and Fermentable Sugars. Appl Biochem Biotechnol 182, 818–830 (2017). https://doi.org/10.1007/s12010-016-2364-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2364-5