Abstract
Airway management and ventilation are central to the resuscitation of the neurologically ill. These patients often have evolving processes that threaten the airway and adequate ventilation. Furthermore, intubation, ventilation, and sedative choices directly affect brain perfusion. Therefore, Airway, Ventilation, and Sedation was chosen as an Emergency Neurological Life Support protocol. Topics include airway management, when and how to intubate with special attention to hemodynamics and preservation of cerebral blood flow, mechanical ventilation settings and the use of sedative agents based on the patient’s neurological status.
Similar content being viewed by others
Introduction
Intubation of the acutely brain injured patient can be a matter of life or death. Failure to intubate a patient with rapidly progressive neurological decline may result in respiratory arrest, secondary brain injury from hypoxia, acidosis, elevated intracranial pressure, or severe aspiration pneumonitis and acute respiratory distress syndrome. Conversely, the process of induction and intubation can elevate intracranial hypertension when a mass lesion is present, complete a massive infarction when brain tissue is marginally perfused, and result in temporary loss of the neurological examination at a time when neurological and neurosurgical decision-making is required.
The goals of airway management in neurological patients are to maintain adequate (but not excessive) oxygenation and ventilation, preserve cerebral perfusion, and prevent aspiration. A neurological assessment prior to the administration of sedating and paralyzing medications should be performed to provide a functional baseline, whereby neurological and neurosurgical decision making may ensue.
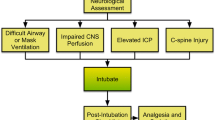
The ENLS suggested algorithm for the initial management of airway, ventilation, and sedation is shown in Fig. 1. Suggested items to complete within the first hour of evaluating a patient are shown in Table 1.
Assessing the Need for Intubation
Patients in severe respiratory distress or impending arrest should be intubated without delay. Additionally, a patient who cannot “protect his airway” because of depressed mental status or vomiting with aspiration may need tracheal intubation. Intubation has the potential for complications, creates significant hemodynamic disturbances, and should not be undertaken without a risk benefit assessment. However, it should not be delayed when necessary. The decision to intubate is influenced by factors specific to patient physiology, clinical environment, and the anticipated course of care.
In the emergency department (ED) or prehospital environment, a stuporous or comatose patient with an unknown diagnosis requiring extended transport, transfer, imaging, or invasive procedures may be most appropriately managed with a secure endotracheal airway. The same patient with a known condition, an anticipated stable or improving course, and no planned transportation may not require intubation and might be managed by a more conservative approach.
With these considerations in mind, there are four commonly accepted indications to intubate:
Failure to Oxygenate
This finding may be determined by pulse oximetry (limitations include regional or systemic hypoperfusion, severe anemia, and opaque nail polish), arterial blood gas analysis, or the patient’s visual appearance (cyanosis).
Failure to Ventilate
Ventilation is assessed by capnometry through nasal cannula or transcutaneous monitoring [1], arterial blood gas analysis, or gross visual appearance (excessive or inadequate work of breathing).
Failure to Protect the Airway
Airway protection is the result of numerous variables including bulbar function, airway anatomy, quantity and quality of secretions, strength of cough reflex, and ability to swallow after suctioning [2]. The presence of a gag reflex is an inadequate method of assessing airway protection [3].
Anticipated Neurological or Cardiopulmonary Decline Requiring Transport or Immediate Treatment
Anticipation of the trajectory of the patient’s condition can avoid rushed or emergent intubations and allow for appropriate preparation for the procedure.
Decision Made to Intubate: Perform Neurological Assessment
Whenever possible, urgent management of the airway should coincide with a focused neurological assessment. The examination can typically be conducted in 2 min or less. The pre-sedation/pre-intubation neurologic exam establishes a baseline that is used to assess therapeutic interventions (e.g., patients with stroke, seizures, hydrocephalus or other disorders) or may identify injuries that are at risk of progressing (e.g., unstable cervical spine fractures). The assessment identifies the type of testing required and may help to limit unnecessary interventions, such as radiological cervical spine clearance. In general, the pre-intubation neurological assessment is the responsibility of the team leader who is coordinating the resuscitation. Findings should be documented and communicated directly to the team that assumes care of the patient. The pre-intubation neurological examination includes an assessment of:
-
Level of arousal, interaction, and orientation, as well as an assessment of simple cortical functions such as vision, attention, and speech comprehension and fluency.
-
Cranial nerve function.
-
Motor function of each individual extremity.
-
Tone and reflexes.
-
Recognition of involuntary movement consistent with tremor or epileptic activity.
-
Cervical tenderness or gross spinal abnormality.
-
Sensory level in patients with suspected spinal cord injury.
Airway Assessment
A difficult airway may be broadly defined as an endotracheal intubation attempt in which a provider who is appropriately trained in airway management experiences difficulty with bag-mask ventilation, tracheal intubation or both [4]. Using this broad definition, up to 30% of ED intubations may involve “difficult airways” [5]. Patients with acute neurological injury may be particularly likely to have a difficult airway, because of the need to immobilize the cervical spine in patients who suffer trauma or are “found down” following neurological emergencies such as strokes and seizures. It is essential that all healthcare providers who manage critically ill neurological patients be able to identify common factors that may increase the complexity of airway management. Identification of the difficult airway is essential for selection of the appropriate technique (awake fiberoptic vs rapid sequence induction), tools (video vs direct laryngoscopy) and operator (anesthesiologist vs other provider, attending vs trainee). Failure to identify a difficult airway prior to induction is one of the most important factors predicting a subsequent failed airway during the intubation attempt [6, 7].
The “LEMON” pneumonic has been shown to successfully predict difficult tracheal intubation in the emergency department [3, 8]:
-
L = Look externally, for features such as abnormal facies, oro-maxillo-facial trauma and abnormal body habitus.
-
E = Evaluate with the 3-2-2 rule.
-
Will 3 of the patient’s fingers fit between the incisors of the open mouth? If not, mouth opening may be too limited to permit adequate laryngoscopic visualization or manipulation of the endotracheal tube.
-
Will 3 of the patient’s fingers fit between the chin (mentum) and the hyoid bone? If not, the airway may be too anterior for easy visualization with direct laryngoscopy (DL).
-
Will 2 of the patient’s fingers fit between the hyoid bone and the superior thyroid notch? If not, the airway may be too high in the neck to permit easy visualization.
-
-
M = Mallampati score assesses the extent of mouth opening in relation to tongue size [9, 10].
-
Grade I: Soft palate, entire uvula, faucial pillars visible.
-
Grade II: Soft palate, entire uvula visible.
-
Grade III: Soft palate, base of uvula visible.
-
Grade IV: Only hard palate visible.
-
Grades 1 and 2 predict easy visualization, 3 predicts difficulty and 4 extreme difficulty. Mallampati grading ideally requires some patient co-operation and may be difficult to assess in patients with acute brain injury.
-
O = Obstruction/obesity. The presence of redundant soft tissue (obesity), a supraglottic mass or trauma/hematoma within the oropharynx may obscure the view of the glottis.
-
N = Neck mobility. Inability to attain the sniffing position because of immobilization of the cervical spine in the trauma patient, ankylosing spondylitis, rheumatoid arthritis or age related degenerative disease.
The “MOANS” pneumonic predicts difficulty of bag mask ventilation [3]:
-
M = Mask seal, may be compromised by abnormal facies, facial hair and body fluids.
-
O = Obesity/obstruction.
-
A = Age >55.
-
N = No teeth.
-
S = Stiff lungs.
When a difficult airway is identified, the most important next step is to call for help, as appropriate. The provider with the most experience in airway management should be present at the bedside, as well as a provider capable of rapidly establishing a surgical (or percutaneous) airway in the event of a failed intubation. Availability of all necessary tools at the bedside, such as a supraglottic airway, endotracheal tube introducer (bougie), cricothyroidotomy tray, and a video laryngoscope should be confirmed. Finally, it is important to remember that prediction of a difficult airway is imperfect and that an unanticipated difficult airway may be encountered [11]. Ready availability of the necessary expertise and equipment in the form of an institutional airway team may increase survival to hospital discharge and decrease the need for a surgical airway [12, 13].
Endotracheal Intubation in the Critically Ill Neurological Patient
Several societies have published guidelines for the management of the difficult airway, particularly in the setting of anesthesia for elective procedures [4, 14,15,16]. Intubation of the critically ill patient is a fundamentally different clinical situation than intubation in the relatively stable environment of the operating room [16]. While over 90% will be technically successful, about 20–25% of critically ill patients will develop severe hypoxemia during intubation, 10–25% will develop severe hypotension and about 2% will suffer cardiac arrest [7, 17,18,19]. More so than other critically ill patients, the patient with acute brain injury is unlikely to tolerate significant periods of hypoxia or hypotension, which may result in secondary injury to the vulnerable brain [20,21,22,23,24,25,26]. The ENLS intubation algorithm, therefore, emphasizes evidence-based best practice for maintenance of adequate oxygenation and perfusion during intubation, as well as the most direct and dependable pathway to a definitive airway for providers with varied experience and backgrounds.
As a first step, at least 2 providers, including at least one provider experienced in airway management, should be present at the bedside. Two-provider presence may decrease complications associated with intubation of the critically ill [17, 27] (Fig. 2).
Awake Intubation
The provider may be “forced to act” in the patient with acute neurological illness who has a compromised airway and rapid progression to cardiovascular or respiratory collapse, necessitating an urgent attempt at laryngoscopy and intubation. When the provider is not “forced to act”, however, and the patient is spontaneously breathing and oxygenating adequately on supplemental oxygen, consideration should be given first to an awake intubation. An awake fiberoptic intubation will avoid the displacement of the cervical spine that is inevitable with the use of any laryngoscope, [28] and may be the intubation technique of choice in the presence of significant cervical spine injury. An awake intubation may also be the procedure of choice when an anticipated difficult airway or bag-mask ventilation makes cessation of spontaneous respiratory effort with rapid sequence intubation (RSI) hazardous. Awake intubation is typically performed using moderate sedation in conjunction with topical anesthesia, most commonly with a flexible endoscope that is navigated into the trachea via an oral or nasal route [29]. An endotracheal tube fitted onto the flexible endoscope is then advanced over the endoscope into the trachea. An awake intubation is not appropriate in patients with elevated intracranial pressure. A fiberoptic intubation requires considerable expertise, and should be performed only with a highly experienced provider at the bedside. Should awake intubation be unsuccessful, the experienced provider must decide on an alternative technique with the greatest likelihood of success, and may consider video laryngoscopy, a supraglottic airway, or a surgical airway.
Pre-oxygenation and Apneic Oxygenation
Maintaining an adequate oxygen saturation during intubation is critical for the patient with acute neurological illness. Any significant period of hypoxia may result in secondary injury to the vulnerable brain and exacerbate any ICP elevation [20, 25]. Three minutes of pre-oxygenation with noninvasive positive pressure ventilation (NIPPV) [30], or a heated high-flow nasal cannula (HHFNC) at 60–70 L/min that is continued following induction [31,32,33], may be more effective at preserving oxygenation during the intubation attempt than pre-oxygenation with a high-flow (non-rebreather) face mask. Pre-oxygenation should be performed with the head of bed elevated to 30°. While randomized trials have had mixed results [31, 34, 35], the preponderance of evidence suggests that apneic oxygenation, performed using either HHFNC at 60–70 L/min or a regular nasal cannula at 15 L/min during laryngoscopy, increases time to desaturation and first-pass success without hypoxia [31,32,33, 36]. Since apneic oxygenation is easy to perform, inexpensive, and without serious adverse effects, its use is recommended during intubation of the critically ill neurological patient.
Intubating the Patient with Intracranial Pathology
Rapid sequence intubation (RSI) consists of the simultaneous administration of a fast-active sedative to induce immediate unresponsiveness and a neuromuscular-blocking agent to achieve optimal intubating conditions, with the goal of attaining control of the airway in the critically ill patient at risk of aspiration of stomach contents. RSI limits the elevation of Intracranial Pressure (ICP) often associated with the physiologic responses to laryngoscopy and is the preferred method for intubation of the patient with elevated ICP [37,38,39,40]. The presence of coma should not justify proceeding without pharmacological agents, or administration of only a neuromuscular blocking agent without appropriate pre-treatment and induction agents. Although the patient may seem unresponsive, laryngoscopy and intubation often provoke reflexes that elevate ICP unless appropriate pre-treatment and induction agents are used [41].
Outcomes in patients with intracranial catastrophes are related to the maintenance of both brain perfusion and oxygenation. It is generally recommended that the ICP be maintained below 22 mmHg, MAP between 80 and 110 mmHg, and Cerebral Perfusion Pressure (CPP = MAP-ICP) at a minimum of 60 mmHg during intubation [42]. Because the intracranial pressure may not be known at the time of urgent intubation, clinicians should anticipate elevated ICP in patients with conditions such as mass lesions, hydrocephalus or extensive cerebral oedema, and choose an appropriate BP target accordingly. When the airway is manipulated, two responses may exacerbate intracranial hypertension. The reflex sympathetic response (RSR) results in increased heart rate, increased blood pressure, and, consequently, increased ICP. The direct laryngeal reflex stimulates an increase in ICP independent of the RSR [3]. Although the RSR may be dangerous in a hypertensive patient, pre-treatment of the RSR is not indicated in a hypotensive patient with known or suspected increased ICP [43]. Elevations in ICP should be mitigated by minimizing airway manipulation (the most experienced person should perform the intubation) and administering medications.
Common pre-medications used to prevent increased ICP during intubation include:
Lidocaine
Administered intravenously at a dose of 1.5 mg/kg 60–90 s before intubation, lidocaine attenuates the direct laryngeal reflex. There is mixed evidence that it mitigates the RSR [44, 45]. It is not associated with drop in MAP.
Fentanyl
At doses of 2–3 μg/kg, fentanyl attenuates the RSR associated with intubation, and is administered as a single pretreatment dose over 30–60 s in order to reduce chances of apnea or hypoventilation prior to induction and paralysis [46]. It is generally not used in patients with incipient or actual hypotension, or those who are dependent on sympathetic drive to maintain an adequate blood pressure for cerebral perfusion.
ICP during intubation also rises due to body positioning and hypoventilation. Hypoventilation immediately causes increased pCO2, a potent acute cerebral vasodilator. When ICP is known or suspected to be elevated, the following approach is suggested (Fig. 3):
Intubation with elevated ICP [212]
-
Duration of time with head of bed lowered should be minimized. Reverse Trendelenberg positioning during intubation may be considered.
-
MAP must be preserved throughout the procedure, with a goal of 80–100 mmHg, but not lower than the pre-intubation blood pressure. If ICP is monitored, keep CPP >60 mmHg.
-
Pain, discomfort, agitation, and fear must be controlled with adequate analgesia and sedation.
-
Hypoventilation must be avoided, and quantitative end-tidal capnography monitoring is recommended.
-
Adequate oxyhemoglobin saturation should be maintained at all times during intubation. In addition to the use of effective pre-oxygenation and apneic oxygenation, bag-mask ventilation should be used between intubation attempts and at any time that the SpO2 is lower than 94%.
Intubating the Patient with Brain Ischemia
In suspected or proven ischaemic stroke, careful attention should be taken to avoid hypotension during induction and post intubation. In the healthy state, the cerebrovascular circulation is well collateralized. During ischaemic stroke, many patients possess an infarct core surrounded by a greater region of ischaemic penumbra. Under these circumstances, the ischaemic penumbra consists of a region of vasodilated vessels, receiving maximal compensatory shunting from the adjacent cerebrovascular circulation. Hypertension and tachycardia often reflect a compensatory, not pathophysiologic, response to this ischemia and may be necessary to maintain perfusion of the ischaemic territory.
Certain vasoactive agents may reverse regional vasoconstriction in normal areas of the brain that is necessary to maintain physiologic shunting of blood to the region of ischemia, even if they do not drop the systemic blood pressure or alter the global CPP. An episode of relative or actual hypotension can dramatically increase brain infarct size by “stealing” blood flow from the maximally dilated watershed territories between vascular distributions.
Brain ischemia is not limited to ischaemic stroke but is also variably present in patients with vasospasm, traumatic brain injury (TBI), intracranial and extracranial cerebrovascular stenosis, and hypoxic-ischaemic encephalopathy following resuscitation from cardiac arrest. Strong correlations between episodic hypotension and poor neurological outcome have been noted in the critical hours following resuscitation from TBI and cardiac arrest [24, 26, 47, 48]. The intubating clinician should be aware of the risks of even a transient decrease in CBF and strive to maintain CBF and systemic vascular tone during airway management. A fluid bolus should be administered prior to intubation in any patient with possible volume depletion. Ketamine or Etomidate are the preferred induction agents in patients with compromised cerebral perfusion. Vasopressors should be administered to prevent hypotension as needed during or following RSI. Brain ischemia is worsened by the effect of hyperventilation upon vascular tone. Normocapnea should be maintained during intubation, and early correlation of an arterial CO2 sample with ETCO2 is suggested to enable non-invasive tracking of ventilation [1, 49].
Conflicting evidence exists regarding the risks of routine intubation and general anesthesia for acute ischaemic stroke patients who require endovascular intervention [50,51,52,53]. Since there is some evidence to suggest harm from routine use of general anesthesia, [50, 52, 53] moderate sedation without intubation may be the technique of choice for most ischaemic stroke patients requiring endovascular therapy. Intubation should be considered, however, for patients with bulbar dysfunction, an inability to protect the airway or control secretions, hypoxia, hypercarbia, a high risk of aspiration (including patients with emesis) or significant agitation [53]. The only randomized trial of conscious sedation versus intubation in patients undergoing urgent endovascular thrombectomy for acute anterior circulation thrombosis did not show a difference in outcomes [50].
Intubating the Patient with Neuromuscular Weakness
Although some patients with neuromuscular disease require immediate intubation, those with preserved bulbar function and reasonable functional ventilatory reserves may undergo a trial of non-invasive ventilation combined with airway clearance by the frequent use of chest physiotherapy and a cough-assist device [54,55,56].
Any patient with neuromuscular weakness that complains of dyspnea, should undergo an assessment of respiratory function that includes (see also the Acute Non-Traumatic Weakness protocol):
-
Arterial blood gas measurement.
-
Serial pulmonary function testing to include negative inspiratory force (NIF), vital capacity (FVC) and maximum expiratory force (MEF).
-
Assessment of bulbar function, neck strength, and cough.
Candidates for intubation include patients with bulbar dysfunction and a demonstrated inability to manage airway secretions or maintain a patent airway, those who have a rapidly progressive course, and those who do not rapidly stabilize gas exchange and work of breathing with non-invasive ventilation [54].
In myasthenia gravis, succinylcholine is safe but requires approximately 2.5 times the dose to get the same effects [57]. Non-depolarizing agents such as Rocuronium are also safe but will have a prolonged duration [57]. In conditions such as Guillain–Barre, succinylcholine can precipitate life-threatening hyperkalemia, and only non-depolarizing agents should be used.
Intubating the Patient with Cervical Spine Injury
Cervical Spine injury should be suspected during direct neck trauma or blunt head trauma resulting in loss of consciousness. During care of these patients, measures must be taken to protect the spinal cord during any movements or procedures, including intubation. Airway maneuvers, including head-tilt/chin lift to achieve the sniffing position, bag-mask ventilation, cricoid pressure, and direct laryngoscopy itself, can all result in displacement of the cervical spine and injure the spinal cord when cervical instability is present [28, 58, 59]. Blade elevation results in the greatest displacement of the spine during laryngoscopy [28]. Awake fiberoptic intubation should, therefore, be considered the best option in patients with significant cervical spine injury who are awake, spontaneously breathing and have stable oxygenation on supplemental oxygen [60,61,62,63]. Patients with acute hypoxic or hypercarbic respiratory failure and rapid clinical decline may not be suitable candidates for an awake fiberoptic intubation; such patients may require RSI with manual in-line stabilization. Similarly, patients who may have raised intracranial pressure and those with impending or established cardiovascular collapse should not undergo awake fiberoptic intubation. When RSI is performed, every precaution should be taken to minimize displacement of the cervical spine, although some movement is inevitable [28, 58, 59]. Prior to intubation, the anterior part of the semi-rigid collar should be removed to permit greater mouth opening during laryngoscopy. The head should then be maintained in the neutral position using Manual In-Line Stabilization (MILS) (demonstrated in Fig. 4), in which an assistant stands by the patient with a hand on either side of the head between the mastoid process and the occiput, [58] The assistant must then hold the head steady while gently opposing the applied forces of airway manipulation.
When a basic maneuver is necessary to open the airway, a jaw-thrust, which may also cause some displacement, should be performed rather than a head-tilt/chin lift. The use of cricoid pressure is no longer recommended during intubation. It definitely should not be implemented in patients with cervical spine injury, as it may cause posterior displacement of the cervical spine [64, 65]. MILS adversely impacts visualization of the glottis, with only the epiglottis visible in about 22% of patients intubated using direct laryngoscopy (DL) [66]. The use of video laryngoscopy (VL) to improve glottic visualization has therefore become common practice when MILS is necessary [67,68,69]. While VL consistently results in a better view of the glottis than DL [70,71,72,73,74,75], manipulation of the ETT into the glottis can be challenging. The anterior part of the semi-rigid collar should be promptly re-applied following intubation.
Rapid Sequence Intubation
Induction Agents
Since hypotension is common following sedation [7, 17,18,19], and may exacerbate secondary injury in patients with acute neurological illness [20,21,22, 24,25,26], the use of a hemodynamically neutral agent such as Etomidate or a sympathetic stimulant such as Ketamine is recommended. Table 4 lists the properties of some medications commonly used for RSI in patients with acute neurological illness.
Etomidate
Etomidate is a short-acting imidazole derivative that provides sedation and muscle relaxation with minimal hemodynamic effect. Despite concerns about adrenal suppression, it is considered to be one of the most hemodynamically neutral of all commonly used induction agents and a drug of choice for patients with elevated ICP or compromised cerebral perfusion [76, 77]. While Etomidate may cause transient myoclonus and regional neuro-excitation [78, 79], the clinical relevance of these phenomena are unknown.
Ketamine
Ketamine is a dissociative agent administered at a dose of 2 mg/kg IV push. Ketamine causes sympathetic stimulation and is therefore the most favorable of all available induction agents from a hemodynamic standpoint [80,81,82]. It is the induction agent of choice for patients with shock or compromised cerebral perfusion. Historically, use of ketamine was avoided in patients with elevated ICP. More recent evidence suggests, however, that when concurrent sedation is provided, it is safe in patients with elevated ICP [83, 84]. In view of the significant sympathetic stimulation that accompanies its use, an alternative to Ketamine should be considered in patients with acute intracerebral hemorrhage and elevated blood pressure, unsecured vascular malformations, or significant ischaemic heart disease.
Neuromuscular Blockade
Succinylcholine
Succinylcholine is a depolarizing neuromuscular blocker with a rapid onset (30–60 s) and short duration of action (5–15 min), making it an ideal agent for RSI. The RSI dose is 1.5–2 mg/kg IV. Although it has been associated with transient increases in ICP, the effect is not considered clinically significant [85]. Immobile and chronically ill neurologic patients are at risk for succinylcholine-induced hyperkalemia, because of an upregulation in extra-junctional acetyl-choline receptors. This includes patients with chronic neurological/neuromuscular disease such as amyotrophic lateral sclerosis, multiple sclerosis and chronic myopathies, as well as those with as little as 24–72 h of immobility following acute brain or spinal cord injury [86]. It is critical, therefore, that the provider performing RSI always screen for contra-indications to succinylcholine in patients with acute neurological illness, to avoid precipitating a life-threatening bradyarrhythmia, ventricular arrhythmia or cardiac arrest. Succinylcholine should be avoided in these patients, and a non-depolarizing agent used [87].
Non-depolarizing Agents
Non-depolarizing agents have a longer duration of action than Succinylcholine. A non-depolarizing agent with rapid onset and relatively short duration of action should be used, such as Rocuronium (at 1.2–1.4 mg/kg IV push) or Vecuronium (at 0.1–0.2 mg/kg IV push). Rocuronium produces optimal intubating conditions almost as quickly (45–60 s) as Succinylcholine, but has a significantly longer duration of action (45–70 min). The novel agent Sugammedex, administered at a dose of 16 mg/kg 3 min following Rocuronium dosing, can reverse neuromuscular blockade and restore muscle function faster than with the use of Succinylcholine [88, 89]. Sugammedex should therefore be available at locations where the use of steroidal neuromuscular blocking agents such as Rocuronium is common, and can be utilized in clinical situations where a rapid return of muscle function is desirable.
Bag-Ventilation and Basic Airway Management
Following induction and paralysis, the use of proper bag-mask-ventilation (BMV) technique is critical. Two-provider BMV is preferable, with one provider entirely focused on attaining an effective mask seal while simultaneously performing a basic airway-opening maneuver, such as the head-tilt/chin-lift, or, when cervical spine immobilization is necessary, a jaw thrust (Fig. 5). A second individual performs bag-ventilation and a third provides MILS when necessary. The morbidly obese patient should be placed in the “ramped” position, with blankets stacked under the upper body and head until horizontal alignment is achieved between the external auditory meatus and the sternal notch [90]. The routine use of an adjuvant such as an oral or nasal airway may greatly facilitate effective BMV. Apneic oxygenation with a HHFNC or a regular nasal cannula at 15 L/min should be performed following induction and continued during BMV and laryngoscopy (Fig. 5).
Technique of 2-provider bag mask ventilation, with a third provider performing MILS. First provider grasps the mask with the thumb and index finger in a “C” hold while the other 3 fingers grasp the mandible in an “E” hold, while performing either jaw thrust, as shown in this patient, or a head-tilt/chin-lift. A third provider provides MILS
Laryngoscopy and Intubation
When BMV is effective, up to 3 attempts at laryngoscopy and intubation are permissible, as long the SpO2 remains >94%. BMV should be performed between attempts and apneic oxygenation continued at all times. With every subsequent attempt, a change in operator (more experienced operator steps in) and/or technique (such as a change from DL to VL, or use of a bougie) should occur. When BMV using optimal technique is ineffective, an experienced operator may make a single attempt at laryngoscopy and intubation. Use of VL should be considered for this “single, best attempt” for optimal glottis visualization. The use of VL consistently results in higher rates of glottis visualization [70,71,72,73,74,75], and may be particularly valuable for less experienced operators and for difficult airways [73, 91]. Randomized trials have not, however, consistently demonstrated superiority in first-pass success rates or a reduction in episodes of hypoxia with the use of VL for intubation of the critically ill [91,92,93,94,95]. When intubation is performed with the Glidescope® (Verathon Medical Inc, Bothell, WA, USA) VL, use of the highly curved rigid Glidescope® stylet is recommended, to facilitate navigation of the ETT past the sharp curve of the VL blade to the glottis [96].
The Cormack-Lehane system is used to grade the direct laryngoscopic view of the glottis [97, 98].
-
Grade 1—Entire glottis visible.
-
Grade 2a—Partial view of the glottis.
-
Grade 2b—Only the posterior extremity of the glottis (or only arytenoids) visible.
-
Grade 3—Only epiglottis visible, no view of glottis inlet.
-
Grade 4—Neither epiglottis nor glottis visible.
Documentation of the direct laryngoscopic grade in the medical record is critical, to inform future airway management decisions.
The Failed Airway
A failed airway is considered to exist in one of two situations—(1) the “cannot intubate, cannot ventilate” scenario, when BMV is ineffective at achieving gas exchange and a “single, best attempt” at intubation by an experienced operator is unsuccessful, and (2) the “cannot intubate, CAN ventilate” scenario, when 3 attempts at intubation (at least one by an experienced operator) using best equipment (including VL) and technique have been unsuccessful, but BMV remains effective. Since both these scenarios may result in death or devastating anoxic injury [6, 7], every provider who participates in airway management of the critically ill neurological patient must have a basic knowledge of the approach to a failed airway. In order to provide the most direct and dependable path to a definitive airway, the ENLS intubation algorithm recommends an attempt at placement of a supraglottic airway (SGA), such as a Laryngeal Mask Airway, in the event of a failed airway. SGAs have a high success rate with inexperienced providers and typically require limited training to use [99, 100]. Second generation SGAs, that include features such as a bite-block and an esophageal/gastric channel, or SGAs that can serve as conduits for subsequent blind or fiberoptic-guided passage of an ETT, are preferable [101]. Up to 2 attempts, with different operators and/or techniques, are permissible as long as the SpO2 remains >94%. Apneic oxygenation should be continued and BMV performed between attempts. If SGA placement is successful and gas-exchange established, insertion of an ETT through the SGA may be attempted. Alternatively, an urgent tracheostomy should be performed. When SGA insertion is unsuccessful, or oxygen desaturation occurs at any time, immediate cricothyroidotomy should be performed, using a surgical or percutaneous technique. Delays in performing a cricothyroidotomy in patients with a failed airway are an important cause of death and severe morbidity [6, 7].
Basic Ventilator Settings
Immediately following intubation, respiratory and hemodynamic homeostasis should be restored. Except in situations of acute brain herniation, the goals of mechanical ventilation are:
-
Normalization of oxygenation utilizing the lowest FiO2 that will maintain hemoglobin saturation >94%.
-
Normalization of ventilation to achieve a systemic pH of 7.3–7.4, and pCO2 or ETCO2 to 30–40 mmHg.
-
Normalization of the work of breathing.
-
Prevention of ventilator induced lung injury.
In most circumstances, clinicians should default to volume-cycled ventilation at 6–8 cc/kg of ideal body weight and a respiratory rate of 12–14 per minute. However, these settings must take into account the patient’s minute ventilation prior to intubation. Normal pCO2 is an appropriate target unless there is chronic hypercarbia (i.e., severe COPD or sleep-disordered breathing). In situations of chronic hypercarbia, the admission bicarbonate level should be used to estimate the “baseline” pCO2, and that level should subsequently be used as the target. When metabolic acidosis is present, ventilation should target normal pH.
Titrate Ventilation
Induced Hyperventilation: Ventilation, Carbon Dioxide Tension, and Clinical Outcome
Hyperventilation causes cerebral vasoconstriction and decreased CBF, while hypoventilation causes cerebral vasodilation and increased ICP [103]. Dysventilation (and especially hyperventilation) is associated with poor outcomes in traumatic brain injury (TBI) [104,105,106]. The Brain Trauma Foundation recommends targeting eucapnea in patients with brain trauma [42].
However, the relationship between arterial and central pH and pCO2 is complex and incompletely understood. During concomitant metabolic acidosis and TBI, CNS pH and CBF are often preserved despite severe systemic acidosis due to the blood brain barrier and the central nervous system (CNS) buffering capacity [107]. Alternatively, in patients with chronic respiratory acidosis, the set-point of cerebral CO2 reactivity changes. It is therefore recommended that mechanical ventilation be adjusted to correct the pH and not the pCO2, or that the estimated “pre-morbid” pCO2 target be used (see Table 2 below). This is a practical goal since ventilating these patients to “normal” pCO2 targets may be extremely difficult or impossible when obstructive lung disease is present.
Herniation: Intentional Hyperventilation to Treat Brain Herniation and Increased ICP
When a patient develops brain herniation with elevated intracranial pressure, hyperventilation is an appropriate temporizing intervention designed to acutely decrease ICP and prevent subsequent neuronal injury and death [108, 109]. Maximal cerebral vasoconstriction is achieved at a pCO2 near 20 mmHg. Therefore, hyperventilation below this level results in no further therapeutic advantage and even less profound pCO2 reductions may impede venous return to the heart, decrease blood pressure, and exacerbate cerebral hypoperfusion.
During hyperventilation, end tidal CO2 monitoring (quantitative capnography) is suggested. As soon as other treatments to control ICP are in place (e.g., blood pressure support, osmotherapy, surgical decompression, hypothermia, metabolic therapy), hyperventilation should be weaned to restore brain perfusion [110].
Hyperventilation for increased ICP is not safe or effective when employed for a prolonged period [42, 111, 112]. Hyperventilation severely reduces CBF, increases the volume of ischaemic tissue, and, may result in rebound elevation of ICP during weaning [113,114,115]. When prolonged (mild) hyperventilation is employed, it is strongly recommended that end-tidal CO2 and cerebral metabolic monitoring (jugular oximetry, CBF, brain tissue oxygen, or cerebral microdialysis) be used to verify the adequacy of tissue perfusion.
Acidemic and Alkalemic Hypocarbia: Potential for Suppression of Spontaneous Hyperventilation
There are two circumstances that should be considered in patients with spontaneous hypocarbia: those whose response to systemic metabolic acidosis accounts for their high ventilatory demand, and those (alkalotic) patients in whom ventilation exceeds systemic metabolic needs.
In patients whose ventilation is driven by metabolic acidosis, suppression of the respiratory drive with sedation or neuromuscular blockade is not recommended, unless direct measurement of brain chemistry suggests that hyperventilation is driving cerebral metabolic crisis. Under these circumstances, clinicians must find another means to buffer pH.
Mechanically ventilated TBI patients presenting with hypocarbia have worse outcomes than their normocarbic peers. However, non-intubated patients presenting with hypocarbia do not exhibit similar findings, suggesting that hypocarbia, in this setting, may be a physiologic response and should not be suppressed [105].
Despite decades of observation and consideration, little is known about alkalemic hypocarbia in patients with an acute brain injury. Alkalemic hypocarbia following brain injury may be theoretically explained by a variety of physiologic and pathophysiologic mechanisms, more than one of which may be present in an individual patient:
-
Brain tissue acidosis requiring acute hyperventilation as a buffer until CNS bicarbonate-generating compensatory mechanisms can catch up.
-
Inadequately treated pain, anxiety, fear, or agitation.
-
Fever.
-
Auto-regulation of elevated ICP.
-
Heme breakdown products or a lactic acid load in the ventricular system.
-
Direct pressure on chemoreceptors present in the floor of the 4th ventricle.
-
Physiologic dysregulation of the medullary respiratory rhythm generator, which has afferent inputs from the pons, mesencephalon, and higher cortical centers.
A recent trial of patients with severe brain injury monitored for brain tissue oxygen showed brain tissue hypoxia worsened when EtCO2 values were reduced by spontaneous alkalemic hyperventilation, suggesting possible harm [116]. It is rarely known whether alkalemic hypocapnia is a physiologic or pathophysiologic process, suppression of this respiratory activity is recommended only in response to evidence that hyperventilation is causing direct harm, either by inducing cerebral ischemia or indirectly by increased systemic metabolic demands and work of breathing.
Oxygenation and Outcomes
Hypoxia is a major source of secondary brain injury [117], and the injured and ischaemic brain is particularly vulnerable to low oxygen levels. Similarly, supra-physiologic levels of oxygen provided to acutely ill patients have the potential to worsen reperfusion injury and outcomes [118, 119]. Hyperoxia drives the formation of reactive oxygen species, overwhelming antioxidants at sites of tissue injury; directly injures respiratory epithelium and alveoli inducing inflammation; drives hypercarbia; and leads to absorption atelectasis in the lung. Hyperoxia (PaO2 > 300 mmHg) immediately following resuscitation is independently associated with poor outcomes in traumatic brain injury (TBI) and cardiac arrest [119, 120], though not all published data are in accordance [121,122,123].
It is recommended that 100% oxygen be provided for pre-oxygenation immediately prior to intubation, but that oxygen be immediately weaned following intubation to 50%, or the lowest FiO2 that will support an oxyhemoglobin saturation of 95–100%. This normoxic resuscitation strategy is recommended in the 2010 American Heart Association Guidelines for post-resuscitation care after cardiac arrest [121].
Oxygenation and Ventilation Monitoring
Oxygenation should be monitored by pulse oximetry or by arterial blood gas analysis when oximetry is suspected to be inaccurate. Conditions of poor perfusion to the extremities, acidosis, vasopressor use, anemia, carboxyhemoglobinemia and methemoglobinemia, and hypoxia all have the potential to compromise the accuracy of pulse oximetry measurements [124].
Ventilation is traditionally monitored by serial arterial blood gas analysis, though venous blood gas analysis may provide an adequate surrogate when arterial samples cannot be obtained. End tidal quantitative capnography of exhaled gases provides an appealing continuous measurement and is extremely useful to monitor trends in ventilation. One study showed that severely hyperventilated head trauma patients (pCO2 < 25 mmHg) in the prehospital environment had higher mortality, and that use of quantitative capnography by paramedics significantly decreased the incidence of hyperventilation [125]. A similar study in patients with major trauma showed a much higher incidence of “normocapnea” on hospital arrival when ETCO2 was monitored by medics.
Because ETCO2 measurements reflect not only ventilation but also systemic perfusion, the correlation between ETCO2 and pCO2 in the blood is variable, especially when severe physiologic derangements are present [126]. In an inpatient environment, ETCO2 measurements should always be correlated with an arterial pCO2 sample. ETCO2 and pCO2 may also vary significantly when lung disease and ventilation-perfusion mismatch are present [127].
Lung Injury
Patients with acute lung injury (ALI) or the acute respiratory distress syndrome (ARDS) are vulnerable to lung injury known variably as ventilator induced lung injury (VILI) or ventilator associated lung injury (VALI). ALI and ARDS represent a spectrum of disease characterized by bilateral parenchymal pulmonary infiltrates, significant hypoxia, absence of left ventricular dysfunction, and hyperacuity of onset [128]. These patients often require high levels of oxygen and elevated mean airway pressures to achieve adequate gas exchange.
Patients with ALI and ARDS often have high circulating levels of inflammatory mediators, which are associated with injury to other vital organs, and are exacerbated by injurious techniques of ventilation. VALI is thought to be caused by:
-
Barotrauma: induced by high ventilator pressures, particularly a high plateau pressure.
-
Volutrauma: induced by higher tidal volumes, often despite low ventilator pressures.
-
Atelectrauma: shearing injury due to recurrent opening and closing of alveolar sacs that may lack adequate surfactant.
-
High inspired fraction of oxygen.
-
High levels of circulating inflammatory cytokines.
Many modes and techniques of ventilation have been proposed to manage the severe gas exchange abnormalities associated with ARDS, though only a few important issues are covered here.
Patients with ALI or ARDS should be ventilated using a strategy of low tidal volumes (6 cc/kg), low plateau pressures (<30 mmHg), adequate positive end expiratory pressure (PEEP) to prevent cyclic collapse of alveolar units, and inhaled oxygen fraction rapidly weaned to 0.6 or less.
Although the landmark study of low tidal volume mechanical ventilation emphasizes permissive hypercarbia, [128] elevation in carbon dioxide is a potent modulator of CBF and this strategy must be balanced with concerns for intracranial hypertension [129]. Several small studies suggest that lung protective ventilation strategies causing mild hypercarbia in patients with elevated ICP may be tolerated [129, 130], but more data are needed before this may be considered safe in routine practice. Prone positioning may increase ICP [131], although several studies show that a small increase in ICP may be more than offset by dramatic improvements in oxygenation [132].
When lung compliance is low, airway pressure has the potential to be transmitted to the intrathoracic vessels and indirectly increase ICP. Although this physiology was once used to justify low-PEEP ventilation in patients with head injury, subsequent research has shown PEEP in brain injured patients to be well tolerated, especially when lung compliance is poor and adequate blood pressure is maintained [133, 134]. Ventilation without PEEP is discouraged, due to the likelihood of atelectrauma. However, the relationship of airway pressure to ICP, CPP, and cerebral perfusion is of concern, and should be individually reviewed based on each patient’s physiology [135,136,137,138].
Sedation
Recent guidelines have reviewed the management pain, agitation and delirium in the critically ill [139].
Necessity of Sedation
Sedation use in the critically ill neurological patient has both benefits and drawbacks. Sedation may be needed to alleviate fear and anxiety, reduce ICP and cerebral oxygen consumption, facilitate tolerance of the endotracheal tube and mechanical ventilation, or to reduce sympathetic nervous activity. Complications associated with under-sedation include ventilator dysynchrony, patient injury, agitation, anxiety, device removal, and elevated ICP [140,141,142,143,144,145,146,147,148,149,150,151,152,153]. Adequate sedation is paramount in all therapeutic algorithms for the treatment of increased ICP [144, 145], since psychomotor restlessness, pain, and autonomic stress all adversely affect ICP, CBF, CPP, and the cerebral metabolic rate for oxygen metabolism (CMRO2). Conversely, sedation makes accurate neurological examination, the cornerstone of clinical assessment, difficult or impossible. Therapeutic and procedural decision making are often contingent upon an accurate neurological assessment. Acute changes in brain physiology become difficult to detect, and the accuracy of neuroprognostication is decreased [138, 154]. Sedation may cause vasodilation, reducing cerebral perfusion due to hypotension and also reversing physiologically advantageous shunting of blood into areas of ischemia. Even short-acting sedatives are known to accumulate in fatty tissues, causing effects beyond their intended duration. Despite each of the competing interests, adequate consideration must be made for patient comfort (Tables 3, 4).
Depth of Sedation and Sedation Interruption
In a general intensive care unit (ICU) population, the use of excessive sedation and analgesia contributes to increased duration of mechanical ventilation, longer length of stay in the ICU and hospital [146,147,148,149], increased rates of depression, post-traumatic stress disorder, infections, and long-term neurocognitive impairments [149,150,151]. Therefore, while some patients with acute neurological illness will require deep sedation for the control of ICP or the management of status epilepticus, most patients should be lightly sedated, so they are easily arousable and attend to voice, to the extent their neurological injury permits. Sedation should be titrated to a validated sedation score, such as the Richmond Agitation Sedation Scale (RASS, Table 5) [155, 156], or the Riker Sedation Agitation Scale (SAS) [157]. A recent review of sedation assessment tools in the neurocritical care setting concluded that the SAS and Richmond Agitation Sedation Scale (RASS) are valid and useful for patients in the neurointensive care unit [158]. A goal RASS of 0 to −2 (light sedation) is recommended for most patients without a specific indication for deep sedation [139]. Sedation should be titrated to an electrophysiological endpoint when neuromuscular blockade is employed or burst-suppression is desired.
Randomized controlled trials demonstrate that protocols requiring decreases in sedative doses or daily interruption of sedative and analgesic drugs can reduce lengths of mechanical ventilation and ICU stay and reduce drug doses administered [146,147,148]. It is important to recognize that these trials did not include patients who required uninterrupted sedation, such as those with status epilepticus and refractory intracranial hypertension. Patients withdrawing from alcohol, benzodiazepines or other substances may also not tolerate abrupt interruption of sedation. While daily sedation lowering in these patient populations is not therapeutically prudent, patients without these conditions should undergo daily sedation interruption, along with a spontaneous breathing trial when appropriate.
Role of Analgesics
Unless deep sedation or general anesthesia is desired, analgesia should precede sedation. Many patients with adequate pain control do not require sedation, and, conversely, most sedative medications provide no analgesia. Sedation without pain control may be an important cause of delirium. Infusion of short acting analgesics allows for interruption and neurological assessment at intervals. Recent studies have demonstrated that analgosedation, a strategy that focuses on using a short-acting opioid infusion (Remifentanil or Fentanyl) alone to manage pain and discomfort, without a sedative, may result in a reduction in duration of mechanical ventilation and ICU length of stay [152, 159,160,161]. The use of analgosedation should therefore be considered first in all mechanically ventilated patients who do not have a specific indication for a sedative infusion, such as raised ICP, seizures or ongoing use of neuromuscular blockade.
Choice of Sedative
Many patients are likely to require ongoing sedation despite the effective use of analgesia. Several randomized trials have compared the use of benzodiazepine infusions, mostly Midazolam, to Propofol and Dexmedetomidine. A meta-analysis of these studies suggests that both Propofol and Dexmedetomidine are associated with shorter ICU length of stay and duration of mechanical ventilation compared to benzodiazepine infusions [139]. Either Dexmedetomidine or Propofol should therefore be utilized as a first-line agent for continuous sedation, rather than a benzodiazepine.
Non-pharmacological Strategies
Environmental stimuli are important triggers of anxiety and agitation. Providing a calm and reassuring environment, with attention to day-night cycles, restriction of noise, use of appropriate music, and/or the reassuring presence of friends and family may decrease agitation and anxiety [162]. These environmental measures are especially important when a dominant-hemisphere lesion causes aphasia and attempts at verbal communication generate agitated behaviors. The use of “sitters” to re-direct and re-orient confused or agitated patients is preferred to the use of sedating medications. Patients with acute brain injury often have deficits in short term memory, concentration, and emotional control, and their confusion may cause agitation. Confused patients require gentle and repeated re-orientation to situation and circumstances, which may obviate the need for sedation [162].
Common Sedatives in Neurological Intensive Care
Propofol
Propofol is among the best studied sedative agents used in neurological critical care. Pharmacologically, its lipid formulation allows for rapid penetration of the blood brain barrier, resulting in rapid onset and cessation of action. It has potent and immediate depressant effects on cerebral electrical and metabolic activity, and it does not require renal or hepatic metabolism for elimination. Disadvantages include robust vasodilating and hypotensive effects, considerable intravenous lipid load, and the potential for the rare, but frequently fatal, propofol infusion syndrome. This syndrome is characterized by acidosis, hepatic failure, hypertriglyceridemia, and elevated creating kinase level. Propofol infusion syndrome may be fatal and is more common in children and adults when used at higher doses [163]. A recent study, comparing propofol to dexmeditomidine sedation in the neurocritically ill, found high (30%) incidences of hypotension in both groups [164]. Caution must be utilized with these medications when concerns for brain ischemia are present.
Remifentanil
Remifentanil hydrochloride is a mu-opioid agonist exhibiting analgesic effects with a rapid onset and a short duration of action. It is an agent which can be used as part of a combined sedative analgesic approach. A recent mechanistic review suggests that remifentanil may be a cost effective sedative alternative when ICU length of stay is considered [152]. Superiority over fentanyl has not been demonstrated [159].
Benzodiazepines
Midazolam is an appealing sedative option given the rapid onset of action and short duration of effect with bolus administration—making it an ideal agent for procedural sedation. Additionally, due to its potent gamma-aminobutyric acid (GABA) activity and relatively benign hemodynamic profile, midazolam is an important drug in treatment of refractory status epilepticus. As a long-term sedative for general ICU use, midazolam accumulates in adipose tissues, significantly prolonging duration of action unless interruptions or down-titration of dose are routinely utilized.
Bolus-dose midazolam is a good choice for intermittent agitation in a NICU population. Conversely, midazolam infusion has been associated with prolonged mechanical ventilation [139, 164]. Though most studies suggest the impact of midazolam on hemodynamics is similar compared to dexmedetomidine or propofol, a recent report suggests less instability compared to dexmedetomidine [164].
Lorazepam is a longer acting benzodiazepine. The strong GABA activity of lorazepam suppresses electrical and metabolic brain activity. Unlike midazolam, lorazepam is formulated in propylene glycol, which can accumulate to toxic levels causing metabolic acidosis and kidney injury. At lorazepam infusion rates above 3 mg/h or daily doses approaching 1 mg/kg, the osmolar gap should be followed, and alternative agents should be used if the osmolar gap rises above 10–12 mOsm/L [165, 166].
Dexmedetomidine
Dexmedetomidine is a centrally acting alpha agonist similar to clonidine, but more specific for the alpha-2 receptor. It is increasingly utilized for ICU sedation. Desirable properties include rapid onset and termination of activity, mild to moderate sedation without significant respiratory depressant action, analgesic effects, and less delirium than the benzodiazepines [164, 167]. Undesirable properties include a high incidence of bradycardia and hypotension [164, 167].
Barbiturates
Use of barbiturates as a sedative in the neurocritical care unit is limited due to its undesirable side effect profile. Immunosuppressant properties and negative inotropic effects are amongst the more concerning limitations. Barbiturates remain second-line therapy for the control of ICP after propofol. They remain in widespread use to control refractory status epilepticus, and their potent effects on cerebral metabolic and electrical activity make them an appealing class of agents for sedation in the NICU. Pentobarbital serves as a potent agent for deep sedation in patients with refractory status epilepticus or elevated ICP [168,169,170].
No tools for the assessment of delirium have been validated in neurocritical care patients, though several studies that used the Intensive Care Delirium Screening Checklist enrolled NICU patients [171, 172].
Many studies have addressed the sedation monitoring approaches and medication choices for NICU patients. One prospective, randomized trial of 67 mechanically ventilated adult patients sedated with propofol showed that the bispectral index (BIS) monitor added to routine sedation scale monitoring significantly reduced the propofol dose and the time to awakening when compared to subjective scale monitoring alone [173].
Pediatric Considerations
Anatomical and physiological differences alter the approach to intubation and mechanical ventilation of children suffering from neurologic injury. While cervical spinal injury is uncommon in children, approximately half of all cervical spinal injuries are associated with simultaneous traumatic brain injury (TBI) [174]. Therefore, cervical spine precautions should be taken when intubating a child with suspected TBI. Criteria for tracheal intubation of children with TBI and other forms of acute brain injury include hypoxemia unresponsive to supplemental oxygen, apnea, hypercarbia (PaCO2 > 45 mmHg), Glasgow Coma Scale Sore (GCS) ≤8, rapid decrease in GCS, anisocoria >1 mm in the context of altered mental status, spinal injury compromising ventilation, abnormal airway reflexes, and any clinical signs of herniation syndrome [175]. A pediatric version of the GCS is recommended when evaluating children infants with acute brain injury.
Several anatomical differences between the pediatric and adult airway should be considered prior to intubating. Children have a proportionally larger tongue, more compliant epiglottis and upper airway tissues, and a prominent occiput. Positioning can be optimized by placing a small shoulder roll prior to intubation. The infant larynx is more anterior and cephalad (C3–4 vs. C4–5 in adults). The narrowest part occurs at the cricoid ring gives a cone shaped appearance that does not become cylindrical until approximately 8 years of age [176]. Pediatric Advanced Life Support Guidelines advise that oral intubation should be performed while maintaining spine immobilization using a cuffed ET tube in children with TBI [177]. Cuff pressures should not be greater than 20 cm H2O due to the risk of mucosal ischemia [178]. A multi-center, randomized control trial demonstrated no increase in post-extubation stridor or long-term complications when using cuffed tubes [179]. For un-cuffed ET tube sizing in children (if cuffed is not available) use the age-based formula: 4+ (age in years/4) [180,181,182]. If inserting a regular cuffed ET tube, select one full size smaller than determined by the age-based formula [116]. If placing a micro-cuffed ET tube, select a tube one half size smaller than the age-based calculation [180,181,182]. A stylet bent in a hockey stick configuration can help reinforce the rigidity of small ET tubes and help direct them anteriorly through the glottis [183].
When intubating a child less than 2 years old, a straight laryngoscope blade directly lifting the epiglottis may be preferred because of the infant’s large and acutely angled epiglottis. Generally, a straight size 00 laryngoscope blade is appropriate for extremely premature infants, a size 0 for average-sized newborns, a size 1 for most infants beyond the immediate newborn period, and a size 2 blade for children over the age of two. If an appropriately sized ET tube is placed, the ideal depth can be achieved by inserting the tube until the centimeter marking at the lip is three times the internal diameter of the ET tube [184].
It is prudent to assume a full stomach and a cervical spinal injury when intubating a child with TBI. Endotracheal intubation should utilize a cerebral-protective rapid sequence induction with cricoid pressure and pre-oxygenation. Bag-valve-mask ventilation should not be attempted unless the child has apnea, hypoxemia, or signs of impending herniation. However, the time to desaturation following pre-oxygenation is shorter in apneic infants compared to older children (less than 100 s), and a modified RSI technique with gentle pressure-limited mask ventilation (10–12 cm H2O) and 100% oxygen may be used to avoid hypoxemia [185, 186]. This technique may also limit hypercarbia and keeps small airways open without the risk of gastric inflation and related morbidity [187,188,189]. Cricoid pressure is routinely applied despite questionable evidence that it improves clinical outcomes [188, 190, 191].
Pretreatment with lidocaine (1.5 mg/kg IV with max dose 100 mg) may be used but its administration should not delay emergent intubation [192]. Atropine (0.02 mg/kg IV with minimum dose 0.1 mg and max single dose 0.5 mg) is recommended in children ≤1 year old or children <5 years old receiving succinylcholine [193]. For hemodynamically unstable children, the combination of etomidate (0.2–0.6 mg/kg) and neuromuscular blockade with rocuronium (1 mg/kg) or vecuronium (0.3 mg/kg) IV is often used. The association between etomidate administration and clinically significant adrenal insufficiency is not well established in children but can be considered when selecting optimal medications for intubation. Succinylcholine is sometimes avoided because of the risk of malignant hyperthermia possible ICP elevation hyperkalemia, and life threatening complications associated with unknown occult metabolic or neuromuscular disease [194,195,196]. Fentanyl (2–4 mg/kg) or ketamine (1–2 mg/kg) IV are alternative sedatives, and recent pediatric studies show that ketamine does not increase ICP and may be neuroprotective [82, 197, 198]. If hemodynamically stable, midazolam (0.1–0.2 mg/kg) can be added to any of the above combinations.
Children less than 6 months old have immature liver metabolism, which places them at high risk for respiratory depression secondary to narcotic administration [199]. Continuous infusions of propofol, especially over a 24 h period, have been associated with lethal cases of propofol infusion syndrome (metabolic acidosis and death). Therefore, continuous infusion of propofol is not recommended for pediatric patients with acute brain injury [200, 201]. Finally, while information is currently limited, concern exists regarding the potential neurotoxicity of sedatives on the developing brain [202]. The strongest evidence for this comes from animal models, with limited evidence in clinical studies [203,204,205,206,207].
After successful intubation, oxygen saturation of 100% and normocarbia (35–39 mmHg) should be confirmed by arterial blood gas. Unless the child has signs of herniation, prophylactic hyperventilation (PaCO2 <35 mmHg) should be avoided [208]. Adequate blood pressure must always be maintained when administering sedatives to assure adequate cerebral perfusion pressure (CPP). A CPP between 40 and 50 mmHg is recommended for children with severe TBI, with infants at the lower end of this range and adolescents at the upper end [175]. Many studies have demonstrated that a CPP ≤ 40 mmHg is associated with higher mortality and morbidity [209, 210]. However, optimal age appropriate CPP thresholds have not been established for TBI and other acute neurological diagnoses. Furthermore, abnormal cerebrovascular autoregulation, which is more common in children less than 4 years old [211], makes establishing such thresholds difficult in the absence of advanced neuromonitoring.
Prophylactic interventions such as hyperosmolar therapy and hyperventilation are not recommended in the absence of signs or symptoms of herniation or neurological deterioration. In children with refractory intracranial hypertension, transient aggressive hyperventilation or barbiturate administration may be useful. Additional sedation and analgesia, together with prophylactic administration of lidocaine (1 mg/kg IV), may prevent blunt rises in ICP during ET tube suctioning.
Communication
When communicating patient information to an accepting or referring physician, consider including the key elements listed in Table 3.
References
Davis DP, Dunford JV, Ochs M, Park K, Hoyt DB. The use of quantitative end-tidal capnometry to avoid inadvertent severe hyperventilation in patients with head injury after paramedic rapid sequence intubation. J Trauma. 2004;56(4):808–14.
Coplin WM, Pierson DJ, Cooley KD, Newell DW, Rubenfeld GD. Implications of extubation delay in brain-injured patients meeting standard weaning criteria. Am J Respir Crit Care Med. 2000;161(5):1530–6. doi:10.1164/ajrccm.161.5.9905102.
Walls RMMM. Manual of emergency airway management. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008.
Apfelbaum JL, Hagberg CA, Caplan RA, Blitt CD, Connis RT, Nickinovich DG, Hagberg CA, Caplan RA, Benumof JL, Berry FA, Blitt CD, Bode RH, Cheney FW, Connis RT, Guidry OF, Nickinovich DG, Ovassapian A, American Society of Anesthesiologists Task Force on Management of the Difficult A. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on management of the difficult airway. Anesthesiology. 2013;118(2):251–70. doi:10.1097/ALN.0b013e31827773b2.
Orebaugh SL. Difficult airway management in the emergency department. J Emerg Med. 2002;22(1):31–48.
Cook TM, Woodall N, Frerk C, Fourth National Audit P. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth. 2011;106(5):617–31. doi:10.1093/bja/aer058.
Cook TM, Woodall N, Harper J, Benger J, Fourth National Audit P. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth. 2011;106(5):632–42. doi:10.1093/bja/aer059.
Reed MJ, Dunn MJ, McKeown DW. Can an airway assessment score predict difficulty at intubation in the emergency department? Emerg Med J. 2005;22(2):99–102. doi:10.1136/emj.2003.008771.
Mallampati SR, Gatt SP, Gugino LD, Desai SP, Waraksa B, Freiberger D, Liu PL. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32(4):429–34.
Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42(5):487–90.
Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, O’Sullivan EP, Woodall NM, Ahmad I, Difficult Airway Society intubation guidelines working g. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. 2015;115(6):827–48. doi:10.1093/bja/aev371.
Sample GMP, Vandruff T. Code critical airway teams improves patient safety. Crit Care. 2010;14(Suppl 1):231.
L R Designated Airway Emergency Team May Improve Survival Rates at Hospital Discharge. In: Proceedings from the annual meeting of the American society anesthesiologists. New Orleans, LA, 17–21 October 2009.
Cooper RM, O’Sullivan E, Popat M, Behringer E, Hagberg CA. Difficult Airway Society guidelines for the management of tracheal extubation. Anaesthesia. 2013;68(2):217. doi:10.1111/anae.12139.
Law JA, Broemling N, Cooper RM, Drolet P, Duggan LV, Griesdale DE, Hung OR, Jones PM, Kovacs G, Massey S, Morris IR, Mullen T, Murphy MF, Preston R, Naik VN, Scott J, Stacey S, Turkstra TP, Wong DT, Canadian Airway Focus G. The difficult airway with recommendations for management–part 1–difficult tracheal intubation encountered in an unconscious/induced patient. Can J Anaesth. 2013;60(11):1089–118. doi:10.1007/s12630-013-0019-3.
Myatra SN, Ahmed SM, Kundra P, Garg R, Ramkumar V, Patwa A, Shah A, Raveendra US, Shetty SR, Doctor JR, Pawar DK, Ramesh S, Das S, Divatia JV. The All India Difficult Airway Association 2016 guidelines for tracheal intubation in the intensive care unit. Indian J Anaesth. 2016;60(12):922–30. doi:10.4103/0019-5049.195485.
Jaber S, Amraoui J, Lefrant JY, Arich C, Cohendy R, Landreau L, Calvet Y, Capdevila X, Mahamat A, Eledjam JJ. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med. 2006;34(9):2355–61. doi:10.1097/01.CCM.0000233879.58720.87.
Jaber S, Jung B, Corne P, Sebbane M, Muller L, Chanques G, Verzilli D, Jonquet O, Eledjam JJ, Lefrant JY. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Intensive Care Med. 2010;36(2):248–55. doi:10.1007/s00134-009-1717-8.
Simpson GD, Ross MJ, McKeown DW, Ray DC. Tracheal intubation in the critically ill: a multi-centre national study of practice and complications. Br J Anaesth. 2012;108(5):792–9. doi:10.1093/bja/aer504.
Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216–22.
Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien). 1993;59:121–5.
Henzler D, Cooper DJ, Tremayne AB, Rossaint R, Higgins A. Early modifiable factors associated with fatal outcome in patients with severe traumatic brain injury: a case control study. Crit Care Med. 2007;35(4):1027–31. doi:10.1097/01.CCM.0000259526.45894.08.
Jones AE, Shapiro NI, Kilgannon JH, Trzeciak S, Emergency Medicine Shock Research Network i. Goal-directed hemodynamic optimization in the post-cardiac arrest syndrome: a systematic review. Resuscitation. 2008;77(1):26–9. doi:10.1016/j.resuscitation.2007.10.021.
Kilgannon JH, Roberts BW, Reihl LR, Chansky ME, Jones AE, Dellinger RP, Parrillo JE, Trzeciak S. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation. 2008;79(3):410–6. doi:10.1016/j.resuscitation.2008.07.019.
Stocchetti N, Furlan A, Volta F. Hypoxemia and arterial hypotension at the accident scene in head injury. J Trauma. 1996;40(5):764–7.
Trzeciak S, Jones AE, Kilgannon JH, Milcarek B, Hunter K, Shapiro NI, Hollenberg SM, Dellinger P, Parrillo JE. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009;37(11):2895–903 ; quiz 2904.
Schmidt UH, Kumwilaisak K, Bittner E, George E, Hess D. Effects of supervision by attending anesthesiologists on complications of emergency tracheal intubation. Anesthesiology. 2008;109(6):973–7. doi:10.1097/ALN.0b013e31818ddb90.
Sawin PD, Todd MM, Traynelis VC, Farrell SB, Nader A, Sato Y, Clausen JD, Goel VK. Cervical spine motion with direct laryngoscopy and orotracheal intubation. An in vivo cinefluoroscopic study of subjects without cervical abnormality. Anesthesiology. 1996;85(1):26–36.
Collins SR, Blank RS. Fiberoptic intubation: an overview and update. Respir Care. 2014;59(6):865–78. doi:10.4187/respcare.03012 ; discussion 878–880.
Baillard C, Fosse JP, Sebbane M, Chanques G, Vincent F, Courouble P, Cohen Y, Eledjam JJ, Adnet F, Jaber S. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174(2):171–7. doi:10.1164/rccm.200509-1507OC.
Jaber S, Monnin M, Girard M, Conseil M, Cisse M, Carr J, Mahul M, Delay JM, Belafia F, Chanques G, Molinari N, De Jong A. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med. 2016;42(12):1877–87. doi:10.1007/s00134-016-4588-9.
Miguel-Montanes R, Hajage D, Messika J, Bertrand F, Gaudry S, Rafat C, Labbe V, Dufour N, Jean-Baptiste S, Bedet A, Dreyfuss D, Ricard JD. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med. 2015;43(3):574–83. doi:10.1097/CCM.0000000000000743.
Mosier JM, Hypes CD, Sakles JC. Understanding preoxygenation and apneic oxygenation during intubation in the critically ill. Intensive Care Med. 2017;43(2):226–8. doi:10.1007/s00134-016-4426-0.
Vourc’h M, Asfar P, Volteau C, Bachoumas K, Clavieras N, Egreteau PY, Asehnoune K, Mercat A, Reignier J, Jaber S, Prat G, Roquilly A, Brule N, Villers D, Bretonniere C, Guitton C. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015;41(9):1538–48. doi:10.1007/s00134-015-3796-z.
Semler MW, Janz DR, Lentz RJ, Matthews DT, Norman BC, Assad TR, Keriwala RD, Ferrell BA, Noto MJ, McKown AC, Kocurek EG, Warren MA, Huerta LE, Rice TW, Investigators F, Pragmatic Critical Care Research G. Randomized trial of apneic oxygenation during endotracheal intubation of the critically Ill. Am J Respir Crit Care Med. 2016;193(3):273–80. doi:10.1164/rccm.201507-1294OC.
Sakles JC, Mosier JM, Patanwala AE, Arcaris B, Dicken JM. First pass success without hypoxemia is increased with the use of apneic oxygenation during rapid sequence intubation in the emergency department. Acad Emerg Med. 2016;23(6):703–10. doi:10.1111/acem.12931.
Sagarin MJ, Barton ED, Chng YM, Walls RM, National Emergency Airway Registry I. Airway management by US and Canadian emergency medicine residents: a multicenter analysis of more than 6000 endotracheal intubation attempts. Ann Emerg Med. 2005;46(4):328–36.
Li J, Murphy-Lavoie H, Bugas C, Martinez J, Preston C. Complications of emergency intubation with and without paralysis. Am J Emerg Med. 1999;17(2):141–3.
Sakles JC, Laurin EG, Rantapaa AA, Panacek EA. Airway management in the emergency department: a one-year study of 610 tracheal intubations. Ann Emerg Med. 1998;31(3):325–32.
Walls RM. Rapid-sequence intubation in head trauma. Ann Emerg Med. 1993;22(6):1008–13.
Bedford RF, Persing JA, Pobereskin L, Butler A. Lidocaine or thiopental for rapid control of intracranial hypertension? Anesth Analg. 1980;59(6):435–7.
Gabriel EJ, Ghajar J, Jagoda A, Pons PT, Scalea T, Walters BC, Brain Trauma F. Guidelines for prehospital management of traumatic brain injury. J Neurotrauma. 2002;19(1):111–74. doi:10.1089/089771502753460286.
Weingart S. Additional thoughts on the controversy of lidocaine administration before rapid sequence intubation in patients with traumatic brain injuries. Ann Emerg Med. 2007;50(3):353. doi:10.1016/j.annemergmed.2007.02.032.
Donegan MF, Bedford RF. Intravenously administered lidocaine prevents intracranial hypertension during endotracheal suctioning. Anesthesiology. 1980;52(6):516–8.
Salhi B, Stettner E. In defense of the use of lidocaine in rapid sequence intubation. Ann Emerg Med. 2007;49(1):84–6. doi:10.1016/j.annemergmed.2006.09.003.
Reynolds SF, Heffner J. Airway management of the critically ill patient: rapid-sequence intubation. Chest. 2005;127(4):1397–412. doi:10.1378/chest.127.4.1397.
Oddo M, Bosel J, Participants in the International Multidisciplinary Consensus Conference on Multimodality M. Monitoring of brain and systemic oxygenation in neurocritical care patients. Neurocrit Care. 2014;21(Suppl 2):S103–20. doi:10.1007/s12028-014-0024-6.
Prough DS, Lang J. Therapy of patients with head injuries: key parameters for management. J Trauma. 1997;42(5 Suppl):S10–8.
Walsh BK, Crotwell DN, Restrepo RD. Capnography/capnometry during mechanical ventilation: 2011. Respir Care. 2011;56(4):503–9. doi:10.4187/respcare.01175.
Brinjikji W, Murad MH, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anesthesia during endovascular acute ischaemic stroke treatment: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(3):525–9. doi:10.3174/ajnr.A4159.
Schonenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, Nagel S, Klose C, Pfaff J, Bendszus M, Ringleb PA, Kieser M, Mohlenbruch MA, Bosel J. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischaemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316(19):1986–96. doi:10.1001/jama.2016.16623.
Abou-Chebl A, Lin R, Hussain MS, Jovin TG, Levy EI, Liebeskind DS, Yoo AJ, Hsu DP, Rymer MM, Tayal AH, Zaidat OO, Natarajan SK, Nogueira RG, Nanda A, Tian M, Hao Q, Kalia JS, Nguyen TN, Chen M, Gupta R. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multicenter study. Stroke. 2010;41(6):1175–9. doi:10.1161/STROKEAHA.109.574129.
Dhakal LP, Diaz-Gomez JL, Freeman WD. Role of anesthesia for endovascular treatment of ischaemic stroke: do we need neurophysiological monitoring? Stroke. 2015;46(6):1748–54. doi:10.1161/STROKEAHA.115.008223.
Seneviratne J, Mandrekar J, Wijdicks EF, Rabinstein AA. Noninvasive ventilation in myasthenic crisis. Arch Neurol. 2008;65(1):54–8. doi:10.1001/archneurol.2007.1.
Flandreau G, Bourdin G, Leray V, Bayle F, Wallet F, Delannoy B, Durante G, Vincent B, Barbier J, Burle JF, Passant S, Richard JC, Guerin C. Management and long-term outcome of patients with chronic neuromuscular disease admitted to the intensive care unit for acute respiratory failure: a single-center retrospective study. Respir Care. 2011;56(7):953–60. doi:10.4187/respcare.00862.
Piastra M, Antonelli M, Caresta E, Chiaretti A, Polidori G, Conti G. Noninvasive ventilation in childhood acute neuromuscular respiratory failure: a pilot study. Respiration. 2006;73(6):791–8. doi:10.1159/000090777.
Abel M, Eisenkraft JB. Anesthetic implications of myasthenia gravis. Mt Sinai J Med. 2002;69(1–2):31–7.
Austin N, Krishnamoorthy V, Dagal A. Airway management in cervical spine injury. Int J Crit Illn Inj Sci. 2014;4(1):50–6. doi:10.4103/2229-5151.128013.
Hauswald M, Sklar DP, Tandberg D, Garcia JF. Cervical spine movement during airway management: cinefluoroscopic appraisal in human cadavers. Am J Emerg Med. 1991;9(6):535–8.
Rosenblatt WH, Wagner PJ, Ovassapian A, Kain ZN. Practice patterns in managing the difficult airway by anesthesiologists in the United States. Anesth Analg. 1998;87(1):153–7.
Malcharek MJ, Rogos B, Watzlawek S, Sorge O, Sablotzki A, Gille J, Larson CP Jr. Awake fiberoptic intubation and self-positioning in patients at risk of secondary cervical injury: a pilot study. J Neurosurg Anesthesiol. 2012;24(3):217–21. doi:10.1097/ANA.0b013e31824da7e5.
Yeganeh N, Roshani B, Azizi B, Almasi A. Target-controlled infusion of remifentanil to provide analgesia for awake nasotracheal fiberoptic intubations in cervical trauma patients. J Trauma. 2010;69(5):1185–90. doi:10.1097/TA.0b013e3181cb4434.
Avitsian R, Lin J, Lotto M, Ebrahim Z. Dexmedetomidine and awake fiberoptic intubation for possible cervical spine myelopathy: a clinical series. J Neurosurg Anesthesiol. 2005;17(2):97–9.
Rice MJ, Mancuso AA, Gibbs C, Morey TE, Gravenstein N, Deitte LA. Cricoid pressure results in compression of the postcricoid hypopharynx: the esophageal position is irrelevant. Anesth Analg. 2009;109(5):1546–52. doi:10.1213/ane.0b013e3181b05404.
Ellis DY, Harris T, Zideman D. Cricoid pressure in emergency department rapid sequence tracheal intubations: a risk-benefit analysis. Ann Emerg Med. 2007;50(6):653–65. doi:10.1016/j.annemergmed.2007.05.006.
Nolan JP, Wilson ME. Orotracheal intubation in patients with potential cervical spine injuries. An indication for the gum elastic bougie. Anaesthesia. 1993;48(7):630–3.
Kill C, Risse J, Wallot P, Seidl P, Steinfeldt T, Wulf H. Videolaryngoscopy with glidescope reduces cervical spine movement in patients with unsecured cervical spine. J Emerg Med. 2013;44(4):750–6. doi:10.1016/j.jemermed.2012.07.080.
Robitaille A, Williams SR, Tremblay MH, Guilbert F, Theriault M, Drolet P. Cervical spine motion during tracheal intubation with manual in-line stabilization: direct laryngoscopy versus GlideScope videolaryngoscopy. Anesth Analg. 2008;106(3):935–41. doi:10.1213/ane.0b013e318161769e.
Turkstra TP, Craen RA, Pelz DM, Gelb AW. Cervical spine motion: a fluoroscopic comparison during intubation with lighted stylet, GlideScope, and Macintosh laryngoscope. Anesth Analg. 2005;101(3):910–5. doi:10.1213/01.ane.0000166975.38649.27.
Brown CA III, Bair AE, Pallin DJ, Laurin EG, Walls RM, National Emergency Airway Registry I. Improved glottic exposure with the Video Macintosh Laryngoscope in adult emergency department tracheal intubations. Ann Emerg Med. 2010;56(2):83–8. doi:10.1016/j.annemergmed.2010.01.033.
Noppens RR, Mobus S, Heid F, Schmidtmann I, Werner C, Piepho T. Evaluation of the McGrath Series 5 videolaryngoscope after failed direct laryngoscopy. Anaesthesia. 2010;65(7):716–20. doi:10.1111/j.1365-2044.2010.06388.x.
Piepho T, Fortmueller K, Heid FM, Schmidtmann I, Werner C, Noppens RR. Performance of the C-MAC video laryngoscope in patients after a limited glottic view using Macintosh laryngoscopy. Anaesthesia. 2011;66(12):1101–5. doi:10.1111/j.1365-2044.2011.06872.x.
Griesdale DE, Liu D, McKinney J, Choi PT. Glidescope(R) video-laryngoscopy versus direct laryngoscopy for endotracheal intubation: a systematic review and meta-analysis. Can J Anaesth. 2012;59(1):41–52. doi:10.1007/s12630-011-9620-5.
Su YC, Chen CC, Lee YK, Lee JY, Lin KJ. Comparison of video laryngoscopes with direct laryngoscopy for tracheal intubation: a meta-analysis of randomised trials. Eur J Anaesthesiol. 2011;28(11):788–95. doi:10.1097/EJA.0b013e32834a34f3.
De Jong A, Molinari N, Conseil M, Coisel Y, Pouzeratte Y, Belafia F, Jung B, Chanques G, Jaber S. Video laryngoscopy versus direct laryngoscopy for orotracheal intubation in the intensive care unit: a systematic review and meta-analysis. Intensive Care Med. 2014;40(5):629–39. doi:10.1007/s00134-014-3236-5.
Bergen JM, Smith DC. A review of etomidate for rapid sequence intubation in the emergency department. J Emerg Med. 1997;15(2):221–30.
Moss E, Powell D, Gibson RM, McDowall DG. Effect of etomidate on intracranial pressure and cerebral perfusion pressure. Br J Anaesth. 1979;51(4):347–52.
Kox WJ, von Heymann C, Heinze J, Prichep LS, John ER, Rundshagen I. Electroencephalographic mapping during routine clinical practice: cortical arousal during tracheal intubation? Anesth Analg. 2006;102(3):825–31. doi:10.1213/01.ane.0000197776.26307.fa.
Reddy RV, Moorthy SS, Dierdorf SF, Deitch RD Jr., Link L. Excitatory effects and electroencephalographic correlation of etomidate, thiopental, methohexital, and propofol. Anesth Analg. 1993;77(5):1008–11.
Langsjo JW, Maksimow A, Salmi E, Kaisti K, Aalto S, Oikonen V, Hinkka S, Aantaa R, Sipila H, Viljanen T, Parkkola R, Scheinin H. S-ketamine anesthesia increases cerebral blood flow in excess of the metabolic needs in humans. Anesthesiology. 2005;103(2):258–68.
Bourgoin A, Albanese J, Wereszczynski N, Charbit M, Vialet R, Martin C. Safety of sedation with ketamine in severe head injury patients: comparison with sufentanil. Crit Care Med. 2003;31(3):711–7. doi:10.1097/01.CCM.0000044505.24727.16.
Bar-Joseph G, Guilburd Y, Tamir A, Guilburd JN. Effectiveness of ketamine in decreasing intracranial pressure in children with intracranial hypertension. J Neurosurg Pediatr. 2009;4(1):40–6. doi:10.3171/2009.1.PEDS08319.
Himmelseher S, Durieux ME. Revising a dogma: ketamine for patients with neurological injury? Anesth Analg. 2005;101(2):524–34. doi:10.1213/01.ANE.0000160585.43587.5B.
Albanese J, Arnaud S, Rey M, Thomachot L, Alliez B, Martin C. Ketamine decreases intracranial pressure and electroencephalographic activity in traumatic brain injury patients during propofol sedation. Anesthesiology. 1997;87(6):1328–34.
Kovarik WD, Mayberg TS, Lam AM, Mathisen TL, Winn HR. Succinylcholine does not change intracranial pressure, cerebral blood flow velocity, or the electroencephalogram in patients with neurologic injury. Anesth Analg. 1994;78(3):469–73.
Martyn JA, Richtsfeld M. Succinylcholine-induced hyperkalemia in acquired pathologic states: etiologic factors and molecular mechanisms. Anesthesiology. 2006;104(1):158–69.
Patanwala AE, Erstad BL, Roe DJ, Sakles JC. Succinylcholine is associated with increased mortality when used for rapid sequence intubation of severely brain injured patients in the emergency department. Pharmacotherapy. 2016;36(1):57–63. doi:10.1002/phar.1683.
Lee C, Jahr JS, Candiotti KA, Warriner B, Zornow MH, Naguib M. Reversal of profound neuromuscular block by sugammadex administered three minutes after rocuronium: a comparison with spontaneous recovery from succinylcholine. Anesthesiology. 2009;110(5):1020–5. doi:10.1097/ALN.0b013e31819dabb0.
Sorensen MK, Bretlau C, Gatke MR, Sorensen AM, Rasmussen LS. Rapid sequence induction and intubation with rocuronium-sugammadex compared with succinylcholine: a randomized trial. Br J Anaesth. 2012;108(4):682–9. doi:10.1093/bja/aer503.
Collins JS, Lemmens HJ, Brodsky JB, Brock-Utne JG, Levitan RM. Laryngoscopy and morbid obesity: a comparison of the “sniff” and “ramped” positions. Obes Surg. 2004;14(9):1171–5. doi:10.1381/0960892042386869.
Lewis SR, Butler AR, Parker J, Cook TM, Smith AF. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database Syst Rev. 2016;11:CD011136. doi:10.1002/14651858.CD011136.pub2.
Janz DR, Semler MW, Lentz RJ, Matthews DT, Assad TR, Norman BC, Keriwala RD, Ferrell BA, Noto MJ, Shaver CM, Richmond BW, Zinggeler Berg J, Rice TW, Facilitating Endotrachea LibLt, apneic Oxygenation Within the ICUI, the Pragmatic Critical Care Research G. Randomized trial of video laryngoscopy for endotracheal intubation of critically ill adults. Crit Care Med. 2016;44(11):1980–7. doi:10.1097/CCM.0000000000001841.
Griesdale DE, Chau A, Isac G, Ayas N, Foster D, Irwin C, Choi P, Canadian Critical Care Trials G. Video-laryngoscopy versus direct laryngoscopy in critically ill patients: a pilot randomized trial. Can J Anaesth. 2012;59(11):1032–9. doi:10.1007/s12630-012-9775-8.
Silverberg MJ, Li N, Acquah SO, Kory PD. Comparison of video laryngoscopy versus direct laryngoscopy during urgent endotracheal intubation: a randomized controlled trial. Crit Care Med. 2015;43(3):636–41. doi:10.1097/CCM.0000000000000751.
Lascarrou JB, Boisrame-Helms J, Bailly A, Le Thuaut A, Kamel T, Mercier E, Ricard JD, Lemiale V, Colin G, Mira JP, Meziani F, Messika J, Dequin PF, Boulain T, Azoulay E, Champigneulle B, Reignier J, Clinical Research in Intensive C, Sepsis G. Video laryngoscopy vs direct laryngoscopy on successful first-pass orotracheal intubation among ICU patients: a randomized clinical trial. JAMA. 2017;317(5):483–93. doi:10.1001/jama.2016.20603.
Sakles JC, Kalin L. The effect of stylet choice on the success rate of intubation using the GlideScope video laryngoscope in the emergency department. Acad Emerg Med. 2012;19(2):235–8. doi:10.1111/j.1553-2712.2011.01271.x.
Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39(11):1105–11.
Yentis SM, Lee DJ. Evaluation of an improved scoring system for the grading of direct laryngoscopy. Anaesthesia. 1998;53(11):1041–4.
Murray MJ, Vermeulen MJ, Morrison LJ, Waite T. Evaluation of prehospital insertion of the laryngeal mask airway by primary care paramedics with only classroom mannequin training. CJEM. 2002;4(5):338–43.
Bosch J, de Nooij J, de Visser M, Cannegieter SC, Terpstra NJ, Heringhaus C, Burggraaf J. Prehospital use in emergency patients of a laryngeal mask airway by ambulance paramedics is a safe and effective alternative for endotracheal intubation. Emerg Med J. 2014;31(9):750–3. doi:10.1136/emermed-2012-202283.
Cook TM, Kelly FE. Time to abandon the ‘vintage’ laryngeal mask airway and adopt second-generation supraglottic airway devices as first choice. Br J Anaesth. 2015;115(4):497–9. doi:10.1093/bja/aev156.
McConnell RA, Kerlin MP, Schweickert WD, Ahmad F, Patel MS, Fuchs BD. Using a post-intubation checklist and time out to expedite mechanical ventilation monitoring: observational study of a quality improvement intervention. Respir Care. 2016;61(7):902–12. doi:10.4187/respcare.04191.
Dumont TM, Visioni AJ, Rughani AI, Tranmer BI, Crookes B. Inappropriate prehospital ventilation in severe traumatic brain injury increases in-hospital mortality. J Neurotrauma. 2010;27(7):1233–41. doi:10.1089/neu.2009.1216.
Davis DP, Idris AH, Sise MJ, Kennedy F, Eastman AB, Velky T, Vilke GM, Hoyt DB. Early ventilation and outcome in patients with moderate to severe traumatic brain injury. Crit Care Med. 2006;34(4):1202–8. doi:10.1097/01.CCM.0000208359.74623.1C.
Davis DP, Stern J, Sise MJ, Hoyt DB. A follow-up analysis of factors associated with head-injury mortality after paramedic rapid sequence intubation. J Trauma. 2005;59(2):486–90.
Rangel-Castilla L, Lara LR, Gopinath S, Swank PR, Valadka A, Robertson C. Cerebral hemodynamic effects of acute hyperoxia and hyperventilation after severe traumatic brain injury. J Neurotrauma. 2010;27(10):1853–63. doi:10.1089/neu.2010.1339.
Koenig MA, Bryan M, Lewin JL III, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70(13):1023–9. doi:10.1212/01.wnl.0000304042.05557.60.
Qureshi AI, Geocadin RG, Suarez JI, Ulatowski JA. Long-term outcome after medical reversal of transtentorial herniation in patients with supratentorial mass lesions. Crit Care Med. 2000;28(5):1556–64.
Oertel M, Kelly DF, Lee JH, McArthur DL, Glenn TC, Vespa P, Boscardin WJ, Hovda DA, Martin NA. Efficacy of hyperventilation, blood pressure elevation, and metabolic suppression therapy in controlling intracranial pressure after head injury. J Neurosurg. 2002;97(5):1045–53. doi:10.3171/jns.2002.97.5.1045.
Muizelaar JP, Marmarou A, Ward JD, Kontos HA, Choi SC, Becker DP, Gruemer H, Young HF. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75(5):731–9. doi:10.3171/jns.1991.75.5.0731.
Stocchetti N, Maas AI, Chieregato A, van der Plas AA. Hyperventilation in head injury: a review. Chest. 2005;127(5):1812–27. doi:10.1378/chest.127.5.1812.
Coles JP, Fryer TD, Coleman MR, Smielewski P, Gupta AK, Minhas PS, Aigbirhio F, Chatfield DA, Williams GB, Boniface S, Carpenter TA, Clark JC, Pickard JD, Menon DK. Hyperventilation following head injury: effect on ischaemic burden and cerebral oxidative metabolism. Crit Care Med. 2007;35(2):568–78. doi:10.1097/01.CCM.0000254066.37187.88.
Coles JP, Minhas PS, Fryer TD, Smielewski P, Aigbirihio F, Donovan T, Downey SP, Williams G, Chatfield D, Matthews JC, Gupta AK, Carpenter TA, Clark JC, Pickard JD, Menon DK. Effect of hyperventilation on cerebral blood flow in traumatic head injury: clinical relevance and monitoring correlates. Crit Care Med. 2002;30(9):1950–9. doi:10.1097/01.CCM.0000026331.91456.9A.
Diringer MN, Videen TO, Yundt K, Zazulia AR, Aiyagari V, Dacey RG Jr., Grubb RL, Powers WJ. Regional cerebrovascular and metabolic effects of hyperventilation after severe traumatic brain injury. J Neurosurg. 2002;96(1):103–8. doi:10.3171/jns.2002.96.1.0103.
Carrera E, Schmidt JM, Fernandez L, Kurtz P, Merkow M, Stuart M, Lee K, Claassen J, Sander Connolly E, Mayer SA, Badjatia N. Spontaneous hyperventilation and brain tissue hypoxia in patients with severe brain injury. J Neurol Neurosurg Psychiatry. 2010;81(7):793–7. doi:10.1136/jnnp.2009.174425.
Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, Parrillo JE, Trzeciak S, Emergency Medicine Shock Research Network I. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165–71. doi:10.1001/jama.2010.707.
Balan IS, Fiskum G, Hazelton J, Cotto-Cumba C, Rosenthal RE. Oximetry-guided reoxygenation improves neurological outcome after experimental cardiac arrest. Stroke. 2006;37(12):3008–13. doi:10.1161/01.STR.0000248455.73785.b1.
Brucken A, Kaab AB, Kottmann K, Rossaint R, Nolte KW, Weis J, Fries M. Reducing the duration of 100% oxygen ventilation in the early reperfusion period after cardiopulmonary resuscitation decreases striatal brain damage. Resuscitation. 2010;81(12):1698–703. doi:10.1016/j.resuscitation.2010.06.027.
Kilgannon JH, Jones AE, Parrillo JE, Dellinger RP, Milcarek B, Hunter K, Shapiro NI, Trzeciak S, Emergency Medicine Shock Research Network I. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123(23):2717–22. doi:10.1161/CIRCULATIONAHA.110.001016.
Bellomo R, Bailey M, Eastwood GM, Nichol A, Pilcher D, Hart GK, Reade MC, Egi M, Cooper DJ, Study of Oxygen in Critical Care G. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15(2):R90. doi:10.1186/cc10090.
Wang CH, Chang WT, Huang CH, Tsai MS, Yu PH, Wang AY, Chen NC, Chen WJ. The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta-analysis of observational studies. Resuscitation. 2014;85(9):1142–8. doi:10.1016/j.resuscitation.2014.05.021.
Elmer J, Scutella M, Pullalarevu R, Wang B, Vaghasia N, Trzeciak S, Rosario-Rivera BL, Guyette FX, Rittenberger JC, Dezfulian C, Pittsburgh Post-Cardiac Arrest S. The association between hyperoxia and patient outcomes after cardiac arrest: analysis of a high-resolution database. Intensive Care Med. 2015;41(1):49–57. doi:10.1007/s00134-014-3555-6.
Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Vanden Hoek TL, Kronick SL, American Heart A. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S768–86. doi:10.1161/CIRCULATIONAHA.110.971002.
CC M Pulse oximetry in adults. http://www.uptodate.com/. Accessed 13 Mar 2017.
Dyer BA, White WA Jr., Lee D, Elkins L, Slayton DJ. The relationship between arterial carbon dioxide tension and end-tidal carbon dioxide tension in intubated adults with traumatic brain injuries who required emergency craniotomies. Crit Care Nurs Q. 2013;36(3):310–5. doi:10.1097/CNQ.0b013e318294ea8f.
Helm M, Schuster R, Hauke J, Lampl L. Tight control of prehospital ventilation by capnography in major trauma victims. Br J Anaesth. 2003;90(3):327–32.
Hardman JG, Aitkenhead AR. Estimating alveolar dead space from the arterial to end-tidal CO(2) gradient: a modeling analysis. Anesth Analg. 2003;97(6):1846–51.
The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342(18):1301–8. doi:10.1056/NEJM200005043421801.
Petridis AK, Doukas A, Kienke S, Maslehaty H, Mahvash M, Barth H, Mehdorn HM. The effect of lung-protective permissive hypercapnia in intracerebral pressure in patients with subarachnoid haemorrhage and ARDS. A retrospective study. Acta Neurochir (Wien). 2010;152(12):2143–5. doi:10.1007/s00701-010-0761-z.
Bennett SS, Graffagnino C, Borel CO, James ML. Use of high frequency oscillatory ventilation (HFOV) in neurocritical care patients. Neurocrit Care. 2007;7(3):221–6. doi:10.1007/s12028-007-0084-y.
Roth C, Ferbert A, Deinsberger W, Kleffmann J, Kastner S, Godau J, Schuler M, Tryba M, Gehling M. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care. 2014;21(2):186–91. doi:10.1007/s12028-014-0004-x.
Reinprecht A, Greher M, Wolfsberger S, Dietrich W, Illievich UM, Gruber A. Prone position in subarachnoid hemorrhage patients with acute respiratory distress syndrome: effects on cerebral tissue oxygenation and intracranial pressure. Crit Care Med. 2003;31(6):1831–8. doi:10.1097/01.CCM.0000063453.93855.0A.
Caricato A, Conti G, Della Corte F, Mancino A, Santilli F, Sandroni C, Proietti R, Antonelli M. Effects of PEEP on the intracranial system of patients with head injury and subarachnoid hemorrhage: the role of respiratory system compliance. J Trauma. 2005;58(3):571–6.
Muench E, Bauhuf C, Roth H, Horn P, Phillips M, Marquetant N, Quintel M, Vajkoczy P. Effects of positive end-expiratory pressure on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation. Crit Care Med. 2005;33(10):2367–72.
Schramm P, Closhen D, Felkel M, Berres M, Klein KU, David M, Werner C, Engelhard K. Influence of PEEP on cerebral blood flow and cerebrovascular autoregulation in patients with acute respiratory distress syndrome. J Neurosurg Anesthesiol. 2013;25(2):162–7. doi:10.1097/ANA.0b013e31827c2f46.
Marik PE, Young A, Sibole S, Levitov A. The effect of APRV ventilation on ICP and cerebral hemodynamics. Neurocrit Care. 2012;17(2):219–23. doi:10.1007/s12028-012-9739-4.
Koutsoukou A, Perraki H, Raftopoulou A, Koulouris N, Sotiropoulou C, Kotanidou A, Orfanos S, Roussos C. Respiratory mechanics in brain-damaged patients. Intensive Care Med. 2006;32(12):1947–54. doi:10.1007/s00134-006-0406-0.
Riker RR, Fugate JE, Participants in the International Multi-disciplinary Consensus Conference on Multimodality M. Clinical monitoring scales in acute brain injury: assessment of coma, pain, agitation, and delirium. Neurocrit Care. 2014;21(Suppl 2):S27–37. doi:10.1007/s12028-014-0025-5.
Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R, American College of Critical Care M. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi:10.1097/CCM.0b013e3182783b72.
Riker RR, Fraser GL. Altering intensive care sedation paradigms to improve patient outcomes. Anesthesiol Clin. 2011;29(4):663–74. doi:10.1016/j.anclin.2011.09.006.
Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM, Crippen DW, Fuchs BD, Kelleher RM, Marik PE, Nasraway SA Jr., Murray MJ, Peruzzi WT, Lumb PD, Task Force of the American College of Critical Care Medicine of the Society of Critical Care Medicine ASoH-SPACoCP. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–41.
Fraser GL, Riker RR, Prato BS, Wilkins ML. The frequency and cost of patient-initiated device removal in the ICU. Pharmacotherapy. 2001;21(1):1–6.
Skoglund K, Enblad P, Marklund N. Effects of the neurological wake-up test on intracranial pressure and cerebral perfusion pressure in brain-injured patients. Neurocrit Care. 2009;11(2):135–42. doi:10.1007/s12028-009-9255-3.
Brain Trauma F, American Association of Neurological S, Congress of Neurological S, Joint Section on N, Critical Care AC, Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37–44. doi:10.1089/neu.2007.9990.
Citerio G, Cormio M. Sedation in neurointensive care: advances in understanding and practice. Curr Opin Crit Care. 2003;9(2):120–6.
Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, Kollef MH. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27(12):2609–15.
Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–7. doi:10.1056/NEJM200005183422002.
Treggiari MM, Romand JA, Yanez ND, Deem SA, Goldberg J, Hudson L, Heidegger CP, Weiss NS. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med. 2009;37(9):2527–34. doi:10.1097/CCM.0b013e3181a5689f.
Mehta S, Burry L, Martinez-Motta JC, Stewart TE, Hallett D, McDonald E, Clarke F, Macdonald R, Granton J, Matte A, Wong C, Suri A, Cook DJ, Canadian Critical Care Trials G. A randomized trial of daily awakening in critically ill patients managed with a sedation protocol: a pilot trial. Crit Care Med. 2008;36(7):2092–9. doi:10.1097/CCM.0b013e31817bff85.
Jones C, Backman C, Capuzzo M, Flaatten H, Rylander C, Griffiths RD. Precipitants of post-traumatic stress disorder following intensive care: a hypothesis generating study of diversity in care. Intensive Care Med. 2007;33(6):978–85. doi:10.1007/s00134-007-0600-8.
Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130(3):869–78. doi:10.1378/chest.130.3.869.
Al MJ, Hakkaart L, Tan SS, Bakker J. Cost-consequence analysis of remifentanil-based analgo-sedation vs. conventional analgesia and sedation for patients on mechanical ventilation in the Netherlands. Crit Care. 2010;14(6):R195. doi:10.1186/cc9313.
Erdman MJ, Doepker BA, Gerlach AT, Phillips GS, Elijovich L, Jones GM. A comparison of severe hemodynamic disturbances between dexmedetomidine and propofol for sedation in neurocritical care patients. Crit Care Med. 2014;42(7):1696–702. doi:10.1097/CCM.0000000000000328.
Samaniego EA, Mlynash M, Caulfield AF, Eyngorn I, Wijman CA. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care. 2011;15(1):113–9. doi:10.1007/s12028-010-9412-8.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–44. doi:10.1164/rccm.2107138.
Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, Sessler CN, Dittus RS, Bernard GR. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289(22):2983–91. doi:10.1001/jama.289.22.2983.
Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–9.
Deogaonkar A, Gupta R, DeGeorgia M, Sabharwal V, Gopakumaran B, Schubert A, Provencio JJ. Bispectral Index monitoring correlates with sedation scales in brain-injured patients. Crit Care Med. 2004;32(12):2403–6.
Spies C, Macguill M, Heymann A, Ganea C, Krahne D, Assman A, Kosiek HR, Scholtz K, Wernecke KD, Martin J. A prospective, randomized, double-blind, multicenter study comparing remifentanil with fentanyl in mechanically ventilated patients. Intensive Care Med. 2011;37(3):469–76. doi:10.1007/s00134-010-2100-5.
Devabhakthuni S, Armahizer MJ, Dasta JF, Kane-Gill SL. Analgosedation: a paradigm shift in intensive care unit sedation practice. Ann Pharmacother. 2012;46(4):530–40. doi:10.1345/aph.1Q525.
Karabinis A, Mandragos K, Stergiopoulos S, Komnos A, Soukup J, Speelberg B, Kirkham AJ. Safety and efficacy of analgesia-based sedation with remifentanil versus standard hypnotic-based regimens in intensive care unit patients with brain injuries: a randomised, controlled trial [ISRCTN50308308]. Crit Care. 2004;8(4):R268–80. doi:10.1186/cc2896.
Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375(9713):475–80. doi:10.1016/S0140-6736(09)62072-9.
Iyer VN, Hoel R, Rabinstein AA. Propofol infusion syndrome in patients with refractory status epilepticus: an 11-year clinical experience. Crit Care Med. 2009;37(12):3024–30. doi:10.1097/CCM.0b013e3181b08ac7.
Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J, Dexmedetomidine for Long-Term Sedation I. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151–60. doi:10.1001/jama.2012.304.
Horinek EL, Kiser TH, Fish DN, MacLaren R. Propylene glycol accumulation in critically ill patients receiving continuous intravenous lorazepam infusions. Ann Pharmacother. 2009;43(12):1964–71. doi:10.1345/aph.1M313.
Yahwak JA, Riker RR, Fraser GL, Subak-Sharpe S. Determination of a lorazepam dose threshold for using the osmol gap to monitor for propylene glycol toxicity. Pharmacotherapy. 2008;28(8):984–91. doi:10.1592/phco.28.8.984.
Grof TM, Bledsoe KA. Evaluating the use of dexmedetomidine in neurocritical care patients. Neurocrit Care. 2010;12(3):356–61. doi:10.1007/s12028-008-9156-x.
Chen HI, Malhotra NR, Oddo M, Heuer GG, Levine JM, LeRoux PD. Barbiturate infusion for intractable intracranial hypertension and its effect on brain oxygenation. Neurosurgery. 2008;63(5):880–6. doi:10.1227/01.NEU.0000327882.10629.06 ; discussion 886–7.
Marshall GT, James RF, Landman MP, O’Neill PJ, Cotton BA, Hansen EN, Morris JA Jr., May AK. Pentobarbital coma for refractory intra-cranial hypertension after severe traumatic brain injury: mortality predictions and one-year outcomes in 55 patients. J Trauma. 2010;69(2):275–83. doi:10.1097/TA.0b013e3181de74c7.
Teitelbaum JS, Ayoub O, Skrobik Y. A critical appraisal of sedation, analgesia and delirium in neurocritical care. Can J Neurol Sci. 2011;38(6):815–25.
Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859–64.
Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13(3):R77. doi:10.1186/cc7892.
Olson DM, Thoyre SM, Peterson ED, Graffagnino C. A randomized evaluation of bispectral index-augmented sedation assessment in neurological patients. Neurocrit Care. 2009;11(1):20–7. doi:10.1007/s12028-008-9184-6.
Brown RL, Brunn MA, Garcia VF. Cervical spine injuries in children: a review of 103 patients treated consecutively at a level 1 pediatric trauma center. J Pediatr Surg. 2001;36(8):1107–14. doi:10.1053/jpsu.2001.25665.
Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, Carson S, Chesnut RM, Ghajar J, Goldstein B, Grant GA, Kissoon N, Peterson K, Selden NR, Tasker RC, Tong KA, Vavilala MS, Wainwright MS, Warden CR, American Academy of Pediatrics-Section on Neurological S, American Association of Neurological Surgeons/Congress of Neurological S, Child Neurology S, European Society of P, Neonatal Intensive C, Neurocritical Care S, Pediatric Neurocritical Care Research G, Society of Critical Care M, Paediatric Intensive Care Society UK, Society for Neuroscience in A, Critical C, World Federation of Pediatric I, Critical Care S. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents–second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82. doi:10.1097/PCC.0b013e31823f435c.
Lucking SMF, Tamburro R, Thomas N. The approach to the critically ill infant. In: Lucking SMF, Tamburro R, Thomas N, editors. Pediatric critical care study guide text and review. London: Springer; 2012. p. 690–712.
Kleinman ME, Chameides L, Schexnayder SM, Samson RA, Hazinski MF, Atkins DL, Berg MD, de Caen AR, Fink EL, Freid EB, Hickey RW, Marino BS, Nadkarni VM, Proctor LT, Qureshi FA, Sartorelli K, Topjian A, van der Jagt EW, Zaritsky AL. Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S876–908. doi:10.1161/CIRCULATIONAHA.110.971101.
Galinski M, Treoux V, Garrigue B, Lapostolle F, Borron SW, Adnet F. Intracuff pressures of endotracheal tubes in the management of airway emergencies: the need for pressure monitoring. Ann Emerg Med. 2006;47(6):545–7. doi:10.1016/j.annemergmed.2005.08.012.
Weiss M, Dullenkopf A, Fischer JE, Keller C, Gerber AC, European Paediatric Endotracheal Intubation Study G. Prospective randomized controlled multi-centre trial of cuffed or uncuffed endotracheal tubes in small children. Br J Anaesth. 2009;103(6):867–73. doi:10.1093/bja/aep290.
Khine HH, Corddry DH, Kettrick RG, Martin TM, McCloskey JJ, Rose JB, Theroux MC, Zagnoev M. Comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology. 1997;86(3):627–31; discussion 627A.
King BR, Baker MD, Braitman LE, Seidl-Friedman J, Schreiner MS. Endotracheal tube selection in children: a comparison of four methods. Ann Emerg Med. 1993;22(3):530–4.
Wheeler MCC, Todres ID. The pediatric airway. In: Cote CLJ, Todres ID, editors. A practice of anesthesia for infants and children. 4th ed. Philadelphia: Saunders-Elsevier; 2009. p. 237.
Levitan RM, Pisaturo JT, Kinkle WC, Butler K, Everett WW. Stylet bend angles and tracheal tube passage using a straight-to-cuff shape. Acad Emerg Med. 2006;13(12):1255–8. doi:10.1197/j.aem.2006.06.058.
Phipps LM, Thomas NJ, Gilmore RK, Raymond JA, Bittner TR, Orr RA, Robertson CL. Prospective assessment of guidelines for determining appropriate depth of endotracheal tube placement in children. Pediatr Crit Care Med. 2005;6(5):519–22.
Patel R, Lenczyk M, Hannallah RS, McGill WA. Age and the onset of desaturation in apnoeic children. Can J Anaesth. 1994;41(9):771–4. doi:10.1007/BF03011582.
Weiss M, Gerber AC. Rapid sequence induction in children—It’s not a matter of time! Paediatr Anaesth. 2008;18(2):97–9. doi:10.1111/j.1460-9592.2007.02324.x.
Lawes EG, Campbell I, Mercer D. Inflation pressure, gastric insufflation and rapid sequence induction. Br J Anaesth. 1987;59(3):315–8.
Moynihan RJ, Brock-Utne JG, Archer JH, Feld LH, Kreitzman TR. The effect of cricoid pressure on preventing gastric insufflation in infants and children. Anesthesiology. 1993;78(4):652–6.
Weiler N, Heinrichs W, Dick W. Assessment of pulmonary mechanics and gastric inflation pressure during mask ventilation. Prehosp Disaster Med. 1995;10(2):101–5.
Brock-Utne JG. Is cricoid pressure necessary? Paediatr Anaesth. 2002;12(1):1–4.
Landsman I. Cricoid pressure: indications and complications. Paediatr Anaesth. 2004;14(1):43–7.
Lev R, Rosen P. Prophylactic lidocaine use preintubation: a review. J Emerg Med. 1994;12(4):499–506.
Sagarin MJ, Chiang V, Sakles JC, Barton ED, Wolfe RE, Vissers RJ, Walls RM, National Emergency Airway Registry i. Rapid sequence intubation for pediatric emergency airway management. Pediatr Emerg Care. 2002;18(6):417–23.
Zelicof-Paul A, Smith-Lockridge A, Schnadower D, Tyler S, Levin S, Roskind C, Dayan P. Controversies in rapid sequence intubation in children. Curr Opin Pediatr. 2005;17(3):355–62.
Gronert GA. Cardiac arrest after succinylcholine: mortality greater with rhabdomyolysis than receptor upregulation. Anesthesiology. 2001;94(3):523–9.
Larach MG, Rosenberg H, Gronert GA, Allen GC. Hyperkalemic cardiac arrest during anesthesia in infants and children with occult myopathies. Clin Pediatr (Phila). 1997;36(1):9–16. doi:10.1177/000992289703600102.
Filanovsky Y, Miller P, Kao J. Myth: ketamine should not be used as an induction agent for intubation in patients with head injury. CJEM. 2010;12(2):154–7.
Sehdev RS, Symmons DA, Kindl K. Ketamine for rapid sequence induction in patients with head injury in the emergency department. Emerg Med Aust. 2006;18(1):37–44. doi:10.1111/j.1742-6723.2006.00802.x.
Pokela ML, Olkkola KT, Seppala T, Koivisto M. Age-related morphine kinetics in infants. Dev Pharmacol Ther. 1993;20(1–2):26–34.
Bray RJ. Propofol infusion syndrome in children. Paediatr Anaesth. 1998;8(6):491–9.
Parke TJ, Stevens JE, Rice AS, Greenaway CL, Bray RJ, Smith PJ, Waldmann CS, Verghese C. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. BMJ. 1992;305(6854):613–6.
Nemergut ME, Aganga D, Flick RP. Anesthetic neurotoxicity: What to tell the parents? Paediatr Anaesth. 2014;24(1):120–6. doi:10.1111/pan.12325.
Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99(23):15089–94. doi:10.1073/pnas.222550499.
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23(3):876–82.
Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106(6):1681–707. doi:10.1213/ane.0b013e318167ad77.
Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W Jr., Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33(2):220–30. doi:10.1016/j.ntt.2011.01.001.
Pohl D, Bittigau P, Ishimaru MJ, Stadthaus D, Hubner C, Olney JW, Turski L, Ikonomidou C. N-Methyl-d-aspartate antagonists and apoptotic cell death triggered by head trauma in developing rat brain. Proc Natl Acad Sci USA. 1999;96(5):2508–13.
Skippen P, Seear M, Poskitt K, Kestle J, Cochrane D, Annich G, Handel J. Effect of hyperventilation on regional cerebral blood flow in head-injured children. Crit Care Med. 1997;25(8):1402–9.
Downard C, Hulka F, Mullins RJ, Piatt J, Chesnut R, Quint P, Mann NC. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma. 2000;49(4):654–8; discussion 658–9.
Elias-Jones AC, Punt JA, Turnbull AE, Jaspan T. Management and outcome of severe head injuries in the Trent region 1985–1990. Arch Dis Child. 1992;67(12):1430–5.
Freeman SS, Udomphorn Y, Armstead WM, Fisk DM, Vavilala MS. Young age as a risk factor for impaired cerebral autoregulation after moderate to severe pediatric traumatic brain injury. Anesthesiology. 2008;108(4):588–95. doi:10.1097/ALN.0b013e31816725d7.
Seder DB, Mayer SA. Critical care management of subarachnoid hemorrhage and ischaemic stroke. Clin Chest Med. 2009;30(1):103–22. doi:10.1016/j.ccm.2008.11.004.
Author information
Authors and Affiliations
Corresponding author
Appendix 1: The 3-min Pre-intubation Neurological Assessment Checklist
Appendix 1: The 3-min Pre-intubation Neurological Assessment Checklist
Level of arousal and interaction |
Basic Cortical functions |
Visual field deficits |
Gaze |
Language |
Neglect/inattention |
Cranial nerves |
Pupils |
Corneal reflex |
Eye movements |
Facial symmetry |
Gag |
Cough |
Motor function |
Face and each extremity |
Sensation |
For symmetry |
Tone |
Neck and each extremity |
Reflexes |
Evidence of subtle or gross seizure activity |
Cervical spine tenderness |
Rights and permissions
About this article
Cite this article
Rajajee, V., Riggs, B. & Seder, D.B. Emergency Neurological Life Support: Airway, Ventilation, and Sedation. Neurocrit Care 27 (Suppl 1), 4–28 (2017). https://doi.org/10.1007/s12028-017-0451-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-017-0451-2