Abstract
Purpose
High-flow nasal cannula oxygen (HFNC) has the potential to provide apnoeic oxygenation. We decided to assess in a proof-of-concept study whether the addition of HFNC to non-invasive ventilation (NIV) could reduce oxygen desaturation during intubation, compared with NIV alone for preoxygenation, in severely hypoxaemic intensive care unit (ICU) patients with respiratory failure.
Methods
We conducted a randomised, controlled, single-centre trial with assessor-blinded outcome assessment in patients admitted to the ICU. Hypoxaemic patients requiring orotracheal intubation for respiratory failure were randomised to receive preoxygenation using HFNC [flow = 60 L/min, fraction of inspired oxygen (FiO2) = 100 %] combined with NIV (pressure support = 10 cmH2O, positive end-expiratory pressure = 5 cmH2O, FiO2 = 100 %) in the intervention group or NIV alone in the reference group prior to intubation. The primary outcome was the lowest oxygen saturation (SpO2) during the intubation procedure. Secondary outcomes were intubation-related complications and ICU mortality.
Results
Between July 2015 and February 2016, we randomly assigned 25 and 24 patients to the intervention and reference groups, respectively. In both groups the main reasons for respiratory failure were pneumonia and ARDS. During the intubation procedure, the lowest SpO2 values were significantly higher in the intervention group than in the reference group [100 (95–100) % vs. 96 (92–99) %, p = 0.029]. After exclusion of two patients from analysis for protocol violation, no (0 %) patients in the intervention group and five (21 %) patients in the reference group had SpO2 below 80 % (p = 0.050). We recorded no significant difference between the groups in intubation-related complications or ICU mortality.
Conclusions
A novel strategy for preoxygenation in hypoxaemic patients, adding HFNC for apnoeic oxygenation to NIV prior to orotracheal intubation, may be more effective in reducing the severity of oxygen desaturation than the reference method using NIV alone.
Similar content being viewed by others
Introduction
Tracheal intubation is needed to provide invasive mechanical ventilation in severely hypoxaemic patients in the intensive care unit (ICU). Orotracheal intubation is one of the most frequent procedures performed in the ICU [1]. Severe hypoxaemia occurring during intubation procedure can result in cardiac arrest [2], cerebral anoxia and death [3]. To prevent and limit the incidence of severe hypoxaemia following the intubation procedure and its complications, several preoxygenation techniques and intubation algorithms have been developed [4–9], and specific risk factors for difficult intubation in the ICU have been identified [10].
Non-invasive ventilation (NIV) for preoxygenation of patients with severe hypoxaemic acute respiratory failure is associated with less hypoxaemia than preoxygenation with oxygen facial mask during intubation procedures [11]. Indeed, combining pressure-support (PS) with positive end-expiratory pressure (PEEP) limits alveolar collapse and atelectasis formation [12, 13], responsible for hypoventilation and low perfusion ventilation ratio [14]. Incidence of severe hypoxaemia defined by a peripheral capillary oxygen saturation (SpO2) of less than 80 % can be reduced by applying NIV preoxygenation, a method which is now used by 36 % of teams [10] for preoxygenation of patients with severe hypoxaemic acute respiratory failure.
However, although NIV can be safely applied for preoxygenation before the intubation procedure, NIV facial mask has to be taken off after preoxygenation in order to allow the passage of the orotracheal tube through the mouth. Furthermore, positioning the orotracheal tube into the trachea may take time, from a few seconds to several minutes in case of difficult intubation [10]. The hypoxaemic patient does not receive oxygen during this period, which contributes to the risk of severe hypoxaemia during intubation procedures [15, 16].
High-flow nasal cannula oxygen therapy (HFNC), which delivers high flow, heated and humidified air via nasal prongs at a prescribed fraction of inspired oxygen (FiO2) and a maximum flow of 60 L/min [17, 18], can be continued during the passage of an orotracheal tube through the mouth. Recent studies suggest that HFNC could allow for apnoeic oxygenation [19, 20], and interestingly as a consequence could be used to continue blood oxygenation during the apnoea period of intubation, especially when the NIV facial mask is removed [15]. Furthermore, previous studies have shown that HFNC oxygen therapy generates a flow-dependent positive airway pressure and improves oxygenation by increasing end-expiratory lung volume [21], thus suggesting possible associated alveolar recruitment. However, the patient’s mouth must be closed to observe this effect [22], suggesting that NIV could be more efficient than HFNC to prevent alveolar derecruitment.
Using HFNC combined with NIV may have potential advantages over conventional NIV alone for preoxygenation before intubation procedures in hypoxaemic ICU patients. Some studies have assessed the preoxygenation effect of HFNC compared to oxygen facial mask or other devices, with conflicting results [19, 23, 24]. However, the technique of preoxygenation combining NIV and HFNC, respectively incorporating the concepts of prevention of alveolar derecruitment and of apnoeic oxygenation, has never been assessed and benefit remains to be established.
We aimed to assess in the OPTINIV study if preoxygenation combining HFNC and NIV compared to NIV alone could prevent desaturation during the intubation procedure and complications related to intubation in ICU severe hypoxaemic patients needing mechanical ventilation for hypoxaemic acute respiratory failure.
We hypothesized that in comparison to the reference preoxygenation method using NIV alone, a novel strategy of preoxygenation which combines HFNC with NIV would allow for a reduction of severe hypoxaemia during the intubation procedure in severe hypoxaemic respiratory failure patients.
Methods
Study design and patients
The HFNC (Optiflow®, Fisher & Paykel Healthcare, Auckland, NZ) combined with NIV for decreasing oxygen desaturation during intubation procedures in ICU hypoxaemic patients (OPTINIV) trial was an investigator-initiated, single-centre, randomised, controlled, two-arm trial. The OPTINIV proof-of-concept study took place in a mixed medical and surgical 16-bed ICU, in France. The OPTINIV protocol was previously published [25]. The Institutional Review Board of the University Hospital of Montpellier (France) approved the trial. On 13 May 2015, the study was approved by a central ethics committee (Comité de Protection des Personnes Sud-Méditerranée IV, Montpellier, France) with the registration number IDRCB 2015-A00708-41. The OPTINIV study was conducted in accordance with the Declaration of Helsinki and was registered at http://www.clinicaltrials.gov with trial identification number NCT02530957. Study design is detailed in the Supplementary Fig. 1.
Three methods of consent were used, as required by the institutional review board in accordance with the 2013 Declaration of Helsinki. If possible, the patient was included after written informed consent. However, the patient often cannot understand information given because of severe hypoxaemia. These patients were included after written informed consent was provided by next of kin or an emergency procedure (investigator signature) if next of kin was not present. When available, after recovery, patients were retrospectively asked for written consent to continue the trial.
Patients admitted to the ICU and requiring mechanical ventilation through an orotracheal tube were eligible in the study. Severe hypoxaemic acute respiratory failure was defined as a respiratory rate higher than 30 per minute, and a FiO2 requirement of 50 % or more [22] to obtain at least 90 % SpO2 (or an impossibility to obtain more than 90 % SpO2) and an estimated partial pressure of arterial oxygen (PaO2)/FiO2 ratio below 300 mmHg, in the 4 h before inclusion [23].
Patients fulfilling one or more of the following criteria were not included: age less than 18 years, pregnant or breastfeeding woman, protected person, intubation procedures in case of cardiocirculatory arrest, nasopharyngeal obstruction contraindicating the use of HFNC, and usual contraindications to NIV [26].
Procedures
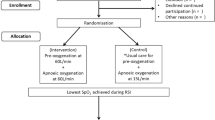
The interventional group received 4 min preoxygenation at 30° of head-up inclination with HFNC (humidified oxygen flow of 60 L/min, FiO2 = 100 %, Fig. 1) combined with NIV (PS of 10 cmH2O, PEEP of 5 cmH2O, FiO2 = 100 %, Fig. 1).
Blinding sequence of the OPTINIV trial. HFNC high-flow nasal cannula oxygen therapy, FiO 2 fraction of inspired oxygen, NIV non-invasive ventilation, PS pressure support, PEEP positive end-expiratory pressure. To allow blinding, a nasal cannula was positioned in each group. The operator performing the intubation blinded the group by placing a large sheet over the oxygen flow meter. Both groups received NIV. a In the interventional group (real HFNC + NIV), the nasal cannula was connected to the oxygen flow meter via a tube and oxygen set at 60 L/min and 100 % of FiO2 which was delivered to the patient. The interventional group consisted in applying a preoxygenation at 30° of head-up inclination with NIV (PS of 10 cmH2O, PEEP of 5 cm H2O, FiO2 = 100 %) and oxygen HFNC set at 60 L/min and 100 % of FiO2. b In the reference group (sham HFNC + NIV), no oxygen flow was administered by the nasal cannula to the patient. The tube connected to the nasal cannula positioned on the patient was hidden under the sheet, without connection to the oxygen flow meter. To mimic the noise of HFNC in the reference group, another nasal cannula was hidden under the sheet and connected to the oxygen flow meter, with a flow also set at 60 L/min. The reference group consisted in applying a preoxygenation at 30° of head-up inclination with NIV only (PS of 10 cmH2O, PEEP of 5 cmH2O, FiO2 = 100 %) without oxygen HFNC (nasal cannula positioned without any flow)
The reference group received 4 min preoxygenation at 30° of head-up inclination with NIV only (same parameters as in the interventional group) without HFNC (nasal cannula positioned without any flow, Fig. 1).
Procedures and description of data collected are detailed in the electronic supplementary material.
Randomisation and masking
Patients eligible for inclusion were randomly assigned to the interventional group or to the reference group (Fig. 1). A computer-generated 1:1 randomisation was used, generated by a statistician who was not involved in determination of patient eligibility or outcome assessment. No stratification was performed. Randomisation was accomplished by using opaque sealed envelopes. The randomisation envelopes contained a card stating the group to which the patient was randomised. The study was blinded to the observer collecting the data (Fig. 1) [25]. NIV was performed and nasal cannula positioned in both groups, to allow blinding. An ICU ventilator with NIV software was used (Evita V500 or XL, Drager Lubeck). The operator performing the intubation procedure blinded the group by placing a large sheet over the oxygen flow meter (Fig. 1). In the interventional group (A. Real HFNC + NIV in Fig. 1), the nasal cannula was connected to the oxygen flow meter via a tube and oxygen set at 60 L/min and 100 % of FiO2. In the reference group (B. Sham HFNC + NIV in Fig. 1), the tube connected to the nasal cannula positioned on the patient was hidden under the sheet, without connection to the oxygen flow meter. No flow of oxygen was administered by the nasal cannula in the reference group. To mimic the noise of HFNC in the reference group, another nasal cannula was hidden under the sheet and connected to the oxygen flow meter, with a flow also set at 60 L/min and delivered into the room atmosphere (Fig. 1). The blinded observer was one of the ICU residents, a nurse or a member of the trained local research team.
Outcomes
The primary outcome was the minimal oxygen saturation indicated by SpO2 during the intubation procedure. Intubation procedures lasted from the beginning of the first laryngoscopy (the end of rapid sequence induction) to the confirmation of the success of intubation by capnography after the patient was connected to mechanical ventilation [23].
Secondary outcomes were preoxygenation quality (ability to improve SpO2, proportion of patients in whom it is impossible to obtain a saturation greater than 90 % during preoxygenation), complications related to intubation procedures (severe and moderate) and morbidity in ICU (ventilator-associated pneumonia, ICU length of stay, length of invasive mechanical ventilation, and mortality rate on day 28). Severe complications were defined as follows: severe hypoxaemia (defined by lowest saturation less than 80 %), severe cardiovascular collapse (defined as systolic blood pressure less than 65 mm Hg recorded at least once or less than 90 mmHg lasting 30 min despite 500–1000 mL of fluid loading (crystalloids solutions) or requiring introduction or increasing doses by more than 30 % of vasoactive support), cardiac arrest, death during intubation. Moderate complications were defined as follows: difficult intubation (more than two attempts), severe ventricular or supraventricular arrhythmia requiring intervention, oesophageal intubation, agitation, pulmonary aspiration, dental injuries.
Statistical analysis
The primary outcome was the minimal SpO2 during intubation procedure. For this study, 23 patients in each group were needed to detect a 5 % difference in the lowest SpO2 during intubation procedure (93 % ± 8 [11] and 88 % ± 5 [10] in previous studies using NIV preoxygenation alone in severe hypoxaemic acute respiratory failure patients), with a standard deviation of 6 %, at a two-sided α level of 0.05 and a statistical power of 80 % [5, 11]. To take into account withdrawn consent after randomisation, inclusions not meeting the inclusion criteria or improvement before intubation, 25 patients were randomised in each group.
Continuous variables were expressed as median (interquartiles 25–75). Qualitative variables were expressed as frequency and percentages. The Mann–Whitney U test was used for primary outcome analysis. The Chi square test (or Fisher’s exact test as appropriate) was used for secondary binary outcomes. Mann–Whitney U test was used for secondary continuous outcomes. A predefined statistical analysis plan was previously published [25]. The statistical analysis incorporated all the elements required by the CONSORT statement for non-pharmacological interventions [25]. Statistical analysis was performed in the intention-to-treat population, including all the randomised patients except those who withdrew their consent, did not meet the inclusion criteria or improved before intubation. After exclusion from analysis of patients with protocol violation, a per-protocol analysis was performed. All analyses were conducted by the medical statistical department of the Montpellier University Hospital using statistical software (SAS, version 9.3; SAS Institute; Cary, NC, USA).
Results
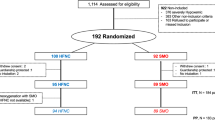
Between July 2015 and February 2016, 50 consecutive patients requiring intubation were enrolled (Fig. 2). In the intention-to-treat analysis, 25 and 24 patients were evaluated in the interventional and reference groups, respectively. In the reference group, the SpO2 per procedure could not be recorded in one patient because of intense peripheral vasoconstriction and this patient was not included in the intention-to-treat analysis. In the per-protocol analysis, two patients were excluded from analysis in the interventional group because of protocol violations: contraindications to NIV (exclusion criteria), i.e. presence of a pneumothorax before intubation, and presence of a leak of an upper oesophageal anastomosis. In the per-protocol analysis, 23 and 24 patients were evaluated in the interventional and reference groups, respectively.
Intention-to-treat analysis
Characteristics of the population before preoxygenation
The baseline characteristics of the two groups were similar in terms of age, disease severity, organ failures, and diagnosis on admission (Table 1). Arterial blood gases and oxygen supply did not differ between the two groups (Table 1 and Supplementary Table 1).
Primary outcome: peripheral capillary oxygen saturation
During the intubation procedure, the distribution of minimal SpO2 values (primary outcome) was significantly higher in the interventional group in comparison with the reference group [100 (95–100) % vs. 96 (92–99) %, p = 0.029, Fig. 3). Changes in distribution of SpO2 values during the entire procedure are shown in Fig. 3. After preoxygenation, SpO2 increased in the interventional group from 89 (87–92) % to 100 (99–100) % and in the reference group from 90 (86–93) % to 100 (95–100) % (Fig. 3).
Variations in minimal SpO2 values from baseline to the end of the intubation procedure. SpO 2 peripheral capillary oxygen saturation, NIV non-invasive ventilation, HFNC high-flow nasal cannula oxygen therapy. At baseline (defined as values obtained at time of randomisation), before the intubation procedure, minimal SpO2 values were similar between groups, and remain similar at the end of preoxygenation. However, during the intubation procedure, minimal SpO2 values were significantly higher in the interventional group compared to the reference group (p = 0.029)
Secondary outcomes: intubation procedure-related complications
One patient (4 %) in the interventional group and five (21 %) in the reference group had an SpO2 below 80 % during the intubation procedure (p = 0.098; Supplementary Fig. 2). The five patients with an SpO2 below 80 % are detailed in the electronic supplementary material.
Descriptions of the preoxygenation and intubation procedures are shown in Table 2. The incidence of intubation procedure-related complications and other secondary outcomes are reported in Table 2. Severe complications occurred in six (24 %) and nine (38 %) patients in the interventional and reference groups, respectively (p = 0.305, Table 2). Duration of mechanical ventilation, ICU length of stay and ICU mortality did not differ between groups (Table 2). No patient experienced barotrauma related to either preoxygenation method.
Per-protocol analysis
Characteristics of the per-protocol population are presented in Supplementary Tables 2 and 3. During intubation, the distribution of minimal SpO2 values was significantly higher in the interventional group when compared with the reference group [100 (95–100) % vs. 95.5 (91.5–99) %, p = 0.010; Supplementary Fig. 3). No patient (0 %) in the interventional group and five (21 %) in the reference group had an SpO2 below 80 % during intubation procedures (p = 0.050; Supplementary Fig. 3). The incidence of intubation procedure-related complications and other secondary outcomes are reported in Supplementary Table 3. Duration of mechanical ventilation, ICU length of stay and ICU mortality did not differ between groups.
Discussion
The present study assessed for the first time a novel strategy of preoxygenation using HFNC and NIV applied together. The results did not demonstrate any safety concerns and show that this novel approach is more effective in increasing minimal SpO2 values during intubation procedure than the preoxygenation reference method using NIV alone in severe hypoxaemic respiratory failure critically ill patients.
OPTINIV is the first randomised controlled trial powered to investigate the effectiveness of combined HFNC and NIV to decrease severe hypoxaemia during the intubation procedure in severe hypoxaemic acute respiratory failure patients in ICU. Supplementary Fig. 4 summarizes the six studies, including the present one, which assessed the minimal SpO2 values during intubation procedure according to the method of preoxygenation used in severe hypoxaemic patients. Oxygen facial mask preoxygenation was assessed in four studies [11, 23, 24, 27],
HFNC alone was assessed in four studies [23, 24, 27, 28] and NIV alone was assessed in three studies (including the present study) [11, 28]. Until the present study, no randomised controlled trial had assessed HFNC combined with NIV in ICU. We chose to compare this new combined method of preoxygenation with the most efficient method reported in the literature in hypoxaemic patients to prevent oxygen desaturation as shown in supplementary Fig. 4, i.e. NIV [11].
Previous clinical studies assessed the effect of apnoeic oxygenation, with conflicting results [29]. Miguel-Montanes et al. [19] compared preoxygenation using oxygen facial mask with HFNC at a flow of 60 L/min in patients with mild-to-moderate hypoxaemia. With the oxygen facial mask, the median lowest SpO2 during intubation was 94 vs. 100 % with HFNC. Similarly to the positive results reported by Miguel-Montanes et al. [19], Sakles et al. [20] found a reduction in the oxygen desaturation using apnoeic oxygenation in the emergency department. However, Vourc’h et al. [23] found no difference for the minimal SpO2 values during intubation in hypoxaemic patients when comparing 60 L/min of HFNC and oxygen facial mask (92 vs. 90 %, p = 0.44). Semler et al. [24] found that the administration of 15 L/min nasal cannula oxygen in the apnoeic oxygenation group was not associated with significantly increased minimal SpO2 values during intubation procedures (92 % in the apnoeic oxygenation group vs. 90 % in the usual care group (p = 0.16)). The discrepancies between the results of these studies [19, 20, 23, 24] could mainly be explained by the oxygen flow used for the apnoeic oxygenation group (from 15 to 60 L/min), the differences between duration of intubation attempts and the different studied populations in terms of hypoxaemia (severe vs mild to moderate). Moreover, the design of these studies differed from that of the current study, which allowed us to specifically assess apnoeic oxygenation using HFNC simultaneously combined with NIV preoxygenation.
The primary endpoint of the trial was the minimal SpO2 value during intubation procedure. The incidence of severe hypoxaemia following intubation is particularly high in ICU, reaching up to 50 % [10, 30]. Severe hypoxaemia can lead to cardiac arrest, neurologic damage, or multiple organ failure and death [10]. However, intubation procedure-related complications and long-term outcome did not differ between groups. A lack of power probably explains the lack of observed differences between the two groups. There was a clinically relevant difference between the rates of severe complications between groups without reaching statistical significance (24 vs. 38 %, p = 0.305, Table 2). To reach significance, inclusion of 169 patients per group would have been required. However, the study was not designed a priori to conclude on this secondary outcome. This was a proof-of-concept study designed to evaluate the safety and efficacy of a new method of preoxygenation which combined apnoeic oxygenation and NIV. The clinical significance of 100 vs. 96 % minimal SpO2 value during intubation could appear low. However, only one patient in the interventional group in the intention-to-treat analysis and no patients in the per-protocol analysis experienced severe oxygen desaturation (SpO2 less than 80 %) during intubation procedure compared to five patients in the reference group (respectively p = 0.098, Supplementary Fig. 2; and p = 0.050, Supplementary Fig. 3).
The present study has some limitations. First, the number of patients included was low and small-sized studies tend to overestimate the treatment effect [31]. Second, there is a theoretical risk of gastric air insufflation and aspiration related to positive airway pressure of NIV. However, as previously reported [11, 23, 32] and in the present study, it was recommended to never exceed a total insufflation airway pressure (PS + PEEP) of 15 cmH2O which has been shown to be effective to avoid gastric air insufflation [33]. Third, the operator performing intubation could be aware of the group of inclusion. However, the assessor was an independent observer who did not know the group of inclusion. One strength of the study was the blinded assessment (Fig. 1), even if the operator could not be blinded, given the flow of the HFNC. Indeed, in order to avoid a PEEP effect, we chose not to administer any flow to the patient in the control group, even at 21 % FiO2. Moreover, the inclusions were performed around the clock, nights and weekends included, which further allows the extrapolation of the results of the present study. Fourth, one could argue that the high flow rate in the intervention group could increase the inspiratory and expiratory pressures in the mask and in the patient’s airway. However, we carefully checked that the ventilator’s safety system could cope with the 60 L/min extra flow without increasing the pressure in the ventilator circuit. Fifth, the adequacy of denitrogenation was not measured with fraction expired in oxygen. Heterogeneity in the aetiology and severity of airspace disease causing the hypoxaemia is such that the study may ultimately be underpowered to show the true effect. Sixth, we did not perform a three-arm study assessing the effect of HFNC alone as a method preoxygenation, because this method was already assessed in other studies in severe hypoxaemic patients [19, 23, 24, 27]. In the same way, we did not use the standard mask ventilation preoxygenation as control because NIV preoxygenation was already found to be superior to standard mask ventilation [11]. Finally, this was a single-centre study, which could limit the generalization of the results.
In conclusion, the OPTINIV randomised trial showed that HFNC combined with NIV, in comparison to NIV alone, allowed significantly higher minimal SpO2 values during the intubation procedure of severe hypoxaemic acute respiratory failure ICU patients. This proof-of-concept study has to be confirmed with a large multicentre randomised controlled trial.
References
Roux D, Reignier J, Thiery G, Boyer A, Hayon J, Souweine B, Papazian L, Mercat A, Bernardin G, Combes A, Chiche JD, Diehl JL, du Cheyron D, L’Her E, Perrotin D, Schneider F, Thuong M, Wolff M, Zeni F, Dreyfuss D, Ricard JD (2014) Acquiring procedural skills in ICUs: a prospective multicenter study. Crit Care Med 42:886–895
Mort TC (2004) The incidence and risk factors for cardiac arrest during emergency tracheal intubation: a justification for incorporating the ASA guidelines in the remote location. J Clin Anesth 16:508–516
Cook TM, Scott S, Mihai R (2010) Litigation related to airway and respiratory complications of anaesthesia: an analysis of claims against the NHS in England 1995–2007. Anaesthesia 65:556–563
De Jong A, Jung B, Jaber S (2014) Intubation in the ICU: we could improve our practice. Crit Care 18:209
De Jong A, Clavieras N, Conseil M, Coisel Y, Moury PH, Pouzeratte Y, Cisse M, Belafia F, Jung B, Chanques G, Molinari N, Jaber S (2013) Implementation of a combo videolaryngoscope for intubation in critically ill patients: a before-after comparative study. Intensive Care Med 39:2144–2152
De Jong A, Molinari N, Conseil M, Coisel Y, Pouzeratte Y, Belafia F, Jung B, Chanques G, Jaber S (2014) Video laryngoscopy versus direct laryngoscopy for orotracheal intubation in the intensive care unit: a systematic review and meta-analysis. Intensive Care Med 40:629–639
Jaber S, Jung B, Corne P, Sebbane M, Muller L, Chanques G, Verzilli D, Jonquet O, Eledjam JJ, Lefrant JY (2010) An intervention to decrease complications related to endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Intensive Care Med 36:248–255
Hypes CD, Stolz U, Sakles JC, Joshi RR, Natt B, Malo J, Bloom JW, Mosier JM (2016) Video laryngoscopy improves odds of first-attempt success at intubation in the intensive care unit. A propensity-matched analysis. Ann Am Thorac Soc 13:382–390
Mosier JM, Malo J, Sakles JC, Hypes CD, Natt B, Snyder L, Knepler J, Bloom JW, Joshi R, Knox K (2015) The impact of a comprehensive airway management training program for pulmonary and critical care medicine fellows. A three-year experience. Ann Am Thorac Soc 12:539–548
De Jong A, Molinari N, Terzi N, Mongardon N, Arnal JM, Guitton C, Allaouchiche B, Paugam-Burtz C, Constantin JM, Lefrant JY, Leone M, Papazian L, Asehnoune K, Maziers N, Azoulay E, Pradel G, Jung B, Jaber S (2013) Early identification of patients at risk for difficult intubation in the intensive care unit: development and validation of the MACOCHA score in a multicenter cohort study. Am J Respir Crit Care Med 187:832–839
Baillard C, Fosse JP, Sebbane M, Chanques G, Vincent F, Courouble P, Cohen Y, Eledjam JJ, Adnet F, Jaber S (2006) Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med 174:171–177
Pepin JL, Timsit JF, Tamisier R, Borel JC, Levy P, Jaber S (2016) Prevention and care of respiratory failure in obese patients. Lancet Respir Med 4:407–418
Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ (2014) High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 384:495–503
De Jong A, Futier E, Millot A, Coisel Y, Jung B, Chanques G, Baillard C, Jaber S (2014) How to preoxygenate in operative room: healthy subjects and situations “at risk”. Ann Fr Anesth Reanim 33:457–461
Papazian L, Corley A, Hess D, Fraser JF, Frat JP, Guitton C, Jaber S, Maggiore SM, Nava S, Rello J, Ricard JD, Stephan F, Trisolini R, Azoulay E (2016) Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med 42:1336–1349
Mosier JM, Hypes CD, Sakles JC (2016) Understanding preoxygenation and apneic oxygenation during intubation in the critically ill. Intensive Care Med. doi:10.1007/s00134-016-4426-0
Chanques G, Constantin JM, Sauter M, Jung B, Sebbane M, Verzilli D, Lefrant JY, Jaber S (2009) Discomfort associated with underhumidified high-flow oxygen therapy in critically ill patients. Intensive Care Med 35:996–1003
Chanques G, Jaber S (2013) Unexpected progress of an old intensive care therapy, oxygen: towards more comfort and less mechanical ventilation. Rev Mal Respir 30:605–608
Miguel-Montanes R, Hajage D, Messika J, Bertrand F, Gaudry S, Rafat C, Labbe V, Dufour N, Jean-Baptiste S, Bedet A, Dreyfuss D, Ricard JD (2015) Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med 43:574–583
Sakles JC, Mosier JM, Patanwala AE, Dicken JM (2016) Apneic oxygenation is associated with a reduction in the incidence of hypoxemia during the RSI of patients with intracranial hemorrhage in the emergency department. Intern Emerg Med 11:983–992
Futier E, Jaber S (2015) High-flow nasal cannula following extubation: is more oxygen flow useful after surgery? Intensive Care Med 41:1310–1313
Chanques G, Riboulet F, Molinari N, Carr J, Jung B, Prades A, Galia F, Futier E, Constantin JM, Jaber S (2013) Comparison of three high flow oxygen therapy delivery devices: a clinical physiological cross-over study. Minerva Anestesiol 79:1344–1355
Vourc’h M, Asfar P, Volteau C, Bachoumas K, Clavieras N, Egreteau PY, Asehnoune K, Mercat A, Reignier J, Jaber S, Prat G, Roquilly A, Brule N, Villers D, Bretonniere C, Guitton C (2015) High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med 41:1538–1548
Semler MW, Janz DR, Lentz RJ, Matthews DT, Norman BC, Assad TR, Keriwala RD, Ferrell BA, Noto MJ, McKown AC, Kocurek EG, Warren MA, Huerta LE, Rice TW (2016) Randomized trial of apneic oxygenation during endotracheal intubation of the critically ill. Am J Respir Crit Care Med 193:273–280
Jaber S, Molinari N, De Jong A (2016) New method of preoxygenation for orotracheal intubation in patients with hypoxaemic acute respiratory failure in the intensive care unit, non-invasive ventilation combined with apnoeic oxygenation by high flow nasal oxygen: the randomised OPTINIV study protocol. BMJ Open 6:e011298
Jaber S, Chanques G, Jung B (2010) Postoperative noninvasive ventilation. Anesthesiology 112:453–461
Simon M, Wachs C, Braune S, de Heer G, Frings D, Kluge S (2016) High-flow nasal cannula versus bag-valve-mask for preoxygenation before intubation in subjects with hypoxemic respiratory failure. Respir Care 61:1160–1167
Besnier E, Guernon K, Bubenheim M, Gouin P, Carpentier D, Beduneau G, Grange S, Declercq PL, Marchalot A, Tamion F, Girault C (2016) Pre-oxygenation with high-flow nasal cannula oxygen therapy and non-invasive ventilation for intubation in the intensive care unit. Intensive Care Med 42:1291–1292
De Jong A, Jaber S (2016) Apneic oxygenation for intubation in the critically ill. Let’s not give up! Am J Respir Crit Care Med 193:230–232
Jaber S, Amraoui J, Lefrant JY, Arich C, Cohendy R, Landreau L, Calvet Y, Capdevila X, Mahamat A, Eledjam JJ (2006) Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med 34:2355–2361
Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14:365–376
Futier E, Constantin JM, Pelosi P, Chanques G, Massone A, Petit A, Kwiatkowski F, Bazin JE, Jaber S (2011) Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiology 114:1354–1363
Delay JM, Sebbane M, Jung B, Nocca D, Verzilli D, Pouzeratte Y, Kamel ME, Fabre JM, Eledjam JJ, Jaber S (2008) The effectiveness of noninvasive positive pressure ventilation to enhance preoxygenation in morbidly obese patients: a randomized controlled study. Anesth Analg 107:1707–1713
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
Dr. Jaber reports receiving consulting fees from Drager, Hamilton, Maquet, and Fisher & Paykel. No potential conflict of interest relevant to this article was reported for the other authors.
Role of the funding source
The study is an investigator-initiated trial. Study promoter is Montpellier University Hospital, Montpellier, France. There is no industry support or involvement in the trial. The funder had no role in the design or conduct of the study, data collection, analysis or interpretation, the writing of the report or in the decision to submit for publication. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Protocol with consort details
Full protocol was published in BMJ Open Access [25] and follows Consort 2010 guidelines.
Ethics committee approval
The Institutional Review Board of the University Hospital of Montpellier (France) approved the trial. On 13 May 2015, the study was approved by a central ethics committee (Comité de Protection des Personnes Sud-Méditerranée IV, Montpellier, France) with the registration number IDRCB 2015-A00708-41. The OPTINIV study was conducted in accordance with the Declaration of Helsinki and was registered at http://www.clinicaltrials.gov with trial identification number NCT02530957.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jaber, S., Monnin, M., Girard, M. et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med 42, 1877–1887 (2016). https://doi.org/10.1007/s00134-016-4588-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4588-9