Abstract
Introduction
Dexmedetomidine is a highly selective α2-adrenoreceptor agonist that produces dose-dependent sedation, anxiolysis, and analgesia without respiratory depression. Dexmedetomidine has been used in critically ill medical, surgical, and pediatric patients, as an adjunct to sedation and/or for treating drug or alcohol withdrawal. Information regarding the dosing and utilization of dexmedetomidine has been derived primarily from studies in critically ill patients in the medical intensive care unit. There has been no study designed specifically to evaluate dexmedetomidine for these therapeutic uses in the neurocritical care population. The primary and secondary objectives were to evaluate the starting dose of dexmedetomidine for neurocritical care patients and to assess the effect on hemodynamic parameters, respectively.
Methods
This was a prospective, observational study conducted from October 2007 to March 2008. Patients were included if they were admitted to the Neuro-Intensive Care Unit and received dexmedetomidine infusion.
Results
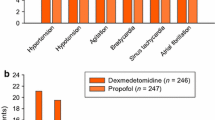
Six patients met the criteria for the study. The mean initial dexmedetomidine infusion rate and mean maximum infusion rate were 0.67 ± 0.2 and 1.3 ± 0.5 mcg/kg/h, respectively. The mean duration of dexmedetomidine infusion was 66 ± 10 h. Two patients (33%) experienced a significant change in mean heart rate and systolic blood pressure after starting dexmedetomidine infusion.
Conclusion
Neurocritically ill patients may require high doses of dexmedetomidine to achieve desired levels of sedation. The high rates and long duration of dexmedetomidine infusion had a statistically, but not clinically, significant impact on hemodynamic parameters.






Similar content being viewed by others
References
Bekker A, Sturaitis MK. Dexmedetomidine for neurological surgery. Neurosurgery. 2005;57(Suppl 1):1–10. doi:10.1227/01.NEU.0000163476.42034.A1.
Prielipp RC, Wall MH, Tobin JR, Groban L, Cannon MA, Fahey FH, et al. Dexmedetomidine-induced sedation in volunteers decreases regional and global cerebral blood flow. Anesth Analg. 2002;95:1052–9. doi:10.1097/00000539-200210000-00048.
Gerlach AT, Dasta JF. Dexmedetomidine: an updated review. Ann Pharmacother. 2007;41:245–54. doi:10.1345/aph.1H314.
Product Information. Precedex (dexmedetomidine). Lake Forest, IL: Hospira; April 2004.
Shehabi Y, Ruettimann U, Adamson H, Innes R, Ickeringill M. Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects. Intensive Care Med. 2004;30:2188–96. doi:10.1007/s00134-004-2417-z.
Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–41. doi:10.1097/00003246-200201000-00020.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi:10.1164/rccm.2107138.
Venn RM, Newman PJ, Grounds RM. A phase II study to evaluate the efficacy of dexmedetomidine for sedation in the medical intensive care unit. Intensive Care Med. 2003;29:201–7.
MacLaren R, Forrest LK, Kiser TH. Adjunctive dexmedetomidine therapy in the intensive care unit: a retrospective assessment of impact on sedative and analgesic requirements, levels of sedation and analgesia, and ventilatory and hemodynamic parameters. Pharmacotherapy. 2007;27(3):351–9. doi:10.1592/phco.27.3.351.
Kim E. Agitation, aggression, and disinhibition syndromes after traumatic brain injury. NeuroRehabilitation. 2002;17:297–310.
Levy M, Berson A, Cook T, Bollegala N, Seto E, Turanski S, et al. Treatment of agitation following traumatic brain injury: a review of the literature. NeuroRehabilitation. 2005;20:279–306.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grof, T.M., Bledsoe, K.A. Evaluating the Use of Dexmedetomidine in Neurocritical Care Patients. Neurocrit Care 12, 356–361 (2010). https://doi.org/10.1007/s12028-008-9156-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-008-9156-x