Abstract
Purpose
Previous observational studies have inconsistently associated early hyperoxia with worse outcomes after cardiac arrest, and have methodological limitations. We tested this association using a high-resolution database controlling for multiple disease-specific markers of severity of illness and care processes.
Methods
This was a retrospective analysis of a single-center, prospective registry of consecutive cardiac arrest patients. We included patients who survived and were mechanically ventilated ≥24 h after arrest. Our main exposure was arterial oxygen tension (PaO2), which we categorized hourly for 24 h as severe hyperoxia (>300 mmHg), moderate or probable hyperoxia (101–299 mmHg), normoxia (60–100 mmHg) or hypoxia (<60 mmHg). We controlled for Utstein-style covariates, markers of disease severity and markers of care responsiveness. We performed unadjusted and multiple logistic regression to test the association between oxygen exposure and survival to discharge, and used ordered logistic regression to test the association of oxygen exposure with neurological outcome and Sequential Organ Failure Assessment (SOFA) score at 24 h.
Results
Of 184 patients, 36 % were exposed to severe hyperoxia and overall mortality was 54 %. Severe hyperoxia, but not moderate or probable hyperoxia, was associated with decreased survival in both unadjusted and adjusted analysis [adjusted odds ratio (OR) for survival 0.83 per hour exposure, P = 0.04]. Moderate or probable hyperoxia was not associated with survival but was associated with improved SOFA score 24 h (OR 0.92, P < 0.01).
Conclusion
Severe hyperoxia was independently associated with decreased survival to hospital discharge. Moderate or probable hyperoxia was not associated with decreased survival and was associated with improved organ function at 24 h.
Similar content being viewed by others
Introduction
Cardiac arrest (CA) is the leading cause of death in North America, with as many as 590,000 arrests annually [1]. Despite advances in care, patient outcomes after CA remain poor. Early arterial hyperoxia has been associated with potentially avoidable mortality after CA, presumably due to increased oxidative stress and ischemia–reperfusion injury [2–4]. However, previous studies testing this association have important methodological limitations and have yielded conflicting results. Therefore, the true clinical significance of hyperoxia in the early post-arrest period remains uncertain.
Animal models evaluating early hyperoxia and post-arrest neurological outcomes have generated mixed results. Some studies report that ventilation with 100 % oxygen versus less oxygen increases oxidative stress and worsens neurological outcomes [5–9], but this has not been consistently replicated [10–12]. The animals used in these models were previously healthy with normal gas exchange. During hyperoxia, the observed partial pressure of arterial oxygen (PaO2) was often >400 mmHg—a finding exaggerated from most clinical experience. Thus, the generalizability of these models to clinical practice has been questioned [13].
Human studies are similarly heterogeneous and inconclusive [14]. The only randomized trial of oxygen titration noted no difference in patient outcomes or biomarkers of neurological injury, but was limited by its small size [15]. Large observational studies have demonstrated an association between hyperoxia and mortality after CA [2, 3]. However, these studies analyzed only a single PaO2 value, either initial or maximum, and the largest studies have not controlled for post-arrest injury severity. Since hyperoxia may be more prevalent in patients with longer arrest durations and delayed intensive care unit (ICU) admission [16], failure to control for injury severity and care processes is a major limitation. Indeed, analyses that have controlled these have not found an association between hyperoxia and outcomes [17, 18]. Unfortunately, no studies have analyzed a dedicated disease-specific CA database. Therefore, important prognostic factors and neurological outcomes were not available for analysis. Furthermore, existing studies have relied on a single time point to define hyperoxia, and could not evaluate the cumulative exposure to oxygen over time.
To overcome these limitations, we used a prospective, disease-specific CA database to examine the association between PaO2 over the first 24 h after CA and patient outcomes. In addition to traditional covariates, we incorporated markers of organ injury severity and critical care processes to adjust our analysis. We tested the null hypothesis that there would be no association between arterial hyperoxia and outcomes.
Methods
Patients and setting
The University of Pittsburgh Medical Center’s Presbyterian Hospital is a 795-bed tertiary care referral center. The Post-Cardiac Arrest Service (PCAS) cares for over 300 survivors of CA annually (150–200 cases annually during the study period), and maintains a prospective database including all post-arrest patients. In the present analysis, we included patients who presented during an 18-month period between October 2008 (when electronic medical records were implemented system-wide, permitting recording of blood gas data, vital signs and ventilator data) and April 2010. We included patients that were successfully resuscitated from CA and were both alive and mechanically ventilated for ≥24 h after return of spontaneous circulation (ROSC). We excluded patients if the time of arrest was unknown, if no arterial blood gas (ABG) or ventilator data were unavailable within 4 h after ROSC, or if extracorporeal membrane oxygenation (ECMO) was used. The University of Pittsburgh Institutional Review Board approved all aspects of this study.
Exposure and covariates
Our primary exposure of interest was arterial oxygen tension. We categorized PaO2 as follows: “severe hyperoxia” (PaO2 ≥ 300 mmHg); “moderate hyperoxia” (PaO2 101–299 mmHg); “normoxia” (PaO2 60–100 mmHg); or, “hypoxia” (PaO2 < 60 mmHg). If PaO2 was not measured during a given hour, we extended the classification for that hour to adjacent hours for up to 2 h before and after the result. If no data were available for a longer period, we used oxygen saturation (SpO2) to classify patients as having “hypoxia” (SpO2 < 90 %); “normoxia” (SpO2 = 90–99 % or 100 % when FiO2 = 0.4); or “probable hyperoxia” (SpO2 = 100 % and FiO2 > 0.4) [19]. We defined “probable hyperoxia” this way because we observed a mean PaO2:FiO2 ratio of 240, which yields a PaO2 of 96 mmHg (i.e. the upper limit of “normoxia”) in a patient with an SaO2 of 100 % and FiO2 = 0.4. For each category of oxygen exposure, we summed the total number of hours spent at that level in the first 24 h, to generate four continuous predictor variables that could range from 0 to 24 h. We used these continuous predictors in our unadjusted models and adjusted models that would include only a single oxygen exposure category predictor.
It is statistically inappropriate to control for multiple non-independent, mutually exclusive predictors in traditional multivariable analysis (i.e. duration of arterial oxygen tension in various categories). Thus, we also calculated a single composite score for each patient’s cumulative exposure to hyperoxia. To do this, we assigned each hour of normoxia a value of 0, moderate or probable hyperoxia a value 1 and severe hyperoxia value of 2, and we summed the result over 24 h. We used this composite score in the adjusted models that would otherwise have included multiple oxygen category predictors. In the absence of an established dose–response relationship between oxygen tension and organ function or toxicity, the weights used to calculate this score were arbitrary. As a sensitivity analysis, we repeated adjusted analyses that used this composite score with alternative scores generated in a number of other manners (assigning normoxia values ranging from 0 to 1, moderate or probable hyperoxia 1 to 2 and severe hyperoxia 2 to 4).
We controlled for covariates including demographic data (age, sex), location of CA (in-hospital or out-of-hospital), initial rhythm (ventricular tachycardia/fibrillation (VT/VF) or pulseless electrical activity/asystole), arrest during a night or weekend [20], use of therapeutic hypothermia, and Pittsburgh Cardiac Arrest Category (PCAC), which we treated categorically [21]. We controlled for severity of initial cardiopulmonary failure, modeled as initial cumulative vasopressor index [22] and dynamic pulmonary compliance.
Greater exposure to hyperoxia may be a marker of less responsive care. To control for this possibility, we recorded the time (in hours) from ROSC to the first FiO2 wean, as well as the total number of times FiO2 was titrated in the first 24 h for each patient. As an additional possible marker of less responsive care previously associated with outcome after cardiac arrest [23], we controlled for the mean of three highest blood glucose levels in the 24 h post-arrest. During the study period, all patients received moderate glucose control targeting values of 110–180 mg/dL with blood sugar checks at least every 6 h, so higher mean glucose might indicate less responsive care by the ICU team.
Outcomes
Our primary outcome was survival to hospital discharge. Secondary outcomes were Pittsburgh Cerebral Performance Category (CPC) at hospital discharge and organ failure 24 h after ROSC, which we modeled using a modified Sequential Organ Failure Assessment (SOFA) score [24]. We treated both as ordinal variables. In calculating SOFA scores, we used the laboratory value obtained closest to 24 h after ROSC (range 18–30 h). Bilirubin values were missing in a majority of patients, so to avoid ascertainment bias we excluded the liver component of the SOFA. Since all patients included in this study were intubated and sedated, GCS was of limited value and therefore the final CPC score minus 1 was used as the neurologic failure component. The result was a SOFA score of 0–20. Since a patient’s respiratory SOFA subscale score could affect the likelihood of exposure to arterial hyperoxia, all tests on the total modified SOFA score were repeated on a score that excluded the respiratory subscale.
Statistical methods
We summarized baseline characteristics and report means with standard deviations for normally distributed data and medians with interquartile ranges for skewed data. In our main analysis, we tested the association between arterial oxygen tension and survival to hospital discharge. Our primary exposure variables were the number of hours spent at each arterial oxygen category, as defined above. First, we used unadjusted logistic regression to test the association between each predictor and survival to discharge. Next, we constructed a multivariable model to determine the independent predictors of outcome. To avoid over-fitting, we only included unadjusted predictors significant at a level of P < 0.2 in the adjusted model. Since ABGs are only intermittently obtained, we report the number obtained each hour and tested the nature of the missingness using Little’s Missing Completely at Random (MCAR) test. We also repeated all test procedures excluding any interpolated data and report the results.
To better determine the average treatment effect of severe hyperoxia and strengthen our ability to make a causal inference between exposure to severe hyperoxia and survival, we performed a propensity score-adjusted analysis. We used unadjusted logistic regression to determine the association between exposure (i.e. >0 h of severe hyperoxia vs. no hours of severe hyperoxia) and survival, and then constructed a propensity score to model the probability of exposure given the baseline covariates (listed in Supplemental Appendix 1). We repeated the logistic regression adjusting for inverse probability of treatment weight (IPTW), and stabilized IPTW to avoid biasing the treatment effect [25, 26].
To evaluate a potential interaction between exposure to severe hyperoxia and prespecified markers of care responsiveness, we used linear regression to test the association between severe hyperoxia and time to first FiO2 wean, number of FiO2 weans in 24 h, and mean highest glucose. To test our secondary outcomes, we repeated unadjusted and multivariable test procedures using unadjusted and multiple ordered logistic regression. We used Stata 13.1 (StataCorp, College Station, TX, USA) for all analyses.
Results
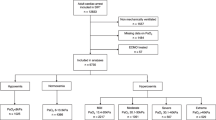
Overall, 232 patients were identified from our registry, of which 48 were excluded, leaving 184 for our analysis. Exclusions were for death <24 h (n = 13), no mechanical ventilation (n = 8) or extubation <24 h (n = 8), unclear time of arrest or transfer from an outside hospital >4 h after ROSC (n = 13), unconfirmed arrest (n = 5) and use of ECMO (n = 1). Mean age was 60 years and 54 % were male (Table 1). Overall, 36 % of patients were exposed to severe hyperoxia with a mean exposure of 1.4 ± 2.2 h (Fig. 1; Table 2). Timing of ABG testing and missing data were MCAR (Little’s P = 0.78). There was no association between exposure to severe hyperoxia and markers of less responsiveness to laboratory values including time to first FiO2 wean (P = 0.09), number of FiO2 weans in 24 h (P = 0.98) or higher mean glucose values (P = 0.83). Overall survival to hospital discharge was 46 % (Supplemental Table 1).
In unadjusted analysis, each hour exposure to severe hyperoxia was associated with an OR of 0.84 (95 %CI 0.72–0.98) for survival to discharge (Table 3). This remained true in logistic regression [unadjusted OR 0.50 (95 %CI 0.27–0.93)], and if we excluded imputed values of the predictor [unadjusted OR 0.51 (95 %CI 0.28–0.95)]. Other unadjusted predictors with P < 0.2 were arrest location, initial rhythm, use of therapeutic hypothermia, PCAC, initial cardiovascular index and mean glucose. Severe hyperoxia remained associated with decreased survival to discharge in adjusted analysis [OR 0.83 (0.69–0.99)] (Table 3), an estimate that did not change when we excluded any imputed data from the model [adjusted OR 0.41 (95 %CI 0.19–0.87)]. Our propensity score (IPTW)-adjusted analysis yielded very similar results [adjusted OR 0.41 (95 %CI 0.28–0.79)] (Supplemental Appendix 1). Results were similar using CPC at hospital discharge as the outcome (data not shown). However, this effect was driven by increased mortality (CPC = 5) and when we restricted our analysis to survivors, we found no association between any level of oxygen exposure and CPC.
When we tested the unadjusted association between oxygen exposure and SOFA score at 24 h, we found that moderate or probable hyperoxia was associated with lower SOFA score at 24 h while exposure to hypoxia was associated with higher SOFA score (Table 4). Based on our a priori statistical analysis plan, since multiple, mutually exclusive levels of oxygen exposure were significant in unadjusted analysis, we built our multivariable model using the composite oxygen exposure score rather than multiple oxygen exposure categories. Doing this, we found that higher composite oxygen score was associated with 24 h SOFA score in both unadjusted and adjusted analysis (both P < 0.01), an effect driven by the association between moderate or probable hyperoxia and lower SOFA score. The results did not change in sensitivity analyses using alternative constructions of cumulative oxygen exposure score or when we excluded the respiratory subscale of the SOFA score (data not shown).
Discussion
We analyzed a high-resolution, prospective disease-specific CA database to test the association between PaO2 and patient outcomes. A key finding of our work is that exposure to severe hyperoxia was associated with decreased survival to discharge. This finding was significant regardless of whether we treated the exposure as a binary variable, as in previous studies [2–4, 18], or modeled exposure as continuous variable, which may better reflect its biological effects. This association remained significant after adjusting for disease-specific markers of severity. Further, since we controlled for time from ROSC to the first FiO2 wean and the number of adjustments in FiO2 made in 24 h, our findings suggest that the deleterious effects of severe hyperoxia are not confounded by increased exposure among patients who may have received less responsive care.
Interestingly, we also observed an association between moderate or probable hyperoxia and improved organ function at 24 h. Because multiple levels of oxygen exposure were significant unadjusted predictors of SOFA, we constructed a multivariable model using a composite score that modeled cumulative exposure. We acknowledge that, in the absence of an established dose–response relationship between oxygen tension and organ function or toxicity, our operationalization of this composite score was somewhat arbitrary. However, given that the results of our analysis did not change after sensitivity analysis, we believe the findings are robust. Since higher SOFA score at 72 h has been associated with worsened survival after CA [27], this effect may be clinically important and warrants further investigation.
Taken together, our results generate several important hypotheses. First, there does not appear to be a linear dose–response relationship between the degree of hyperoxia and worse outcomes. Rather, poor outcomes may occur only when the oxygen tension exceeds a certain threshold. This is biologically plausible since at low levels oxidative stress is countered by endogenous antioxidants. CA and ROSC generate reactive oxygen species and deplete plasma antioxidant potential [28], which may lower the threshold for subsequent oxidative injury. In animal models, resuscitation with 100 % oxygen has been shown to increase this oxidative stress and decrease cerebral antioxidant reserves [9]. Since moderate hyperoxia may be associated with improved extracerebral organ function without worsening neurologic injury or patient outcomes, quantifying cerebral oxidative stress in response to moderate hyperoxia merits further evaluation. Notably, neurological injury is the major driver of morbidity and mortality after out-of-hospital CA, while multiple organ dysfunction is a major cause of death after in-hospital CA [29, 30]. Therefore, future work evaluating post-arrest oxygen exposure may need to be targeted to specific patient subpopulations or even individual patient characteristics.
Our results are consistent with those of Janz et al. [4], who report equivalent neurological outcomes in patients exposed to moderate hyperoxia, but differ from Kilgannon et al. [2, 3], who suggest that even moderate hyperoxia is associated with increased mortality. It should be noted that both of these reports used the highest PaO2 in 24 h, whereas we examined cumulative effects over time. Since oxygen delivery and utilization may be impaired in critical illness [31], and hypoxemia almost certainly exacerbates neurological injury [32, 33], it is reasonable to postulate that moderate levels of hyperoxia that do not overwhelm antioxidant reserves may be beneficial after CA. Our results are consistent with this overall pattern. We submit that our findings support clinical equipoise for a randomized trial comparing a normoxia to moderate hyperoxia for management of early post-arrest patients.
Our study has several strengths. First, we controlled for both disease-specific factors and general physiological markers of severity. We included proxy markers for responsiveness of care, which were not associated with exposure to hyperoxia. Assuming that our markers were valid, this is supportive of the hypothesis that exposure to hyperoxia is not simply a surrogate for less attentive care. Marginal structural models such as our propensity score (IPWT)-adjusted analysis further support this causal influence, and provide a robust measure of the average treatment effect of exposure to severe hyperoxia on survival [25, 26]. Additionally, a core group of experienced physicians on the PCAS cared all the patients in our study. The PCAS is the result of our effort to improve and standardize post-arrest care, and we have implemented multiple hospital-wide care protocols [34]. In this regard, our single-center design is advantageous insofar as it minimizes confounding by practice variation. Another strength is our use of high-resolution patient-level data. In addition to controlling for multiple baseline factors, we abstracted hourly oxygen data for each patient, allowing us to examine the cumulative effects of PaO2. This is in contrast to previous studies that have evaluated only a single PaO2 value [2–4], and helps explain our statistically significant results despite a comparatively small sample size. Although this method required imputation of missing oxygen data, our analysis of missingness indicated that data were MCAR and our results did not change when we restricted our analysis to only measured values, supporting the validity of our method.
We acknowledge several important limitations of our study. First, although our single center database has benefits, it limits the generalizability of our findings compared to previous multicenter studies [2, 3, 18]. However, both the incidence of severe hyperoxia and patient outcomes are similar to those reported previously, suggesting that our cohort and care practices are similar [2–4]. Second, the relatively small number of patients in certain sub-populations (e.g., shockable initial rhythm) increases the probability of making type 2 errors when analyzing these predictors. This likely explains why we did not find a significant association between these well-validated predictors and patient outcomes. Also, although we controlled for biologically plausible confounders, we cannot assert causality in the observed associations. Furthermore, our findings may have been biased by excluding patients who died in <24 h and those who were not mechanically ventilated for >24 h. Patients who recover quickly after ROSC with little or no requirement for mechanical ventilation are likely physiologically different from the patients included in our analysis, with different potential to benefit from hyperoxia or normoxia. The same may be true of those who do not survive >24 h. Our findings cannot be reliably generalized to these subpopulations. Additionally, a majority of our patients were managed with mild therapeutic hypothermia, which has been demonstrated to reduce oxidative stress after CA [28, 34]. Our local practice during this study period was to target a temperature of 33 °C for 24 h regardless of arrest rhythm. Given the recent findings of Nielsen et al. [35], targeting a core temperature of 33 °C may become less common, and we advocate caution in generalizing our findings to patients treated with different management strategies.
In conclusion, we found that severe hyperoxia is independently associated with decreased survival to hospital discharge. By contrast, moderate or probable hyperoxia was not associated with decreased survival but was associated with improved organ function at 24 h. Our findings suggest that a PaO2 >300 mmHg is potentially dangerous after CA, but that exposure to moderate hyperoxia may improve organ function and survival.
References
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S (2012) Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 125:e2–e220
Kilgannon JH, Shapiro NI, Angelos MG, Milcarek B, Hunter K, Parrillo JE, Trzeciak S, For the Emergency Medicine Shock Research Network Investigators (2010) Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA 303:2165–2171
Kilgannon JH, Jones AE, Parrillo JE, Dellinger RP, Milcarek B, Hunter K, Shapiro NI, Trzeciak S (2011) Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest/clinical perspective. Circulation 123:2717–2722
Janz DR, Hollenbeck RD, Pollock JS, McPherson JA, Rice TW (2012) Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Crit Care Med 40:3135–3139
Balan IS, Fiskum G, Hazelton J, Cotto-Cumba C, Rosenthal RE (2006) Oximetry-guided reoxygenation improves neurological outcome after experimental cardiac arrest. Stroke 37:3008–3013
Richards EM, Fiskum G, Rosenthal RE, Hopkins I, McKenna MC (2007) Hyperoxic reperfusion after global ischemia decreases hippocampal energy metabolism. Stroke 38:1578–1584
Richards EM, Rosenthal RE, Kristian T, Fiskum G (2006) Postischemic hyperoxia reduces hippocampal pyruvate dehydrogenase activity. Free Radic Biol Med 40:1960–1970
Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G (2006) Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab 26:821–835
Walson KH, Tang M, Glumac A, Alexander H, Manole MD, Ma L, Hsia CJ, Clark RS, Kochanek PM, Kagan VE, Bayr H (2011) Normoxic versus hyperoxic resuscitation in pediatric asphyxial cardiac arrest: effects on oxidative stress. Crit Care Med 39:335–343. doi:10.1097/CCM.1090b1013e3181ffda1090e
Lipinski C, Hicks S, Callaway C (1999) Normoxic ventilation during resuscitation and outcome from asphyxial cardiac arrest in rats. Resuscitation 42:221–229
Zwermer C, Whitesall S, D’Alecy L (1995) Hypoxic cardiopulmonary-cerebral resuscitaiton fails to improve neurological outcome following cardiac arrest in dogs. Resuscitation 29:225–236
Rosenthal RE, Silbergleit R, Hof PR, Haywood Y, Fiskum G (2003) Hyperbaric oxygen reduces neuronal death and improves neurological outcome after canine cardiac arrest. Stroke 34:1311–1316
Pilcher J, Weatherall M, Shirtcliffe P, Bellomo R, Young P, Beasley R (2012) The effect of hyperoxia following cardiac arrest—a systematic review and meta-analysis of animal trials. Resuscitation 83:417–422
Wang CH, Chang WT, Huang CH, Tsai MS, Yu PH, Wang AY, Chen NC, Chen WJ (2014) The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta-analysis of observational studies. Resuscitation 85:1142–1148
Kuisma M, Boyd J, Voipio V, Alaspää A, Roine RO, Rosenberg P (2006) Comparison of 30 and the 100% inspired oxygen concentrations during early post-resuscitation period: a randomised controlled pilot study. Resuscitation 69:199–206
Nelskyla A, Parr MJ, Skrifvars MB (2013) Prevalence and factors correlating with hyperoxia exposure following cardiac arrest—an observational single centre study. Scand J Trauma, Resusc Emerg Med 21:35
Ihle JF, Bernard S, Bailey MJ, Pilcher DV, Smith K, Scheinkestel CD (2013) Hyperoxia in the intensive care unit and outcome after out-of-hospital ventricular fibrillation cardiac arrest. Crit Care Acad Crit Care Med 15:186–190
Bellomo R, Bailey M, Eastwood G, Nichol A, Pilcher D, Hart G, Reade M, Egi M, Cooper DJ, Group tSoOiCC (2011) Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care 15:R90
Pandharipande PP, Shintani AK, Hagerman HE, St Jacques PJ, Rice TW, Sanders NW, Ware LB, Bernard GR, Ely EW (2009) Derivation and validation of SpO2/FiO2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the sequential organ failure assessment score. Crit Care Med 37:1317–1321
Peberdy MA, Ornato JP, Larkin GL, Braithwaite RS, Kashner TM, Carey SM, Meaney PA, Cen L, Nadkarni VM, Praestgaard AH, Berg RA, National Registry of Cardiopulmonary Resuscitation I (2008) Survival from in-hospital cardiac arrest during nights and weekends. JAMA 299:785–792
Rittenberger JC, Tisherman SA, Holm MB, Guyette FX, Callaway CW (2011) An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation 82:1399–1404
Trzeciak S, McCoy J, Phillip Dellinger R, Arnold R, Rizzuto M, Abate N, Shapiro N, Parrillo J, Hollenberg S, Resuscitation obotMAi, Shock i (2008) Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Int Care Med 34:2210–2217
Daviaud F, Dumas F, Demars N, Geri G, Bougle A, Morichau-Beauchant T, Nguyen YL, Bougouin W, Pene F, Charpentier J, Cariou A (2014) Blood glucose level and outcome after cardiac arrest: insights from a large registry in the hypothermia era. Int Care Med 40:855–862
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Int Care Med 22:707–710
Harder VS, Stuart EA, Anthony JC (2010) Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 15:234–249
Robins JM, Hernan MA, Brumback B (2000) Marginal structural models and causal inference in epidemiology. Epidemiology 11:550–560
Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Parrillo JE, Trzeciak S (2013) Multiple organ dysfunction after return of spontaneous circulation in postcardiac arrest syndrome. Crit Care Med 41:1492–1501
Dohi K, Miyamoto K, Fukuda K, Nakamura S, Hayashi M, Ohtaki H, Shioda S, Aruga T (2013) Status of systemic oxidative stress during therapeutic hypothermia in patients with post-cardiac arrest syndrome. Oxid Med Cell Longev 2013:562429
Laver S, Farrow C, Turner D, Nolan J (2004) Mode of death after admission to an intensive care unit following cardiac arrest. Int Care Med 30:2126–2128
Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche JD, Carli P, Mira JP, Nolan J, Cariou A (2013) Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Int Care Med 39:1972–1980
Nelson DP, Samsel RW, Wood LD, Schumacker PT (1988) Pathological supply dependence of systemic and intestinal O2 uptake during endotoxemia. J Appl Physiol 64:2410–2419
Tsui SS, Schultz JM, Shen I, Ungerleider RM (2004) Postoperative hypoxemia exacerbates potential brain injury after deep hypothermic circulatory arrest. Ann Thorac Surg 78:188–196 discussion 188–196
Stahel PF, Smith WR, Moore EE (2008) Hypoxia and hypotension, the “lethal duo” in traumatic brain injury: implications for prehospital care. Int Care Med 34:402–404
Rittenberger JC, Guyette FX, Tisherman SA, DeVita MA, Alvarez RJ, Callaway CW (2008) Outcomes of a hospital-wide plan to improve care of comatose survivors of cardiac arrest. Resuscitation 79:198–204
Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Aneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Kober L, Langorgen J, Lilja G, Moller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H, Investigators TTMT (2013) Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. New Engl J Med 369:2197–2206
Conflicts of interest
We have no financial or non-financial competing interests to disclose. Dr. Dezfulian’s research time is supported by the NHLBI K08NS069817. Dr. Elmer’s research time is supported by the NHLBI 5K12HL109068. Dr. Rosario-Rivera’s time is supported by the NIH CTSA program grants UL1 RR024153 and UL1TR000005.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Take-home message: Severe hyperoxia is independently associated with decreased survival to hospital discharge. By contrast, moderate or probable hyperoxia was not associated with decreased survival and was associated with improved organ function at 24 h.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
The Pittsburgh Post-Cardiac Arrest Service investigators: Clifton W. Callaway, Cameron Dezfulian, Ankur A. Doshi, Jonathan Elmer, Francis X. Guyette, and Jon C. Rittenberger.
Rights and permissions
About this article
Cite this article
Elmer, J., Scutella, M., Pullalarevu, R. et al. The association between hyperoxia and patient outcomes after cardiac arrest: analysis of a high-resolution database. Intensive Care Med 41, 49–57 (2015). https://doi.org/10.1007/s00134-014-3555-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3555-6