Abstract
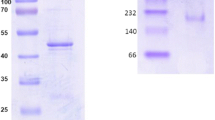
1,3-Propanediol dehydrogenase (PDOR) is important in the biosynthesis of 1,3-propanediol. In the present study, the dhaT gene encoding PDOR was cloned from Lactobacillus brevis 6239 and expressed in Escherichia coli for the first time. Sequence analysis revealed that PDOR containing two Fe2+-binding motifs and a cofactor motif belongs to the type III alcohol dehydrogenase. The purified recombinant PDOR exhibited a single band of 42 kDa according to SDS-PAGE. Optimal temperatures and pH values of this dehydrogenase are 37 °C, 7.5 for reduction and 25 °C, 9.5 for oxidation, respectively. We found that PDOR was more stable in acid buffer than in alkaline condition, and 60 % of its relative activity still remained after a 2-h incubation at 37 °C. The activity of PDOR can be enhanced in the presence of Mn2+ or Fe2+ iron and inhibited by EDTA or PMSF by different degrees. The K m and V max of this dehydrogenase are 1.25 mM, 64.02 μM min−1 mg−1 for propionaldehyde and 2.26 mM, 35.05 μM min−1 mg−1 for 1,3-PD, respectively. Substrate specificity analysis showed that PDOR has a broad range of substrate specificities. The modeling superposition indicated that the structural differences may account for the diversity of PDORs’ properties. Thus, our PDOR is a potential candidate for facilitating the 1,3-PD biosynthesis.





Similar content being viewed by others
References
Barbirato, F., Larguier, A., Conte, T., Astruc, S., & Bories, A. (1997). Sensitivity to pH, product inhibition, and inhibition by NAD+ of 1,3-propanediol dehydrogenase purified from Enterobacter agglomerans CNCM 1210. Archives of Microbiology, 168, 160–163.
Boenigk, R., Bowien, S., & Gottschalk, G. (1993). Fermentation of glycerol to 1,3-propanediol in continuous cultures of Citrobacter freundii. Applied Microbiology and Biotechnology, 38, 453–457.
Chatzifragkou, A., Dietz, D., Komaitis, M., Zeng, A. P., & Papanikolaou, S. (2010). Effect of biodiesel-derived waste glycerol impurities on biomass and 1,3-propanediol production of Clostridium butyricum VPI 1718. Biotechnology and Bioengineering, 107, 76–84.
Daniel, R., Boenigk, R., & Gottschalk, G. (1995). Purification of 1,3-propanediol dehydrogenase from Citrobacter freundii and cloning, sequencing, and overexpression of the corresponding gene in Escherichia coli. Journal of Bacteriology, 177, 2151–2156.
Elleuche, S., Fodor, K., Klippel, B., von der Heyde, A., Wilmanns, M., & Antranikian, G. (2013). Structural and biochemical characterisation of a NAD(+)-dependent alcohol dehydrogenase from Oenococcus oeni as a new model molecule for industrial biotechnology applications. Applied Microbiology and Biotechnology, 97, 8963–8975.
Fenghuan, W., Huijin, Q., He, H., & Tan, T. (2005). High-level expression of the 1,3-propanediol oxidoreductase from Klebsiella pneumoniae in Escherichia coli. Molecular Biotechnology, 31, 211–219.
Johnson, E. A., & Lin, E. C. (1987). Klebsiella pneumoniae 1,3-propanediol: NAD+ oxidoreductase. Journal of Bacteriology, 169, 2050–2054.
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N., & Sternberg, M. J. (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nature Protocols, 10, 845–858.
Luthi-Peng, Q., Dileme, F. B., & Puhan, Z. (2002). Effect of glucose on glycerol bioconversion by Lactobacillus reuteri. Applied Microbiology and Biotechnology, 59, 289–296.
Ma, C., Zhang, L., Dai, J., & Xiu, Z. (2010). Relaxing the coenzyme specificity of 1,3-propanediol oxidoreductase from Klebsiella pneumoniae by rational design. Journal of Biotechnology, 146, 173–178.
Malaoui, H., & Marczak, R. (2000). Purification and characterization of the 1-3-propanediol dehydrogenase of Clostridium butyricum E5. Enzyme and Microbial Technology, 27, 399–405.
Marcal, D., Rego, A. T., Carrondo, M. A., & Enguita, F. J. (2009). 1,3-Propanediol dehydrogenase from Klebsiella pneumoniae: decameric quaternary structure and possible subunit cooperativity. Journal of Bacteriology, 191, 1143–1151.
Montella, C., Bellsolell, L., Perez-Luque, R., Badia, J., Baldoma, L., Coll, M., & Aguilar, J. (2005). Crystal structure of an iron-dependent group III dehydrogenase that interconverts L-lactaldehyde and L-1,2-propanediol in Escherichia coli. Journal of Bacteriology, 187, 4957–4966.
Oh, B. R., Seo, J. W., Heo, S. Y., Luo, L. H., Hong, W. K., Park, D. H., & Kim, C. H. (2013). Efficient production of 1,3-propanediol from glycerol upon constitutive expression of the 1,3-propanediol oxidoreductase gene in engineered Klebsiella pneumoniae with elimination of by-product formation. Bioprocess and Biosystems Engineering, 36, 757–763.
Qi, X., Deng, W., Wang, F., Guo, Q., Chen, H., Wang, L., He, X., & Huang, R. (2013). Molecular cloning, co-expression, and characterization of glycerol dehydratase and 1,3-propanediol dehydrogenase from Citrobacter freundii. Molecular Biotechnology, 54, 469–474.
Qi, X., Guo, Q., Wei, Y., Xu, H., & Huang, R. (2012). Enhancement of pH stability and activity of glycerol dehydratase from Klebsiella pneumoniae by rational design. Biotechnology Letters, 34, 339–346.
Ringel, A. K., Wilkens, E., Hortig, D., Willke, T., & Vorlop, K. D. (2012). An improved screening method for microorganisms able to convert crude glycerol to 1,3-propanediol and to tolerate high product concentrations. Applied Microbiology and Biotechnology, 93, 1049–1056.
Robert, X., & Gouet, P. (2014). Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Research, 42, W320–W324.
Talarico, T. L., Axelsson, L. T., Novotny, J., Fiuzat, M., & Dobrogosz, W. J. (1990). Utilization of glycerol as a hydrogen acceptor by Lactobacillus reuteri: purification of 1,3-propanediol-NAD+ oxidoreductase. Applied and Environmental Microbiology, 56, 943–948.
van der Oost, J., Voorhorst, W. G., Kengen, S. W., Geerling, A. C., Wittenhorst, V., Gueguen, Y., & de Vos, W. M. (2001). Genetic and biochemical characterization of a short-chain alcohol dehydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus. European Journal of Biochemistry, 268, 3062–3068.
Veiga-da-Cunha, M., & Foster, M. A. (1992). 1,3-Propanediol: NAD+ oxidoreductases of Lactobacillus brevis and Lactobacillus buchneri. Applied and Environmental Microbiology, 58, 2005–2010.
Zeng, A. P., & Sabra, W. (2011). Microbial production of diols as platform chemicals: recent progresses. Current Opinion in Biotechnology, 22, 749–757.
Zhou, S., Catherine, C., Rathnasingh, C., Somasundar, A., & Park, S. (2013). Production of 3-hydroxypropionic acid from glycerol by recombinant Pseudomonas denitrificans. Biotechnology and Bioengineering, 110, 3177–3187.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 31571806 and 21006041), Project of Jiangsu University (No. 08JDG009), and Jiangsu Government Scholarship for Overseas Studies and PAPD.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Qi, X., Yun, J., Qi, Y. et al. Expression and Characterization of a Novel 1,3-Propanediol Dehydrogenase from Lactobacillus brevis . Appl Biochem Biotechnol 179, 959–972 (2016). https://doi.org/10.1007/s12010-016-2043-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2043-6