Abstract
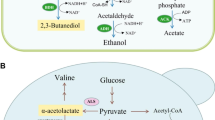
The stereochemistry of 2,3-butanediol (2,3-BD) synthesis in microbial fermentations is important for many applications. In this work, we showed that Corynebacterium glutamicum endowed with the Lactococcus lactis genes encoding α-acetolactate synthase and decarboxylase activities produced meso-2,3-BD as the major end product, meaning that (R)-acetoin is a substrate for endogenous 2,3-butanediol dehydrogenase (BDH) activity. This is curious in view of the reported absolute stereospecificity of C. glutamicum BDH for (S)-acetoin (Takusagawa et al. Biosc Biotechnol Biochem 65:1876–1878, 2001). To resolve this discrepancy, the enzyme encoded by butA Cg was produced in Escherichia coli and purified, and the stereospecific properties of the pure protein were examined. Activity assays monitored online by 1H-NMR using racemic acetoin and an excess of NADH showed an initial, fast production of (2S,3S)-2,3-BD, followed by a slow (∼20-fold lower apparent rate) formation of meso-2,3-BD. Kinetic parameters for (S)-acetoin, (R)-acetoin, meso-2,3-BD and (2S,3S)-BD were determined by spectrophotometric assays. V max values for (S)-acetoin and (R)-acetoin were 119 ± 15 and 5.23 ± 0.06 μmol min−1 mg protein−1, and K m values were 0.23 ± 0.02 and 1.49 ± 0.07 mM, respectively. We conclude that C. glutamicum BDH is not absolutely specific for (S)-acetoin, though this is the preferred substrate. Importantly, the low activity of BDH with (R)-acetoin was sufficient to support high yields of meso-2,3-BD in the engineered strain C. glutamicum ΔaceEΔpqoΔldhA(pEKEx2-als,aldB,butA Cg). Additionally, we found that the BDH activity was nearly abolished upon inactivation of butA Cg (from 0.30 ± 0.03 to 0.004 ± 0.001 μmol min−1 mg protein−1), indicating that C. glutamicum expresses a single BDH under the experimental conditions examined.





Similar content being viewed by others
References
Celińska E, Grajek W (2009) Biotechnological production of 2,3-butanediol - current state and prospects. Biotechnol Adv 27:715–725
Chen C, Wei D, Shi J, Wang M, Hao J (2014) Mechanism of 2,3-butanediol stereoisomer formation in Klebsiella pneumoniae. Appl Microbiol Biotechnol 98:4603–4613
Crout DHG, Rathbone DL (1988) Biotransformations with acetolactate decarboxylase: unusual conversions of both substrate enantiomers into products of high optical purity. J Chem Soc Chem Commun (2):98
Crow VL (1990) Properties of 2,3-butanediol dehydrogenases from Lactococcus lactis subsp. lactis in relation to citrate fermentation. Appl Environ Microbiol 56:1656–1665
Dickschat JS, Wickel S, Bolten CJ, Nawrath T, Schulz S, Wittmann C (2010) Pyrazine biosynthesis in Corynebacterium glutamicum. Eur J Org Chem 2010:2687–2695
Eikmanns BJ, Kleinertz E, Liebl W, Sahm H (1991) A family of Corynebacterium glutamicum/Escherichia coli shuttle vectors for cloning, controlled gene expression, and promoter probing. Gene 102:93–98
Gao J, Yang H-H, Feng X-H, Li S, Xu H (2013) A 2,3-butanediol dehydrogenase from Paenibacillus polymyxa ZJ-9 for mainly producing R,R-2,3-butanediol: purification, characterization and cloning. J Basic Microbiol 53:733–741
Gaspar P, Neves AR, Gasson MJ, Shearman CA, Santos H (2011) High yields of 2,3-butanediol and mannitol in Lactococcus lactis through engineering of NAD+ cofactor recycling. Appl Environ Microbiol 77:6826–6835
Gonzalez E, Fernandez MR, Larroy C, Sola L, Pericas MA, Pares X, Biosca JA (2000) Characterization of a (2R,3R)-2,3-butanediol dehydrogenase as the Saccharomyces cerevisiae YAL060W gene product: disruption and induction of the gene. J Biol Chem 275:35876–35885
Green MR, Sambrook J (2012) Molecular cloning: a laboratory manual, 4th edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Hao W, Ji F, Wang J, Zhang Y, Wang T, Bao Y (2014) Biochemical characterization of unusual meso-2,3-butanediol dehydrogenase from a strain of Bacillus subtilis. J Mol Catal B Enzym 109:184–190
Häßler T, Schieder D, Pfaller R, Faulstich M, Sieber V (2012) Enhanced fed-batch fermentation of 2,3-butanediol by Paenibacillus polymyxa DSM 365. Bioresour Technol 124:237–244
Höhn-Bentz H, Radler F (1978) Bacterial 2,3-butanediol dehydrogenases. Arch Microbiol 116:197–203
Hugenholtz J, Starrenburg MC (1992) Diacetyl production by different strains of Lactococcus lactis subsp. lactis var. diacetylactis and Leuconostoc spp. Appl Microbiol Biotechnol 38:17–22
Ji X-J, Huang H, Ouyang P-K (2011) Microbial 2,3-butanediol production: a state-of-the-art review. Biotechnol Adv 29:351–364
Johansen E, Kibenich A (1992) Isolation and characterization of IS1165, an insertion sequence of Leuconostoc mesenteroides subsp. cremoris and other lactic acid bacteria. Plasmid 27:200–206
Jojima T, Igari T, Moteki Y, Suda M, Yukawa H, Inui M (2015) Promiscuous activity of (S,S)-butanediol dehydrogenase is responsible for glycerol production from 1,3-dihydroxyacetone in Corynebacterium glutamicum under oxygen-deprived conditions. Appl Microbiol Biotechnol 99:1427–1433
Jung M-Y, Ng CY, Song H, Lee J, M-K O (2012) Deletion of lactate dehydrogenase in Enterobacter aerogenes to enhance 2,3-butanediol production. Appl Microbiol Biotechnol 95:461–469
Lian J, Chao R, Zhao H (2014) Metabolic engineering of a Saccharomyces cerevisiae strain capable of simultaneously utilizing glucose and galactose to produce enantiopure (2R,3R)-butanediol. Metab Eng 23:92–99
Liebl W, Bayerl A, Schein B, Stillner U, Schleifer KH (1989) High efficiency electroporation of intact Corynebacterium glutamicum cells. FEMS Microbiol Lett 53:299–303
Liu JC, Siu HJ, Brock-Nannestad T, Chen J, Lee SY, Solem C, Jensen PR (2016) Combining metabolic engineering and biocompatible chemistry for high-yield production of homo-diacetyl and homo-(S,S)-2,3-butanediol. Metab Eng 36:57–67
Marlow VA, Rea D, Najmudin S, Wills M, Fülöp V (2013) Structure and mechanism of acetolactate decarboxylase. ACS Chem Biol 8:2339–2344
Nicholson WL (2008) The Bacillus subtilis ydjL (bdhA) gene encodes acetoin reductase/2,3-butanediol dehydrogenase. Appl Environ Microbiol 74:6832–6838
Park C, Lu M, Yun S, Park K, Lee J (2013) Effect of pH on the metabolic flux of Klebsiella oxytoca producing 2,3-butanediol in continuous cultures at different dilution rates. Bioprocess Biosyst Eng 36:845–855
Qi G, Kang Y, Li L, Xiao A, Zhang S, Wen Z, Xu D, Chen S (2014) Deletion of meso-2,3-butanediol dehydrogenase gene budC for enhanced D-2,3-butanediol production in Bacillus licheniformis. Biotechnol Biofuels 7:16
Radoš D, Carvalho AL, Wieschalka S, Neves AR, Blombach B, Eikmanns BJ, Santos H (2015) Engineering Corynebacterium glutamicum for the production of 2,3-butanediol. Microb Cell Factories 14(1):171
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Stormer FC (1975) 2,3-Butanediol biosynthetic system in Aerobacter aerogenes. Methods Enzymol 41:518–532
Takusagawa Y, Otagiri M, Ui S, Ohtsuki T, Mimura A, Ohkuma M, Kudo T (2001) Purification and characterization of L-2,3-butanediol dehydrogenase of Brevibacterium saccharolyticum C-1012 expressed in Escherichia coli. Biosci Biotechnol Biochem 65:1876–1878
Ui S, Takusagawa Y, Sato T, Ohtsuki T, Mimura A, Ohkuma M, Kudo T (2004) Production of L-2,3-butanediol by a new pathway constructed in Escherichia coli. Lett Appl Microbiol 39:533–537
Van der Rest ME, Lange C, Molenaar D (1999) A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl Microbiol Biotechnol 52:541–545
Voloch M, Ladisch MR, Rodwell VW, Tsao GT (1983) Reduction of acetoin to 2,3-butanediol in Klebsiella pneumoniae: a new model. Biotechnol Bioeng 25:173–183
Wang Z, Song Q, Yu M, Wang Y, Xiong B, Zhang Y, Zheng J, Ying X (2014a) Characterization of a stereospecific acetoin(diacetyl) reductase from Rhodococcus erythropolis WZ010 and its application for the synthesis of (2S,3S)-2,3-butanediol. Appl Microbiol Biotechnol 98:641–650
Wang Y, Tao F, Xu P (2014b) Glycerol dehydrogenase plays a dual role in glycerol metabolism and 2,3-butanediol formation in Klebsiella pneumoniae. J Biol Chem 289:6080–6090
Wang A, Xu Y, Ma C, Gao C, Li L, Wang Y, Tao F, Xu P (2012) Efficient 2,3-butanediol production from cassava powder by a crop-biomass-utilizer, Enterobacter cloacae subsp. dissolvens SDM. PLoS One 7:e40442. doi:10.1371/journal.pone.0040442
Westerfeld WW (1945) A colorimetric determination of paraldehyde. J Lab Clin Med 30:1076
Wieschalka S, Blombach B, Eikmanns BJ (2012) Engineering Corynebacterium glutamicum for the production of pyruvate. Appl Microbiol Biotechnol 94:449–459
Xu Y, Chu H, Gao C, Tao F, Zhou Z, Li K, Li L, Ma C, Xu P (2014) Systematic metabolic engineering of Escherichia coli for high-yield production of fuel bio-chemical 2,3-butanediol. Metab Eng 23:22–33
Yang J, Kim B, Kim H, Kweon Y, Lee S, Lee J (2015) Industrial production of 2,3-butanediol from the engineered Corynebacterium glutamicum. Appl Biochem Biotechnol 176:2303–2313
Yu M, Huang M, Song Q, Shao J, Ying X (2015a) Characterization of a (2R,3R)-2,3-Butanediol dehydrogenase from Rhodococcus erythropolis WZ010. Molecules 20:7156–7173
Zhang L, Xu Q, Zhan S, Li Y, Lin H, Sun S, Sha L, Hu K, Guan X, Shen Y (2014) A new NAD(H)-dependent meso-2,3-butanediol dehydrogenase from an industrially potential strain Serratia marcescens H30. Appl Microbiol Biotechnol 98:1175–1184
Zhang L, Yang Y, Sun J, Shen Y, Wei D, Zhu J, Chu J (2010) Microbial production of 2,3-butanediol by a mutagenized strain of Serratia marcescens H30. Bioresour Technol 101:1961–1967
Acknowledgments
Technical assistance by Sara Rebelo is gratefully acknowledged. The generous advice provided by Dr. Nuno Borges was very important to the development of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Fundação para a Ciência e a Tecnologia, Portugal, project MOSTMICRO UID/CBQ/04612/2013. The NMR spectrometers are part of the National NMR Facility, supported by Fundação para a Ciência e a Tecnologia (RECI/BBB-BQB/0230/2012).
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 339 kb)
Rights and permissions
About this article
Cite this article
Radoš, D., Turner, D.L., Catarino, T. et al. Stereospecificity of Corynebacterium glutamicum 2,3-butanediol dehydrogenase and implications for the stereochemical purity of bioproduced 2,3-butanediol. Appl Microbiol Biotechnol 100, 10573–10583 (2016). https://doi.org/10.1007/s00253-016-7860-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7860-6