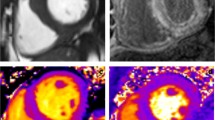
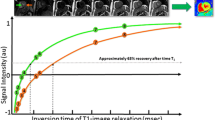
Abstract
Purpose of review
In this review, we will focus the role of CMR in dilated cardiomyopathy and genetic cardiomyopathy.
Recent findings
Non-invasive imaging plays a crucial rule in the diagnostic workup of cardiomyopathies. In these entities, echocardiography is the first-line imaging tool for diagnostic assessment, but CMR has the unique capability to identify and differentiate the underlying pathology, mainly through tissue characterization, even if the EF is preserved. Myocardial tissue characterization is crucial for adequate prognostication and guiding of therapy. Visual, semi-quantitative, and quantitative methods allow the accurate description of myocardial pathologies such as edema, hyperemia, hypoperfusion, and fibrosis. Basic CMR protocols and standardized post-processing methods are well established and routinely performed.
Summary
CMR has the ability to characterize concomitantly the myocardial tissue characteristics using techniques such as LGE, T1 mapping with ECV measurements, and T2 mapping, and also deformation functional parameters (i.e., strain) and thus provides important insights into the underlying etiology of cardiomyopathy and prognosis. The results of several studies show that CMR findings are associated with clinical outcomes and can inform the management of these patients, including longitudinal assessment of treatment response. CMR should be routinely used in the workup of patients with non-ischemic cardiomyopathy for both diagnostic and prognostic applications.
The presence of LV dilatation and systolic dysfunction in the absence of significant coronary artery disease together with valvular diseases represents a significant etiology to cause cardiomyopathy in cardiology practices. Additionally, other etiologies as infiltrative heart diseases, channelopathy/genetic cardiomyopathy and cardiac involvement in systemic diseases and oncologic process complete the spectrum that may range from isolated LV involvement or biventricular failure. Non-invasive imaging plays a crucial rule in the diagnostic workup of cardiomyopathies. In these entities, echocardiography is the first-line imaging tool for diagnostic assessment, but CMR has the unique capability to identify and differentiate the underlying pathology, mainly through tissue characterization, even if the EF is preserved. Myocardial tissue characterization is crucial for adequate prognostication and guiding of therapy. Visual, semi-quantitative, and quantitative methods allow the accurate description of myocardial pathologies such as edema, hyperaemia, hypoperfusion, and fibrosis. Basic CMR protocols and standardized post-processing methods are well established and routinely performed.




Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–7.
• Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2016;18(1):89 Reader can find these references helpful to understand the role of CMR in different disease process with different and newer CMR applications.
Brown PF, Miller C, Di Marco A, et al. Towards cardiac MRI based risk stratification in idiopathic dilated cardiomyopathy. Heart. 2019;105:270–5.
Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908.
Iles LM, Ellims AH, Llewellyn H, Hare JL, Kaye DM, McLean CA, et al. Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. Eur Heart J Cardiovasc Imaging. 2015;16:14–22.
Moon JC, Messroghli DR, Kellman P, Piechnik SK, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. JCMR. 2013;15:92.
Kammerlander AA, Marzluf BA, Zotter- Tufaro C, et al. T1 mapping by CMR imaging: from histological validation to clinical implication. JACC Card Imag 2016 Jan;9(1):14–23.
McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9.
Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardio- myopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; quality of care and outcomes re- search and functional genomics and translation- al biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–16.
Shehata ML, Turkbey EB, Vogel-Claussen J, Bluemke DA. Role of cardiac magnetic resonance imaging in assessment of nonischemic cardiomyo- pathies. Top Magn Reson Imaging. 2008;19:43–57.
Ordovas KG, Reddy GP, Higgins CB. MRI in nonischemic acquired heart disease. J Magn Reson Imaging. 2008;27:1195–213.
Cowie MR, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, Suresh V, et al. Incidence and aetiology of heart failure; a population-based study. Eur Heart J. 1999;20:421–8.
Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ, et al. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–74.
Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, et al. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverterdefibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011;57:821–8.
Pedretti S, Vargiu S, Baroni M, Dellegrottaglie S, Lanzarin B, Roghi A, et al. Complexity of scar and ventricular arrhythmias in dilated cardiomyopathy of any etiology: long-term data from the SCARFEAR (Cardiovascular Magnetic Resonance Predictors of Appropriate Implantable Cardioverter-Defibrillator Therapy Delivery) Registry. Clin Cardiol. 2018;41(4):494–501.
Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–30.
Barison A, Aimo A, Castiglione V, Arzilli C, et al. Late gadolinium enhancement predicts appropriate defibrillator interventions in noni- schaemic dilated cardiomyopathy. European Heart Journal - Cardiovascular Imaging. 2019;20(Issue Supplement 2):jez102.002.
Leong DP, Chakrabarty A, Shipp N, Molaee P, Madsen PL, Joerg L, et al. Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in newpresentation idiopathic dilated cardiomyopathy: insights from cardiovascular magnetic resonance and echocardiography. Eur Heart J. 2012;33:640–8.
Pi SH, Kim SM, Choi JO, Kim EK, Chang SA, Choe YH, et al. Prognostic value of myocardial strain and late gadolinium enhancement on cardiovascular magnetic resonance imaging in patients with idiopathic dilated cardiomyopathy with moderate to severely reduced ejection fraction. J Cardiovasc Magn Reson. 2018;20(1):36.
Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–20.
Barison A, Aimo A, Castiglione V, Arzilli C, et al. Late gadolinium enhancement predicts appropriate defibrillator interventions in noni- ischemic dilated cardiomyopathy. European Heart Journal - Cardiovascular Imaging. 2019;20(Issue Supplement 2):jez102.002.
•Pontone G, Guaricci AI, Andreini D, et al. Prognostic benefit of cardiac magnetic resonance over transthoracic echocardiography for the assessment of ischemic and nonischemic dilated cardiomyopathy patients referred for the evaluation of primary prevention implantable cardioverter-defibrillator therapy. Circ Cardiovasc Imaging. 2016;9(10) Reader can find these references helpful to understand the role of CMR in different disease process with different and newer CMR applications.
Leyva F, Foley PW, Chalil S, et al. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:29.
Sandeep P, Andrew J, Taylor B, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction. The CAMERA-MRI Study. JACC. 2017;70(16):1950–61.
Siontis KC, Kim HM, Sharaf Dabbagh G, Latchamsetty R, Stojanovska J, Jongnarangsin K, et al. Association of preprocedural cardiac magnetic resonance imaging with outcomes of ventricular tachycardia ablation in patients with idiopathic dilated cardiomyopathy. Heart Rhythm. 2017 Oct;14(10):1487–93.
Youn JC, Yj H, Lee HJ, Lee HJ, et al. Contrast-enhanced T1 mapping based extracellular volume fraction independently predicts clinical outcome in patients with non-ischemic dilated cardiomyopathy: a prospective a cohort study. Eur Radiol. 2017;27(9):3924–33.
Zhuang B, Sirajuddin A, Wang S, et al. Prognostic value of T1 mapping and extracellular volume fraction in cardiovascular disease: a systematic review and meta-analysis. Heart rEV. 2018;23(5):723–31.
Chen R, Wang J, Du Z, Juan YH, Chan CW, Fei H, et al. The comparison of short-term prognostic value of T1 mapping with feature tracking by cardiovascular magnetic resonance in patients with severe dilated cardiomyopathy. Int J Cardiovasc Imaging. 2019;35(1):171–8.
Inui K, Asai K, Tachi M, et al. Extracellular volume fraction assessed using cardiovascular magnetic resonance can predict improvement in left ventricular ejection fraction in patients with dilated cardiomyopathy. Heart Vessels. 2018;33(10):1195–203.
Spieker M, Katsianos E, Gastl M, Behm P, et al. T2 mapping cardiovascular magnetic resonance identifies the presence of myocardial inflammation in patients with dilated cardiomyopathy as compared to endomyocardial biopsy. Eur Heart J Cardiovascular Imaging. 2018;195(5):574–82.
Nakamori S, Bui AH, Jang J, el-Rewaidy HA, Kato S, Ngo LH, et al. Increased myocardial native Ti relaxation time in patients with nonischemic dilated cardiomyopathy with complex ventricular arrythmias. J Magn Imaging. 2018;47(3):779–86.
Kato S, Nakamori S, Roujol S, Delling FN, Akhtari S, Jang J, et al. Relationship between native papillary muscle T1 time and severity of functional mitral regurgitation in patients with non-ischemic dilated cardiomyopathy. J Cardiovasc Magn Reson. 2016;18(1):79.
Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:307–15.
Pan J, Wan Q, Li J, Wu H, Gao C, Tao Y, et al. Strain values of the left ventricular segments reduces non-homogeneosly in dilated cardiomyopathy with moderately and severely deteriorated heart function assessed by MRI tissue tracking imaging. Int Heart J. 2018;59:1312–9.
Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–54.
Lima JA, Desai MY. Cardiovascular magnetic resonance imaging: current and emerging applications. J Am Coll Cardiol. 2004;44:1164–71.
Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:559–66.
Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112:855–61.
Kwon DH, Setser RM, Popovic ZB, et al. Association of myocardial fibrosis, electrocardiography and ventricular tachyarrhythmia in hypertrophic cardiomyopathy: a delayed contrast enhanced MRI study. Int J Cardiovasc Imaging. 2008;24:617–25.
Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–74.
Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51–8.
O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–74.
Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875–87.
Avegliano G, Politi MT, Costabel JP, Kuschnir P, Trivi M, Ronderos R. Differences in the extent of fibrosis in obstructive and nonobstructive hypertrophic cardiomyopathy. J Cardiovasc Med (Hagerstown). 2019 Jun;20(6):389–96.
Raman B, Ariga R, Spartera M, Sivalokanathan S, Chan K, Dass S, et al. Progression of myocardial fibrosis in hypertrophic cardiomyopathy: mechanisms and clinical implications. Eur Heart J Cardiovasc Imaging. 2019;20(2):157–67.
Hinojar R, Fernández-Golfín C, González-Gómez A, Rincón LM, Plaza-Martin M, Casas E, et al. Prognostic implications of global myocardial mechanics in hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking. Relations to left ventricular hypertrophy and fibrosis. Int J Cardiol. 2017;249:467–72.
Sanaani A, Fuisz A. Cardiac magnetic resonance for diagnosis and risk stratification. Cardiol Clin. 2019;37(1):27–33.
Ismail TF, Jabbour A, Gulati A, Mallorie A, Raza S, Cowling TE, et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart. 2014;100:1851–8.
Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, et al. Prognostic value of LGE-CMR in HCM: a meta-analysis. J Am Coll Cardiol Img. 2016;9:1392–402.
Patel AR, Kramer CM. Role of the cardiac magnetic resonance in the diagnosis and prognosis of nonischemic cardiomyopathy. J Am Coll Cardiol: Imaging. 2017;10:1180–93.
Arbustini E, Di Toro A, Giuliani L, et al. Cardiac phenotypes in hereditary muscle disorders. J Am Coll. 2018;72:2485–506.
Amano Y, Suzuki Y, Yanagisawa F, Omori Y, Matsumoto N. Relationship between extension or texture features of late gadolinium enhancement and ventricular tachyarrhythmias in hypertrophic cardiomyopathy. Biomed Res Int. 2018;2018:4092469.
Cochet H, Morlon L, Vergé MP, Salel M, Camaioni C, Reynaud A, et al. Predictors of future onset of atrial fibrillation in hypertrophic cardiomyopathy. Arch Cardiovasc Dis. 2018;111(10):591–600.
Cheng S, Fang M, Cui C, Chen X, Yin G, Prasad SK, et al. LGE-CMR-derived texture features reflect poor prognosis in hypertrophic cardiomyopathy patients with systolic dysfunction: preliminary results. Eur Radiol. 2018;28(11):4615–24.
Swoboda PP, McDiarmid AK, Erhayiem B, Law GR, et al. Effect of cellular and extracellular pathology assessed by T1 mapping on regional contractile function in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2017;19(1):16.
Hinojar R, Varma N, Child N, et al. T1 Mapping in discrimination of hypertrophic phenotypes: hypertensive heart disease and hypertrophic cardiomyopathy: findings from the International T1 Multicenter Cardiovascular Magnetic Resonance Study. Circ Cardiovasc Imaging. 2015;8:e003285.
McLellan A, Ellims AH, Prabhu S, et al. Diffuse ventricular fibrosis on cardiac magnetic resonance imaging associates with ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2016;27:571–80.
Avanesov M, Münch J, Weinrich J, Well L, Säring D, Stehning C, et al. Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur Radiol. 2017;27(12):5136–45.
Gommans DHF, Cramer GE, Bakker J, Dieker HJ, Michels M, Fouraux MA, et al. High T2-weighted signal intensity for risk prediction of sudden cardiac death in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2018;34(1):113–20.
Authors/Task Force Members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, et al. Authors/Task Force Members. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–79.
Nistri S, Olivotto I, Betocchi S, Losi MA, Valsecchi G, Pinamonti B, et al. Prognostic significance of left atrial size in patients with hypertrophic cardiomy- opathy (from the Italian Registry for hypertrophic cardiomyopathy). Am J Cardiol. 2006;98:960–5.
Woo A, Williams WG, Choi R, Wigle ED, Rozenblyum E, Fedwick K, et al. Clinical and echocardiographic determinants of long-term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation. 2005;111:2033–41.
Williams LK, et al. Effect of left ventricular outflow tract obstruction on left atrial mechanics in hypertrophic cardiomyopathy. Biomed Res Int. 2015;2015:481245.
Hage FG, Karakus G, Luke WD Jr, et al. Effect of alcohol-induced septal ablation on left atrial volume and ejection fraction assessed by real time three-dimensional transthoracic echocardiography in patients with hypertrophic cardiomyopathy. Echocardiography. 2008;25(7):784–9.
Girasis C, Vassilikos V, Efthimiadis GK, Papadopoulou SL, Dakos G, Dalamaga EG, et al. Patients with hypertrophic cardiomyopathy at risk for paroxysmal atrial fibrillation: advanced echocardiographic evaluation of the left atrium combined with non-invasive P-wave analysis. Eur Heart J Cardiovasc Imaging. 2013;14(5):425–34.
Debonnaire P, Joyce E, Hiemstra Y, Mertens BJ, Atsma DE, Schalij MJ, et al. Left atrial size and function in hypertrophic cardiomyopathy patients and risk of new-onset atrial fibrillation. Circ Arrhythm Electrophysiol. 2017.
Leng S, Tan RS, Zhao X, Allen JC, Koh AS, Zhong L. Validation of a rapid semi-automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2018;20(1):71.
Hinojar R, Zamorano JL, Fernández-Méndez M, Esteban A, Plaza-Martin M, González-Gómez A, et al. Prognostic value of left atrial function by cardiovascular magnetic resonance feature tracking in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2019;35:1055–65.
Farhad H, Seidelmann SB, Vigneault D, Abbasi SA, Yang E, Day SM, et al. Left atrial structure and function in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. J Cardiovasc Magn Reson. 2017 Dec 28;19(1):107.
Jenni R, Oechslin EN, van der Loo B. Isolated ventricular non-compaction of the myocardium in adults. Heart. 2007;93:11–5.
Samsa LA, Yang B, Liu J. Embryonic cardiac chamber maturation: trabeculation, conduction and cardiomyocyte proliferation. Am J Med Genet C Semin Med Genet. 2013;163c:157–68.
Gati S, Chandra N, Bennett RL, Reed M, Kervio G, Panoulas VF, et al. Increased left ventricular trabeculation in highly trained athletes: do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart. 2013;99:401–8.
Kawel N, Nacif M, Arai AE, Gomes AS, Hundley WG, Johnson WC, et al. Trabeculated (noncompacted) and compact myocardium in adults: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2012;5:357–66.
Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–5.
Jacquier A, Thuny F, Jop B, Giorgi R, Cohen F, Gaubert JY, et al. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur Heart J. 2010;31:1098–104.
Andre F, Burger A, et al. References values for the left and right ventricular trabeculation and non-compacted myocardium. Int J Cardiol. 2015;185:240–7.
Captur G, Muthurangu V, Cook C, Flett AS, Wilson R, Barison A, et al. Quantification of left ventricular trabeculae using fractal analysis. J Cardiovasc Magn Reson. 2013;15:36.
Dellegrottaglie S, Pedrotti P, Roghi A, Pedretti S, Chiariello M, Perrone-Filardi P. Regional and global ventricular systolic function in isolated ventricular non-compaction: pathophysiological insights from magnetic resonance imaging. Int J Cardiol. 2012;158:394–9.
Nucifora G, Aquaro GD, Pingitore A, Masci PG, Lombardi M. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur J Heart Fail. 2011;13:170–6.
Boban M, Pesa V, Gabric ID, Manola S, Persic V, Antic-Kauzlaric H, et al. Auxiliary diagnostic potential of ventricle geometry and late gadolinium enhancement in left ventricular non-compaction; non-randomized case control study. BMC Cardiovasc Disord. 2017 Dec 6;17(1):286.
Andreini D, Pontone G, Bogaert J, Roghi A, Barison A, Schwitter J, et al. Long-term prognostic value of cardiac magnetic resonance in left ventricle noncompaction: a prospective multicenter study. J Am Coll Cardiol. 2016;68:2166–8.
Mathis S, Tazir M, Magy L, Duval F, le Masson G, Duchesne M, et al. History and current difficulties in classifying inherited myopathies and muscular dystrophies. J Neurol Sci. 2018;384:50–4.
Malfatti E, Romero NB. Diseases of the skeletal muscle. Handb Clin Neurol. 2017:429–51.
Ashford MW Jr, Liu W, Lin SJ, Abraszewski P, Caruthers SD, Connolly AM, et al. Occult cardiac contractile dysfunction in dystrophin deficient children revealed by cardiac magnetic resonance strain imaging. Circulation. 2005;112:2462–7.
Becker S, Florian A, Patrascu A, Rösch S, Waltenberger J, Sechtem U, et al. Identification of cardiomyopathy associated circulating miRNA biomarkers in patients with muscular dystrophy using a complementary cardiovascular magnetic resonance and plasma profiling approach. J Cardiovasc Magn Reson. 2016;18:25.
Tandon A, Villa CR, Hor KN, et al. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in Duchenne muscular dystrophy. J Am Heart Assoc. 2015;4:e001338.
Hor KN, Taylor MD, Al-Khalidi HR, et al. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function. J Cardiovasc Magn Reson. 2013;15:107.
Panovský R, Pešl M, Holeček T, Máchal J, Feitová V, Mrázová L, et al. Cardiac profile of the Czech population of Duchenne muscular dystrophy patients: a cardiovascular magnetic resonance study with T1 mapping. Orphanet J Rare Dis. 2019;14(1):10.
Starc JJ, Moore RA, Rattan MS, Villa CR, Gao Z, Mazur W, et al. Elevated myocardial extracellular volume fraction in Duchenne muscular dystrophy. Pediatr Cardiol. 2017;38(7):1485–92.
Soslow JH, Damon SM, Crum K, Lawson MA, et al. Increased myocardial native T1 and extracellular volume in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson. 2016;18:5.
Olivieri LJ, Kellman P, McCarter RJ, Cross RR, Hansen MS, Spurney CF. Native T1 values identify myocardial changes and stratify disease severity in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson. 2016;18(1):72.
Siegel B, Olivieri L, Gordish-Dressman H, Spurney CF, et al. Myocardial strain using cardiac MR feature tracking and speckle tracking echocardiography in Duchenne muscular dystrophy patients. Pediatr Cardiol. 2018;39(3):478–83.
Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J. 2010;31:806–14.
Pieroni M, Dello Russo A, Marzo F, et al. High prevalence of myocarditis mimicking arrhythmogenic right ventricular cardiomyopathy differential diagnosis by electroanatomic mapping-guided endomyocardial biopsy. J Am Coll Cardiol. 2009;53:681–9.
Fontaine GH, Andreoletti L, Redheuil A. Genetics of myocarditis in cardiomyopathies. Heart Rhythm. 2015;12:774–5.
Lopez-Ayala JM, Pastor-Quirante F, Gonzalez-Carrillo J, et al. Genetics of myocarditis in arrhythmogenic right ventricular dysplasia. Heart Rhythm. 2015;12:766–73.
Ott P, Marcus FI, Sobonya RE, Morady F, Knight BP, Fuenzalida CE. Cardiac sarcoidosis masquerading as right ventricular dysplasia. Pacing Clin Electrophysiol. 2003;26:1498–503.
Kumar S, Baldinger SH, Kapur S, Romero J, Mehta NK, Mahida S, et al. Right ventricular scar-related ventricular tachycardia in nonischemic cardiomyopathy: electrophysiological characteristics, mapping, and ablation of underlying heart disease. J Cardiovasc Electrophysiol. 2018;29:79–89.
Deac M, Alpendurada F, Fanaie F, Vimal R, Carpenter JP, Dawson A, et al. Prognostic value of cardiovascular magnetic resonance in patients with suspected arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol. 2013;168:3514–21.
Kellman P, Hernando D, Shah S, Zuehlsdorff S, Jerecic R, Mancini C, et al. Multiecho Dixon fat and water separation method for detecting fibro-fatty infiltration in the myocardium. Magn Reson Med. 2009;61:215–21.
Tandri H, Saranathan M, Rodriguez ER, Martinez C, Bomma C, Nasir K, et al. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005;45:98–103.
Xie S, Desjardins B, Kubala M, Liang J, Yang J, van der Geest R, et al. Association of regional epicardial right ventricular electrogram voltage amplitude and late gadolinium enhancement distribution on cardiac magnetic resonance in patients with arrhythmogenic right ventricular cardiomyopathy: implications for ventricular tachycardia ablation. Heart Rhythm. 2018;15(7):987–93.
Åström Aneq M, Maret E, Brudin L, Svensson A, Engvall J. Right ventricular systolic function and mechanical dispersion identify patients with arrhythmogenic right ventricular cardiomyopathy. Clin Physiol Funct Imaging. 2018;38(5):779–87.
Prati G, Vitrella G, Allocca G, et al. Right ventricular strain and dyssynchrony assessment in arrhythmogenic right ventricular cardiomyopathy: cardiac magnetic resonance feature-tracking study. Circ Cardiovasc Imaging. 2015;8:e003647 discussion e003647.
Bourfiss M, Vigneault DM, Aliyari Ghasebeh M, Murray B, James CA, Tichnell C, et al. Feature tracking CMR reveals abnormal strain in preclinical arrhythmogenic right ventricular dysplasia/ cardiomyopathy: a multisoftware feasibility and clinical implementation study. J Cardiovasc Magn Reson. 2017;19(1):66.
Lamy J, Soulat G, Evin M, Huber A, de Cesare A, Giron A, et al. Scan-rescan reproducibility of ventricular and atrial MRI feature tracking strain. Comput Biol Med. 2018;92:197–203.
Heermann P, Hedderich DM, Paul M, Schülke C, Kroeger JR, Baeßler B, et al. Biventricular myocardial strain analysis in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) using cardiovascular magneticresonance feature tracking. J Cardiovasc Magn Reson. 2014;16:75.
Czimbalmos C, Csecs I, Dohy Z, Toth A, Suhai FI, Müssigbrodt A, et al. Cardiac magnetic resonance based deformation imaging: role of feature tracking in athletes with suspected arrhythmogenic right ventricular cardiomyopathy. Int J Cardiovasc Imaging. 2019;35(3):529–38.
McLeod K, Wall S, Leren IS, Saberniak J, Haugaa KH. Ventricular structure in ARVC: going beyond volumes as a measure of risk. J Cardiovasc Magn Reson. 2016;18(1):73.
Te Riele AS, Tandri H, Bluemke DA. Arrhythmogenic right ventricular cardiomyopathy (ARVC): cardiovascular magnetic resonance update. J Cardiovasc Magn Reson. 2014;16:50.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Rodriguez-Ortiz, J., Abuzaid, A., Brian, AE. et al. Cardiovascular Magnetic Resonance Imaging Tissue Characterization in Non-ischemic Cardiomyopathies. Curr Treat Options Cardio Med 22, 16 (2020). https://doi.org/10.1007/s11936-020-00813-1
Published:
DOI: https://doi.org/10.1007/s11936-020-00813-1