Abstract
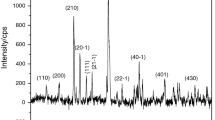
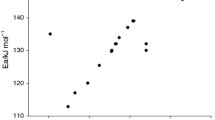
The hydrolysis of copper(II) chloride is the water splitting process in the copper–chlorine thermochemical hydrogen production cycle. In this paper, a simultaneous differential scanning calorimetry and thermogravimetric analysis (DSC/TG) technique is used to determine the transitional temperature and kinetics of the thermal decomposition of CuCl2. Thermodynamic analysis is performed on the decomposition reaction for a comparison with the thermogravimetric analysis results. The CuCl2 decomposition temperature obtained from thermogravimetric experiments is found to be higher than that predicted from the thermodynamic analysis. This broadens the available operating temperature range of the CuCl2 hydrolysis step for the Cu–Cl cycle. It is also found that the decomposition product CuCl may completely evaporate if the temperature is higher than its melting point, so the CuCl2 hydrolysis reaction should be operated below the melting point of CuCl (430 °C) to avoid the undesirable CuCl and Cl2 byproducts. A preliminary correlation is proposed in this paper for the decomposition kinetics in terms of the extent of converting CuCl2 to CuCl and Cl2.








Similar content being viewed by others
Abbreviations
- Cp :
-
Specific heat/kJ mol−1K−1
- G :
-
Gibbs free energy/kJ mol−1
- J r :
-
Reaction quotient
- H :
-
Enthalpy/kJ mol−1
- K :
-
Reaction equilibrium constant
- N Cl2,f :
-
Final amount of Cl2/mol
- N S,i :
-
Initial sample amount/mol
- R TG :
-
Reading of TG/mass%
- S :
-
Entropy/kJ mol−1K−1
- T :
-
Temperature/°C or K
- T :
-
Time/min
- λ :
-
Decomposition extent/%
References
Zhang H, Xiao R, Song M, Shen D, Liu J. Hydrogen production from bio-oil by chemical looping reforming: characteristics of the synthesized metal organic frameworks for CO2 removal. J Therm Anal Calorim. 2014;115:1921–7.
US Energy Information Administration. The impact of increased Use of hydrogen on petroleum consumption and carbon dioxide emissions. Report number: SR-OIAF-CNEAF/2008–04, energy information administration, official energy statistics from the US government. Released date: Aug, 2008.
Kothari R, Buddhiand D, Sawhney RL. Comparison of environmental and economic aspects of various hydrogen production methods. Renew Sustain Energy Rev. 2008;12:553–63.
Schultz K. Thermochemical production of hydrogen from solar and nuclear energy, presentation to the Stanford global climate and energy project. San Diego: General Atomics; 2003.
Huang C, Raissi AT. Analysis of sulfur iodine thermochemical cycle for solar hydrogen production. Part I: decomposition of sulfuric acid. Sol Energy. 2005;78:632–46.
Wu X, Kaoru O. Thermochemical water splitting for hydrogen production utilizing nuclear heat from an HTGR. Tsinghua Sci Technol. 2005;10:270–6.
Abanades S, Charvin P, Lemont F, Flamant G. Novel two-step SnO2/SnO water-splitting cycle for solar thermochemical production of hydrogen. Int J Hydrog Energy. 2008;33:6021–30.
Galvez ME, Frei A, Albisetti G, Lunardi G, Steinfeld A. Solar hydrogen production via a two-step thermochemical process based on MgO/Mg redox reactions-thermodynamic and kinetic analyses. Int J Hydrog Energy. 2008;33:2880–90.
Wang Z, Naterer GF, Gabriel KS, Secnik E, Gravelsins R, Daggupati V. Thermal design of a solar hydrogen plant with a copper–chlorine cycle and molten salt energy storage. Int J Hydrog Energy. 2011;36:11258–72.
Lewis MA, Sink C. High temperature thermochemical processes. DOE hydrogen program, annual progress report. 2008;240–244.
Wang ZL, Naterer G, Rosen M, Gabriel K. Recent research advances in system integration and scale-up. In: Proceedings of 2013 ORF workshop on clean hydrogen production with water splitting technologies. AECL Chalk River Laboratories, Chalk River, Ontario, 22 April 2013.
Wang Z, Daggupati VN, Marin G, Pope K, Xiong Y, Secnik E, Naterer GF, Gabriel KS. Towards integration of hydrolysis, decomposition and electrolysis processes of the Cu–Cl thermochemical water splitting cycle. Int J Hydrog Energy. 2012;37:16557–69.
Lewis MA, Masin JG, O’Hare PA. Evaluation of alternative thermo chemical cycles. Part I: the methodology. Int J Hydrog Energy. 2009;34:4115–24.
Lewis MA. Update on the Cu–Cl Cycle R& D effort, workshop of the ORF hydrogen project at AECL Chalk River Laboratories, Chalk River, 17 Oct 2008. p. 25–33.
Šesták J. Thermal science and analysis. J Therm Anal Calorim. 2013;113:1049–54.
Muraleedharan K. Thermal decomposition kinetics of potassium iodate: part II. Effect of gamma-irradiation on the rate and kinetics of decomposition. J Therm Anal Calorim. 2013;114:491–6.
Tian L, Chen H, Chen Z, Wang X, Zhang S. A study of non-isothermal kinetics of limestone decomposition in air (O2/N2) and oxy-fuel (O2/CO2) atmospheres. J Therm Anal Calorim. 2014;115:45–53.
Mehta SK, Kaur R, Singh S. Thermogravimetric evaluation of decomposition kinetics of metal surfactant complexes. J Therm Anal Calorim. 2012;107:69–75.
Mehdi M, Fekecs T, Zapf I, Ferencz A, Lőrinczy D. Differential scanning calorimetry (DSC) analysis of human plasma in different psoriasis stages. J Therm Anal Calorim. 2013;111:1801–4.
Saleh S, Mantheni DR, Maheswaram MPK, Moreno-Molek S, Sam-Yellowe T, Riga AT. Human cytokines characterized by dielectric thermal analysis, thermogravimetry, and differential scanning calorimetry. J Therm Anal Calorim. 2013;111:1707–16.
Zlá S, Smetana B, Žaludová M, Dobrovská J, Vodárek V, Konečná K, Matějka V, Francová H. Determination of thermophysical properties of high temperature alloy IN713LC by thermal analysis. J Therm Anal Calorim. 2012;110:211–9.
Wang Z, Xiong Y, Daggupati VN, Secnik E, Naterer G. F. Non-equilibrium CuCl2 crystallization process in the Cu–Cl hydrogen production cycle. International Conference on Hydrogen Production, Seoul, 24–27 June 2012.
NIST (National Institute of Standards and Technology). Chemistry WebBook. [Online] NIST. Available from: http://webbook.nist.gov/chemistry/. Cited 2 Feb 2011.
Serban M, Lewis MA, Basco JK. Kinetic study of the hydrogen and oxygen production reactions in the copper-chloride thermochemical cycle. AIChE 2004 Spring National Meeting, New Orleans, April 25–29, 2004.
Ball MC, Coultard RFM J. Thermal studies on halides and basic halides of copper (ii). Journal of the Chemical Society A. 1968;1417–1419.
Li Z, Wang R, Zheng H, Xie K. Preparation of CuIY catalyst using CuCl2 as precursor for vapor phase oxidative carbonylation of methanol to dimethyl carbonate. Fuel. 2010;89:1339–43.
Polyachenok OG, Dudkina EN, Polyachenok LD. Thermal stability and thermodynamics of copper(II) chloride dehydrate. J Chem Thermodyn. 2009;41:74–9.
Melnikov P, Melnikov VA, Nascimento P, Arkhangelsky IV, Zanoni Consolo LZ, de Oliveira LCS. Thermolysis mechanism of chromium nitrate nonahydrate and computerized modeling of intermediate products. J Therm Anal Calorim. 2013;114:1021–7.
Chovanec J, Chromčíková M, Pilný P, Shánělova J, Málek J, Liška M. As2Se3 melt crystallization studied by quadratic approximation of nucleation and growth rate temperature dependence. J Therm Anal Calorim. 2013;114:971–7.
Xiao WZ. Effect of source water blending on copper release in pipe distribution system: thermodynamic and empirical models. PhD Thesis. University of Central Florida, Orlando, Florida, 2004. p. 6–8 and 67–69.
Basir SMA. Recovery of cupric chloride from spent copper etchant solution: a mechanistic study. Hydrometallurgy. 2003;69:135–43.
Richardson HW. Handbook of copper compounds and applications. Published by CRC Press, ISBN 0824789989. 1997; p. 64–92.
Ulmanu C. Procedeu de obtinere directâ a clorurii cuprice anhidre. Romanian patent. Patent number 119755, issued date: 30 Sep 1987.
Leray JL. Growth kinetics of hydrated cupric chloride. J Cryst Growth. 1968;3:344–9.
Marin GD, Wang Z, Naterer GF, Gabriel K. X-ray diffraction study of multiphase reverse reaction with molten CuCl and oxygen. Thermochim Acta. 2011;524:109–16.
Marin GD, Wang Z, Naterer GF, Gabriel K. Coupled multiphase heat and mass transfer of a solid particle decomposition reaction with phase change. Int J Hydrogen Energy. 2012;55:4323–33.
Frost RL, Wills RA, Kloprogge JT, Martens W. Thermal decomposition of ammonium jarosite (NH4)Fe3(SO4)2(OH)6. J Therm Anal Calorim. 2006;84:489–96.
Acknowledgements
Support of this research from Atomic Energy of Canada Limited and the Ontario Research Excellence Fund is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z., Marin, G., Naterer, G.F. et al. Thermodynamics and kinetics of the thermal decomposition of cupric chloride in its hydrolysis reaction. J Therm Anal Calorim 119, 815–823 (2015). https://doi.org/10.1007/s10973-014-3929-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3929-6