Abstract
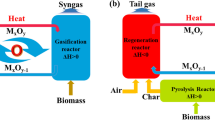
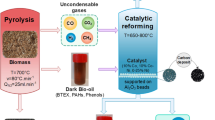
Hydrogen is a green energy carrier. Chemical looping reforming of biomass and its derivatives is a promising way for hydrogen production. However, the removal of carbon dioxide is costly and inefficient with the traditional chemical absorption methods. The objective of this article is to find a new material with low energy consumption and high capacity for carbon dioxide storage. A metal organic framework (MOF) material (e.g., CuBTC) was prepared using the hydrothermal synthesis method. The synthesized material was characterized by X-ray diffraction, −196 °C N2 adsorption/desorption isotherms, and thermogravimetry analysis to obtain its physical properties. Then BET, t-plot, and density functional theory (DFT) methods were used to acquire its specific surface area and pore textural properties. Its carbon dioxide adsorption capacity was evaluated using a micromeritics ASAP 2000 instrument. The results show that the decomposition temperature of the synthesized CuBTC material is 300 °C. Besides, high CO2 adsorption capacity (4 mmol g−1) and low N2 adsorption capacity were obtained at 0 °C and atmospheric pressure. These results indicate that the synthesized MOF material has a high efficiency for CO2 separation. From this study, it is expected that this MOF material could be used in adsorption and separation of carbon dioxide in chemical looping reforming process for hydrogen production in the near future.








Similar content being viewed by others
References
Gaudernack B, Lynum S. Hydrogen from natural gas without release of CO2 to the atmosphere. Int J Hydrogen Energy. 1998;23:1087–93.
Arjharn W, Hinsui T, Liplap P, Raghavan G. Evaluation of electricity production from different biomass feedstocks using a pilot-scale downdraft gasifier. J Biobased Mater Bioenerg. 2012;6:309–18.
Bridgwater A. The technical and economic feasibility of biomass gasification for power generation. Fuel. 1995;74:631–53.
Zhang HY, Carlson TR, Xiao R, Huber GW. Catalytic fast pyrolysis of wood and alcohol mixtures in a fluidized bed reactor. Green Chem. 2012;14:98–110.
Sinem T, Yuda Y. Co-firing of biomass with coals. J Therm Anal Calorim. 2012;107:293–8.
Açıkalın O. Pyrolytic characteristics and kinetics of pistachio shell by thermogravimetric analysis. J Therm Anal Calorim. 2012;109:227–35.
Figen AK, İsmail O, Pişkin S. Devolatilization non-isothermal kinetic analysis of agricultural stalks and application of TG-FTIR analysis. J Therm Anal Calorim. 2012;107:1177–89.
Liu Z, Zhang YS, Zhong LC, Orndroff W, Zhao HY, Cao Y, Zhang K, Pan WP. Synergistic effects of mineral matter on the combustion of coal blended with biomass. J Therm Anal Calorim. 2013;113:489–96.
Nowak B, Karlström O, Backman P, Brink A, Zevenhoven M, Voglsam S, Winter F, Hupa M. Mass transfer limitation in thermogravimetry of biomass gasification. J Therm Anal Calorim. 2013;111:183–92.
Zhao HY, Cao Y, Zhang K, Orndorff W, Chen JF, Pan WP. Fast pyrolysis characteristics of miscanthus over M/ZSM-5 (M = La and Ca). J Therm Anal Calorim. 2013;113:511–7.
Nakanishi M, Ogi T, Fukuda Y. Thermogravimetric analysis in steam and oxygen with gas chromatograph mass spectrometry for basic study of biomass gasification. J Therm Anal Calorim. 2010;101:391–6.
Vispute TP, Zhang HY, Sanna A, Xiao R, Huber GW. Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils. Science. 2010;330:1222–7.
Zhang HY, Cheng YT, Vispute TP, Xiao R, Huber GW. Catalytic conversion of biomass-derived feedstocks into olefins and aromatics with ZSM-5: the hydrogen to carbon effective ratio. Energy Environ Sci. 2011;4:2297–307.
Millward AR, Yaghi OM. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J Am Chem Soc. 2005;127:17998–9.
Férey G. Hybrid porous solids: past, present, future. Chem Soc Rev. 2008;37:191–214.
Hwang YK, Hong DY, Chang JS, Seo H, Yoon M, Kim J, Jhung SH, Serre C, Férey G. Selective sulfoxidation of aryl sulfides by coordinatively unsaturated metal centers in chromium carboxylate MIL-101. Appl Catal A. 2009;358:249–53.
Fang QR, Zhu GS, Jin Z, Xue M, Wei X, Wang DJ, Qiu SL. A novel metal–organic framework with the diamondoid topology constructed from pentanuclear zinc–carboxylate clusters. Cryst Growth Des. 2007;7:1035–7.
Qiu S, Zhu G. Molecular engineering for synthesizing novel structures of metal–organic frameworks with multifunctional properties. Coord Chem Rev. 2009;253:2891–911.
Desiraju GR. Crystal engineering. From molecules to materials. J Mol Struct. 2003;656:5–15.
Grajciar L, Wiersum AD, Llewellyn PL, Chang JS, Nachtigall P. Understanding CO2 adsorption in CuBTC MOF: comparing combined DFT–ab initio calculations with microcalorimetry experiments. J Phys Chem C. 2011;115:17925–33.
Liang Z, Marshall M, Chaffee AL. CO2 adsorption-based separation by metal organic framework (Cu-BTC) versus zeolite (13X). Energy Fuel. 2009;23:2785–9.
Xu X, Zhao X, Sun L, Liu X. Adsorption separation of carbon dioxide, methane, and nitrogen on Hβ and Na-exchanged β-zeolite. J Nat Gas Chem. 2008;17:391–6.
Guo B, Chang L, Xie K. Adsorption of carbon dioxide on activated carbon. J Nat Gas Chem. 2006;15:223–9.
Bae YS, Farha OK, Spokoyny AM, Mirkin CA, Hupp JT, Snurr RQ. Carborane-based metal–organic frameworks as highly selective sorbents for CO2 over methane. Chem Commun. 2008;35:4135–7.
Acknowledgements
This study is financially supported by the National Basic Research Program of China (973 Program) (Grant No. 2010CB732206), the National Natural Science Foundation of China (Grant No. 51076031), the Jiangsu Natural Science Foundation (Grant No. BK20130615), and the China Postdoctoral Science Foundation (Grant No. 2012M520978).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Xiao, R., Song, M. et al. Hydrogen production from bio-oil by chemical looping reforming. J Therm Anal Calorim 115, 1921–1927 (2014). https://doi.org/10.1007/s10973-013-3497-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3497-1