Abstract
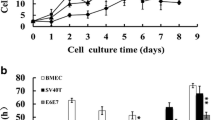
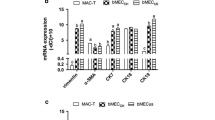
This study aimed to develop a bovine mammary epithelial (BME) cell line model, which provides a possibility to determine functional properties of the bovine mammary gland. The primary cell culture was derived from bovine mammary gland tissues and processed enzymatically to obtain cell colonies with epithelial-like morphology. The cultures of BME cells were purified and optimally cultured at 37 °C in DMEM/F12 medium supplemented with 10% fetal bovine serum. The BME cells were identified as epithelial cell line by the evaluating the expression of keratin-18 using immunofluorescence staining. A novel gene expression system strongly enhances the expression of telomerase, has been used to immortalize BME cell line termed hTBME cell line. Interestingly, telomerase remained active even after over 60 passages of hTBME cell line, required for immortalization of BME cells. In addition, the hTBME cell line was continuously subcultured with a spontaneous epithelial-like morphology, with a great proliferation activity, and without evidence of apoptotic and necrotic effects. Further characterization showed that hTBME cell line can be continuously propagated in culture with constant chromosomal features and without tumorigenic properties. Finally, established hTBME cell line was evaluated for mammary gland specific functions. Our results demonstrated that the hTBME cell line was able to retain functional-morphological structure, and functional differentiation by expression of beta (β)-casein as in the bovine mammary gland in vivo. Taken together, our findings suggest that the established hTBME cell line can serve as a valuable tool for the study of bovine mammary gland functions.







Similar content being viewed by others
References
Ahn JY, Aoki N, Adachi T, Mizuno Y, Nakamura R, Matsuda T (1995) Isolation and culture of bovine mammary epithelial cells and establishment of gene transfection conditions in the cells. Biosci Biotechnol Biochem 59:59–64. doi:10.1271/bbb.59.59
Anand V, Dogra N, Singh S, Kumar SN, Jena MK, Malakar D, Dang AK, Mishra BP, Mukhopadhyay TK, Kaushik JK, Mohanty AK (2012) Establishment and characterization of a buffalo (Bubalus bubalis) mammary epithelial cell line. PLoS ONE 7:e40469. doi:10.1371/journal.pone.0040469
Aoki N (2006) Regulation and functional relevance of milk fat globules and their components in the mammary gland. Biosci Biotechnol Biochem 70:2019–2027. doi:10.1271/bbb.60142
Barber MC, Clegg RA, Finley E, Vernon RG, Flint DJ (1992) The role of growth hormone, prolactin and insulin-like growth factors in the regulation of rat mammary gland and adipose tissue metabolism during lactation. J Endocrinol 135:195–202
Bernstein P, Peltz SW, Ross J (1989) The poly(A)-poly(A)-binding protein complex is a major determinant of mRNA stability in vitro. Mol Cell Biol 9:659–670
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 16:349–352
Booth C, O’Shea JA (2002) Isolation and culture of intestinal epithelial cells. In: Freshney RI, Freshney MG (eds) Culture of epithelial cells, 2nd edn. Copyright 2002 Wiley-Liss Inc (2002) ISBNs: 0-471-40121-8 (Hardback); 0-471-22120-1 (Electronic)
Brinster RL, Allen JM, Behringer RR, Gelinas RE, Palmiter RD (1988) Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A 85:836–840
Capuco AV, Ellis S, Wood DL, Akers RM, Garrett W (2002) Postnatal mammary ductal growth: three-dimensional imaging of cell proliferation, effects of estrogen treatment, and expression of steroid receptors in prepubertal calves. Tissue Cell 34:143–154
Christman SA, Kong BW, Landry MM, Kim H, Foster DN (2006) Contributions of differential p53 expression in the spontaneous immortalization of a chicken embryo fibroblast cell line. BMC Cell Biol 7:27. doi:10.1186/1471-2121-7-27
Davis T, Kipling D (2005) Telomeres and telomerase biology in vertebrates: progress towards a non-human model for replicative senescence and ageing. Biogerontology 6:371–385. doi:10.1007/s10522-005-4901-4
Fu M, Chen Y, Xiong X, Lan D, Li J (2014) Establishment of mammary gland model in vitro: culture and evaluation of a yak mammary epithelial cell line. PLoS ONE 9:e113669. doi:10.1371/journal.pone.0113669
Fuchs E (1988) Keratins as biochemical markers of epithelial differentiation. Trends Genet 4:277–281
Gao K, Lu YR, Wei LL, Lu XF, Li SF, Wan L, Li YP, Cheng JQ (2008) Immortalization of mesenchymal stem cells from bone marrow of rhesus monkey by transfection with human telomerase reverse transcriptase gene. Transplant Proc 40:634–637. doi:10.1016/j.transproceed.2008.01.053
Geraghty RJ, Capes-Davis A, Davis JM, Downward J, Freshney RI, Knezevic I, Lovell-Badge R, Masters JR, Meredith J, Stacey GN, Thraves P, Vias M; Cancer Research UK (2014) Guidelines for the use of cell lines in biomedical research. Br J Cancer 111:1021–1046. doi:10.1038/bjc.2014.166
German T, Barash I (2002) Characterization of an epithelial cell line from bovine mammary gland. In Vitro Cell Dev Biol Anim 38:282–292. doi:10.1290/1071-2690(2002)038<0282:COAECL>2.0.CO;2
Gibson CA, Vega JR, Baumrucker CR, Oakley CS, Welsch CW (1991) Establishment and characterization of bovine mammary epithelial cell lines. In Vitro Cell Dev Biol 1993 Jan;29A:86.
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell Biol Int 100:57–70
Herbert BS, Wright WE, Shay JW (2002) p16(INK4a) inactivation is not required to immortalize human mammary epithelial cells. Oncogene 21:7897–7900. doi:10.1038/sj.onc.1205902
Hong HX, Zhang YM, Xu H, Su ZY, Sun P (2007) Immortalization of swine umbilical vein endothelial cells with human telomerase reverse transcriptase. Mol Cells 24:358–363
Hou M, Xu D, Bjorkholm M, Gruber A (2001) Real-time quantitative telomeric repeat amplification protocol assay for the detection of telomerase activity. Clin Chem 47:519–524
Hu H, Wang J, Bu D, Wei H, Zhou L, Li F, Loor JJ (2009) In vitro culture and characterization of a mammary epithelial cell line from Chinese Holstein dairy cow. PLoS ONE 4:e7636. doi:10.1371/journal.pone.0007636
Huynh H, Pollak M (1995) HH2A, an immortalized bovine mammary epithelial cell line, expresses the gene encoding mammary derived growth inhibitor (MDGI). In Vitro Cell Dev Biol Anim 31:25–29. doi:10.1007/BF02631334
Huynh HT, Robitaille G, Turner JD (1991) Establishment of bovine mammary epithelial cells (MAC-T): an in vitro model for bovine lactation. Exp Cell Res 197:191–199
Iannuzzi L (1994) Standard karyotype of the river buffalo (Bubalus bubalis L., 2n = 50). Report of the committee for the standardization of banded karyotypes of the river buffalo. Cytogenet Cell Genet 67:102–113
Jedrzejczak MA (2010) Bovine mammary epithelial cell culture an alternative model for study of modification and mammary gland functions. PhD dissertation, West Pomeranian University of Technology, Szczecin
Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD, Chiu CP (1999) Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet 21:111–114. doi:10.1038/5056
Johnson TL, Fujimoto BA, Jimenez-Flores R, Peterson DG (2010) Growth hormone alters lipid composition and increases the abundance of casein and lactalbumin mRNA in the MAC-T cell line. J Dairy Res 77:199–204. doi:10.1017/S0022029910000087
Kim H, Farris J, Christman SA, Kong BW, Foster LK, O’Grady SM, Foster DN (2002) Events in the immortalizing process of primary human mammary epithelial cells by the catalytic subunit of human telomerase. Biochem J 365:765–772. doi:10.1042/BJ20011848
Kozłowski M, Motyl T (2007) Use of three-dimensional cultures (3D) in the investigation of bovine mammary gland biology. Med Weter 63:1417–1420
Kwak S, Jung JE, Jin X, Kim SM, Kim TK, Lee JS, Lee SY, Pian X, You S, Kim H, Choi YJ (2006) Establishment of immortal swine kidney epithelial cells. Anim Biotechnol 17:51–58
Lee KM, Choi KH, Ouellette MM (2004) Use of exogenous hTERT to immortalize primary human cells. Cytotechnology 45:33–38. doi:10.1007/10.1007/s10616-004-5123-3
Moore CM, Janish C, Eddy CA, Hubbard GB, Leland MM, Rogers J (1999) Cytogenetic and fertility studies of a rheboon, rhesus macaque (Macaca mulatta) x baboon (Papio hamadryas) cross: further support for a single karyotype nomenclature. Am J Phys Anthropol 110:119–127. doi:10.1002/(SICI)1096-8644(199910)110:2<119:AID-AJPA1>3.0.CO;2-S
Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW (1999) Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet 21:115–118. doi:10.1038/5063
Morales CP, Gandia KG, Ramirez RD, Wright WE, Shay JW, Spechler SJ (2003) Characterisation of telomerase immortalised normal human oesophageal squamous cells. Gut 52:327–333
Neville MC, McFadden TB, Forsyth I (2002) Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia 7:49–66
Ouellette MM, McDaniel LD, Wright WE, Shay JW, Schultz RA (2000) The establishment of telomerase-immortalized cell lines representing human chromosome instability syndromes. Hum Mol Genet 9:403–411
Povey RC, Osborne AD (1969) Mammary gland neoplasia in the cow. A review of the literature and report of a fibrosarcoma. Pathol Vet 6:502–512
Ramirez RD, Herbert BS, Vaughan MB, Zou Y, Gandia K, Morales CP, Wright WE, Shay JW (2003) Bypass of telomere-dependent replicative senescence (M1) upon overexpression of Cdk4 in normal human epithelial cells. Oncogene 22:433–444. doi:10.1038/sj.onc.1206046
Richert MM, Schwertfeger KL, Ryder JW, Anderson SM (2000) An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia 5:227–241
Riley LG, Gardiner-Garden M, Thomson PC, Wynn PC, Williamson P, Raadsma HW, Sheehy PA (2010) The influence of extracellular matrix and prolactin on global gene expression profiles of primary bovine mammary epithelial cells in vitro. Anim Genet 41:55–63. doi:10.1111/j.1365-2052.2009.01964.x
Said A, Osterrieder N (2014) Equine herpesvirus type 1 (EHV-1) open reading frame 59 encodes an early protein that is localized to the cytosol and required for efficient virus growth. Virology 449:263–269. doi:10.1016/j.virol.2013.11.035
Said A, Damiani A, Osterrieder N (2014) Ubiquitination and degradation of the ORF34 gene product of equine herpesvirus type 1 (EHV-1) at late times of infection. Virology 460:11–22. doi:10.1016/j.virol.2014.05.009
Schmid E, Schiller DL, Grund C, Stadler J, Franke WW (1983) Tissue type-specific expression of intermediate filament proteins in a cultured epithelial cell line from bovine mammary gland. J Cell Biol 96:37–50
Senapathy P, Shapiro MB, Harris NL (1990) Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol 183:252–278
Serakinci N, Christensen R, Graakjaer J, Cairney CJ, Keith WN, Alsner J, Saretzki G, Kolvraa S (2007) Ectopically hTERT expressing adult human mesenchymal stem cells are less radiosensitive than their telomerase negative counterpart. Exp Cell Res 313:1056–1067. doi:10.1016/j.yexcr.2007.01.002
Shi H, Shi H, Luo J, Wang W, Haile AB, Xu H, Li J (2014) Establishment and characterization of a dairy goat mammary epithelial cell line with human telomerase (hT-MECs). Anim Sci J 85:735–743. doi:10.1111/asj.12206
Tait L, Soule HD, Russo J (1990) Ultrastructural and immunocytochemical characterization of an immortalized human breast epithelial cell line, MCF-10. Cancer Res 50:6087–6094
Talhouk RS, Neiswander RL, Schanbacher FL (1990) In vitro culture of cryopreserved bovine mammary cells on collagen gels: synthesis and secretion of casein and lactoferrin. Tissue Cell 22:583–599
Taylor-Papadimitriou J, Stampfer M, Bartek J, Lewis A, Boshell M, Lane EB, Leigh IM (1989) Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J Cell Sci 94:403–413
Tong HL, Li QZ, Gao XJ, Yin DY (2012) Establishment and characterization of a lactating dairy goat mammary gland epithelial cell line. In Vitro Cell Dev Biol Anim 48:149–155. doi:10.1007/s11626-012-9481-4
Toouli CD, Huschtscha LI, Neumann AA, Noble JR, Colgin LM, Hukku B, Reddel RR (2002) Comparison of human mammary epithelial cells immortalized by simian virus 40 T-Antigen or by the telomerase catalytic subunit. Oncogene 21:128–139. doi:10.1038/sj.onc.1205014
Vaziri H, Benchimol S (1998) Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol 8:279–282
Wang W, Said A, Wang Y, Fu Q, Xiao Y, Lv S, Shen Z (2015) Establishment and characterization of duck embryo epithelial (DEE) cell line and its use as a new approach toward DHAV-1 propagation and vaccine development. Virus Res 29:260–268
Wong SC, Ong LL, Er CP, Gao S, Yu H, So JB (2003) Cloning of rat telomerase catalytic subunit functional domains, reconstitution of telomerase activity and enzymatic profile of pig and chicken tissues. Life Sci 73:2749–2760
Yang J, Chang E, Cherry AM, Bangs CD, Oei Y, Bodnar A, Bronstein A, Chiu CP, Herron GS (1999) Human endothelial cell life extension by telomerase expression. J Biol Chem 274:26141–26148
Yudoh K, Matsuno H, Nakazawa F, Katayama R, Kimura T (2001) Reconstituting telomerase activity using the telomerase catalytic subunit prevents the telomere shorting and replicative senescence in human osteoblasts. J Bone Miner 16:1453–1464. doi:10.1359/jbmr.2001.16.8.1453
Zavizion B, Gorewit RC, Politis I (1995a) Subcloning the MAC-T bovine mammary epithelial cell line: morphology, growth properties, and cytogenetic analysis of clonal cells. J Dairy Sci 78:515–527. doi:10.3168/jds.S0022-0302(95)76662-0
Zavizion B, van Duffelen M, Schaeffer W, Politis I (1995b) Use of microinjection to generate an immortalized bovine mammary cell line with both epithelial and myoepithelial charcteristics. Methods Cell Sci 17:271–282
Zavizion B, van Duffelen M, Schaeffer W, Politis I (1996) Establishment and characterization of a bovine mammary epithelial cell line with unique properties. In Vitro Cell Dev Biol Anim 32:138–148
Zhao CF, Hu HY, Meng L, Li QQ, Lin AX (2010a) Immortalization of bovine mammary epithelial cells alone by human telomerase reverse transcriptase. Cell Biol Int 34:579–586. doi:10.1042/CBI20100006
Zhao K, Liu HY, Zhou MM, Liu JX (2010b) Establishment and characterization of a lactating bovine mammary epithelial cell model for the study of milk synthesis. Cell Biol Int 34:717–721. doi:10.1042/CBI20100023
Zheng YM, He XY (2010) Characteristics and EGFP expression of porcine mammary gland epithelial cells. Res Vet Sci 89:383–390. doi:10.1016/j.rvsc.2010.03.023
Acknowledgements
The work was supported by the Key Program of the National Science Foundation of China [Grant No: 2008AA101006, China], and supported by grants from the National Natural Science Foundation of China (Grant Nos. 31572492, 31072109), the Natural Science Foundation of Tianjin, China (Grant No. 12JCZDJC22100), the Veterinary Biotechnology Scientific Research Innovation Team of Tianjin, China (Grant No. TD12-5019), and the Veterinary “Leading Talent Culture Project” of Tianjian, China.
Authors’ contribution
JL and AS contributed equally to this work by designing, carrying out experiments, analyzing and interpreting the data. XG and WW contributed to analyze and interpret the data of this work. AS, JL, YZ and TJ contributed to the drafting of the manuscript, revising it critically, and giving final approval of the version to be published. All authors read and approved the final manuscript. All contributors who do not meet the criteria for authorship should be listed in an acknowledgements section.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical standard
Bovine mammary gland tissue was obtained from a local slaughterhouse in Shannxi, Xian, China. Animals were not killed for this scientific research, therefore, no ethical approval was needed for tissue collection.
Additional information
Ji-Xia Li and Abdelrahman Said have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, JX., Said, A., Ge, XG. et al. Development and validation of immortalized bovine mammary epithelial cell line as an in vitro model for the study of mammary gland functions. Cytotechnology 70, 67–82 (2018). https://doi.org/10.1007/s10616-017-0114-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-017-0114-3