Summary
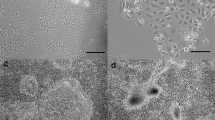
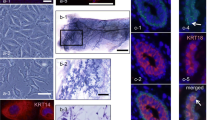
Clonal cell lines (BME-UV) were established from primary epithelial cells by stable transfection with a plasmid, carrying the sequence of the simian virus 40 early region mutant tsA58, encoding the thermolabile large T antigen. The BME-UV cells have undergone more than 300 population doublings and produce intranuclear large T antigen. At low confluency, growing islands of cells are apparent exhibiting the characteristic cobblestone morphology of epithelial cells. The BME-UV cells expressed functional markers such as microvilli and desmosomes and biochemical markers of mammary epithelial cells such as a repertoire of cytokeratins. The BME-UV cells are capable of synthesizing low levels of α-lactalbumin and α8l (50 ng/ml of medium/24 h). One of the cell lines, BME-UV1 showed enhanced proliferation in the presence of epidermal growth factor (EGF) and insulinlike growth factor I (IGF-I). The BME-UV1 cell line is the only known bovine mammary epithelial cell line responsive to EGF. The BME-UV cells grown on collagen at low confluency are capable of developing very long projections that most likely allow for communication between cells at a distance from each other. The BME-UV cells may become a valid model system to examine bovine mammary epithelial proliferation and differentiation and cell-to-cell communication.
Similar content being viewed by others
References
Akers, R. M. Lactation physiology: a ruminant animal perspective. Protoplasma 159:96–111; 1990.
Bausher, J.; Schaeffer, W. I. A diploid rat liver cell culture. 1. Characterization and sensitivity to aflatoxin B1. In Vitro 9:286–293; 1974.
Byatt, J. C.; Larson, B. R.; Baganoff, M. P., et al. Purification and partial characterization of a bovine epidermal growth factor-like polypeptide. Biochem. Int. 20:1179–1187; 1990.
Chomczynski, P.; Sacchi, N. Single step method of RNA isolation by acid guanididium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:56–61; 1987.
Cohen, S. H. Dry ice fixation of myofibrils for scanning electron microscopy. Stain Technol. 51:43–45; 1976.
Danielson, K. G.; Oborn, C. J.; Durban, E. M., et al. Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc. Natl. Acad. Sci. USA 81:3756–3760; 1984.
Graessmann, M.; Graessman, A. Microinjection of tissue culture cells. Methods Enzymol. 101:482–492; 1983.
Hurley, W. L. Mammary gland function during involution. J. Dairy Sci. 72:1637–1646; 1989.
Huynh, H.; Pollak, M. HH2A, an immortalized bovine mammary epithelial cell line, expresses the gene encoding mammary derived growth inhibitor (MDGI). In Vitro Cell. Dev. Biol. 31A:25–29; 1995.
Huynh, H. T.; Robitaille, G.; Turner, J. D.: Establishment of bovine mammary epithelial cells (MAC-T): an in vitro model for bovine lactation. Exp. Cell Res. 197:191–199; 1991.
Jat, P. S.; Sharp, P. A. Cell lines established by a temperature-sensitive simian virus 40 large T-antigen gene are growth restricted at the nonpermissive temperature. Mol. Cell. Biol. 9:1672–1681; 1989.
Larson, B. I. Biosynthesis and cellular secretion of milk. In: Larson, B. L., ed. Lactation. Ames, IA: Iowa State University Press; 1985:129–163.
Levine, A.; Momand, J.; Findlay, C. The p53 tumour suppressor gene. Nature (Lond.) 351:453–456; 1991.
Medina, D.; Li, M. L.; Oborn, C. J., et al. Casein gene expression in mouse mammary epithelial cell lines: dependence upon the extracellular matrix and cell type. Exp. Cell Res. 172:192–203; 1987.
Michalopoulos, G.; Pitot, H. C. Primary culture of parenchymal liver cells on collagen membranes. Exp. Cell Res. 94:70–78; 1975.
Ossowski, L.; Biegel, D.; Reich, E. Mammary plasminogen activator: correlation with involution, hormonal modulation and comparison between normal and neoplastic tissue. Cell 72:1929–1940; 1979.
Paul, E. C. A.; Hochman, J.; Quaroni, A. Conditionally immortalized intestinal epithelial cells: novel approach for study of differentiated enterocytes. Am. J. Physiol. 265:C266-C278; 1993.
Politis, I.; Zavizion, B.; White, J. H., et al. Hormonal and extracellular matrix regulation of plasminogen activator in a bovine mammary epithelial cell line. Endocrine 3:345–350; 1985.
Schmid, E.; Franke, W. W.; Grund, C., et al. An epithelial cell line with elongated myoid morphology derived from bovine mammary gland. Exp. Cell Res. 146:309–328; 1983.
Schmid, E.; Schiller, D. L.; Grund, C., et al. Tissue-specific expression of intermediate filament proteins in a cultured epithelial cell line from the bovine mammary gland. J. Cell Biol. 96:37–50; 1983.
Spitzer, E.; Grosse, R. EGF receptors on plasma membranes purified from the bovine mammary gland of lactating and pregnant animals. Biochem. Int. 14:581–588; 1987.
Talhouk, R. S.; Neiswander, R. L.; Schanbacher, F. L. In vitro culture of cryopreserved bovine mammary cells on collagen gels: synthesis and secretion of casein and lactoferrin. Tissue & Cell 22:583–599; 1990.
Wiens, D. J.; Brooks, C. L.; Hodgson, C. P. Casein, actin, and tubulin expression during early involution in bovine and murine mammary tissue. J. Dairy Sci. 75:1857–1869; 1992.
Woodward, T. L.; Akers, R. M.; Turner, J. D. Lack of mitogenic response to EGF, pituitary and ovarian hormones in bovine mammary epithelial cells. Endocrine 2:529–535; 1994.
Zavizion, B.; Gorewit, R. C.; Politis, I. Subcloning the MAC-T bovine mammary epithelial cell line: morphology, growth properties, and cytogenetic analysis of clonal cells. J. Dairy Sci. 78:515–527; 1995.
Zavizion, B.; Politis, I.; Gorewit, R. C. Bovine mammary myoepithelial cells. I. Isolation, culture, and characterization. J. Dairy Sci. 75:3367–3880; 1992.
Zavizion, B.; Politis, I.; Gorewit, R. C. Bovine mammary myoepithelial cells. 2. Interactions with epithelial cells in vitro. J. Dairy Sci. 75:3381–3392; 1992.
Zavizion, B.; van Duffelen, M.; Schaeffer, W., et al. Establishment and characterization of a bovine mammary myoepithelial cell line. In Vitro Cell. Dev. Biol. (accepted for publication, companion paper).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zavizion, B., van Duffelen, M., Schaeffer, W. et al. Establishment and characterization of a bovine mammary epithelial cell line with unique properties. In Vitro Cell.Dev.Biol.-Animal 32, 138–148 (1996). https://doi.org/10.1007/BF02723679
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02723679