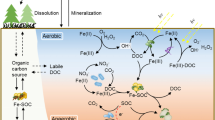
Abstract
Estimates of gaseous carbon (C) fluxes in wetlands are heavily based on temperature. However, isolating specific effects of temperature on anaerobic C processing from other controls (C quality and nutrients) has proven difficult. Here, we test the hypothesis that temperature sensitivity of soil organic matter (SOM) decomposition is more influenced by C quality than nutrient availability in subtropical freshwater, sawgrass (Cladium jamaicense)-based peats. Carbon age (characterized by depth: 0–10 and 10–20 cm) was used as a surrogate of C quality while two sites were selected with contrasting levels of nutrient (P) availability. In anaerobic laboratory incubations temperature was increased in 5 °C steps to assess the proportion of C available at a given temperature (i.e. thermo-labile C) as productions of gaseous (CO2 and CH4) and dissolved organic C (DOC) fractions. Thermo-labile C increased 3.1–3.6 times from 15 °C to 30 °C in all soils. Disproportionate increase in the production of gaseous forms versus DOC as well as CH4:CO2 was observed with warming. Observed Q10 values followed the trend of CH4 (~14) ≫ CO2 (~2.5) > DOC (~1.7) and temperature sensitivity was more dependent on C quality than nutrient availability over the entire temperature range. Spectral analysis indicated more bio-available DOC production at higher temperature. Regression analysis also indicated that C quality primarily influenced SOM decomposition at lower temperature, while at higher temperature nutrient limitation dominantly controlled SOM decomposition. These findings confirm the role of C quality in temperature sensitivity of warm peat soils, but also indicate an increased importance of nutrient limitation at higher temperature.




Similar content being viewed by others
References
Bergamaschi P, Frankenberg C, Meirink JF, Krol M, Dentener F, Wagner T, Platt U, Kaplan JO, Körner S, Heimann M, Dlugokencky EJ, Goede A (2007) Satellite chartography of atmospheric methane from SCIAMACHY on board ENVISAT: 2. Evaluation based on inverse model simulations. J Geophys Res 112(D2)
Allison SD, Martiny JB (2008) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci 105:11512–11519
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340
Andersen J (1976) An ignition method for determination of total phosphorus in lake sediments. Water Res 10:329–331
Andrews JA, Matamala R, Westover KM, Schlesinger WH (2000) Temperature effects on the diversity of soil heterotrophs and the δ13C of soil-respired CO2. Soil Biol Biochem 32:699–706. doi:10.1016/S0038-0717(99)00206-0
Bianchi TS et al (2013) Enhanced transfer of terrestrially derived carbon to the atmosphere in a flooding event. Geophys Res Lett 40:116–122
Billings SA, Ballantyne F (2013) How interactions between microbial resource demands, soil organic matter stoichiometry, and substrate reactivity determine the direction and magnitude of soil respiratory responses to warming. Glob Change Biol 19:90–102
Birdwell JE, Engel AS (2010) Characterization of dissolved organic matter in cave and spring waters using UV–Vis absorbance and fluorescence spectroscopy. Org Geochem 41:270–280
Bloom AA, Palmer PI, Fraser A, Reay DS, Frankenberg C (2010) Large-scale controls of methanogenesis inferred from methane and gravity spaceborne data. Science 327:322–325
Bosatta E, Ågren GI (1999) Soil organic matter quality interpreted thermodynamically. Soil Biol Biochem 31:1889–1891
Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, Mohan JE, ReynoldsJF Treseder KK, Wallenstein MD (2008) Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett 11:1316–1327
Bradford MA, Watts BW, Davis CA (2010) Thermal adaptation of heterotrophic soil respiration in laboratory microcosms. Glob Change Biol 16:1576–1588
Bridgham SD, Richardson CJ (1992) Mechanisms controlling soil respiration (CO2 and CH4) in southern peatlands. Soil Biol Biochem 24:1089–1099. doi:10.1016/0038-0717(92)90058-6
Bridgham SD, Johnston CA, Pastor J, Updegraff K (1995) Potential feedbacks of northern wetlands on climate change. BioScience 45:262–274
Bridgham S, Pastor J, Janssens J, Chapin C, Malterer T (1996) Multiple limiting gradients in peatlands: a call for a new paradigm. Wetlands 16:45–65. doi:10.1007/BF03160645
Bridgham S, Megonigal JP, Keller J, Bliss N, Trettin C (2006) The carbon balance of North American wetlands. Wetlands 26:889–916. doi:10.1672/0277-5212(2006)26[889:TCBONA]2.0.CO;2
Brookes P, Powlson D, Jenkinson D (1982) Measurement of microbial biomass phosphorus in soil. Soil Biol Biochem 14:319–329
Conant RT et al (2011) Temperature and soil organic matter decomposition rates—synthesis of current knowledge and a way forward. Glob Change Biol 17:3392–3404
Conen F, Leifeld J, Seth B, Alewell C (2006) Warming mobilises young and old soil carbon equally. Biogeosci Discuss 3:1355–1366
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19:988–995
Craft C, Richardson C (1993) Peat accretion and phosphorus accumulation along a eutrophication gradient in the northern Everglades. Biogeochemistry 22:133–156
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Davidson EA, Janssens IA, Luo Y (2006) On the variability of respiration in terrestrial ecosystems:moving beyond Q10. Glob Change Biol 12:154–164
Davidson EA, Samanta S, Caramori SS, Savage K (2012) The dual arrhenius and Michaelis-Menten kinetics model for decomposition of soil organic matter at hourly to seasonal time scales. Glob Change Biol 18:371–384
Davis SM (1991) Growth, decomposition, and nutrient retention of Cladium jamaicense Crantz and Typha domingensis Pers. in the Florida Everglades. Aquat Bot 40:203–224. doi:10.1016/0304-3770(91)90059-E
DeBusk W, Reddy K (1998) Turnover of detrital organic carbon in a nutrient-impacted Everglades marsh. Soil Sci Soc Am J 62:1460–1468
DeBusk W, Newman S, Reddy K (2001) Spatio–temporal patterns of soil phosphorus enrichment in Everglades Water Conservation Area 2A. J Environ Qual 30:1438–1446
DeBusk W, Reddy K (2003) Nutrient and hydrology effects on soil respiration in a northern Everglades marsh. J Environ Qual 32:702–710
DeBusk WF, Reddy KR (2005) Litter decomposition and nutrient dynamics in a phosphorus enriched everglades marsh. Biogeochemistry 75:217–240
Dodla SK, Wang JJ, DeLaune RD (2012) Characterization of labile organic carbon in coastal wetland soils of the Mississippi River deltaic plain: Relationships to carbon functionalities. Sci Total Environ 435:151–158
Eliasson PE, McMurtrie RE, Pepper DA, Strömgren M, Linder S, Ågren GI (2005) The response of heterotrophic CO2 flux to soil warming. Glob Change Biol 11:167–181
Fang C, Smith P, Moncrieff JB, Smith JU (2005) Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature 433:57–59
Fichot CG, Benner R (2012) The spectral slope coefficient of chromophoric dissolved organic matter (S275–295) as a tracer of terrigenous dissolved organic carbon in river-influenced ocean margins. Limnol Oceanogr 57:1453–1466
Fierer N, Craine JM, McLauchlan K, Schimel JP (2005) Litter quality and the temperature sensitivity of decomposition. Ecology 86:320–326
Fisher JB, Badgley G, Blyth E (2012) Global nutrient limitation in terrestrial vegetation. Glob Biogeochem Cycles 26:GB3007 doi:10.1029/2011GB004252
Freeman C, Evans C, Monteith D, Reynolds B, Fenner N (2001) Export of organic carbon from peat soils. Nature 412:785–785
Fröberg M, Berggren D, Bergkvist B, Bryant C, Knicker H (2003) Contributions of Oi, Oe and Oa horizons to dissolved organic matter in forest floor leachates. Geoderma 113:311–322
Geisseler D, Horwath WR (2009) Relationship between carbon and nitrogen availability and extracellular enzyme activities in soil. Pedobiologia 53:87–98. doi:10.1016/j.pedobi.2009.06.002
German DP, Marcelo KR, Stone MM, Allison SD (2012) The Michaelis-Menten kinetics of soil extracellular enzymes in response to temperature: a cross-latitudinal study. Glob Change Biol 18:1468–1479
Ghani A, Dexter M, Perrott K (2003) Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol Biochem 35:1231–1243
Giardina CP, Ryan MG (2000) Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 404:858–861
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1:182–195
Grzymski JJ et al (2008) Metagenome analysis of an extreme microbial symbiosis reveals eurythermal adaptation and metabolic flexibility. Proc Natl Acad Sci. doi:10.1073/pnas.0802782105
Gujer W, Zehnder A (1983) Conversion processes in anaerobic digestion. Water Sci Technol 15:127–167
Hamdi S, Moyano F, Sall S, Bernoux M, Chevallier T (2013) Synthesis analysis of the temperature sensitivity of soil respiration from laboratory studies in relation to incubation methods and soil conditions. Soil Biol Biochem 58:115–126
Hartley IP, Ineson P (2008) Substrate quality and the temperature sensitivity of soil organic matter decomposition. Soil Biol Biochem 40:1567–1574
Hartley IP, Hopkins DW, Garnett MH, Sommerkorn M, Wookey PA (2008) Soil microbial respiration in arctic soil does not acclimate to temperature. Ecol Lett 11:1092–1100
Helms JR, Stubbins A, Ritchie JD, Minor EC, Kieber DJ, Mopper K (2008) Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol Oceanogr 53:955
Hernández D, Hobbie S (2010) The effects of substrate composition, quantity, and diversity on microbial activity. Plant Soil 335:397–411. doi:10.1007/s11104-010-0428-9
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Hochachka P, Somero G (2002) Biochemical adaptation, mechanism and process in physiological evolution. Oxford University Press, Oxford, p 478
Inglett PW, Reddy KR (2006) Investigating the use of macrophyte stable C and N isotopic ratios as indicators of wetland eutrophication: Patterns in the P-affected Everglades. Limnol Oceanogr 51:2380–2387
Inglett P, Reddy K, McCormick P (2004) Periphyton chemistry and nitrogenase activity in a northern Everglades ecosystem. Biogeochemistry 67:213–233
Inglett P, Rivera-Monroy V, Wozniak J (2011) Biogeochemistry of nitrogen across the Everglades landscape. Crit Rev Environ Sci Technol 41:187–216
Inglett K, Inglett P, Reddy K, Osborne T (2012) Temperature sensitivity of greenhouse gas production in wetland soils of different vegetation. Biogeochemistry 108:77–90
Jinbo Z, Changchun S, Wenyan Y (2006) Land use effects on the distribution of labile organic carbon fractions through soil profiles. Soil Sci Soc Am J 70:660–667
Kadlec RH, Reddy K (2001) Temperature effects in treatment wetlands. Water Environ Res 73:543–557
Karhu K, Fritze H, Tuomi M, Vanhala P, Spetz P, Kitunen V, Liski J (2010) Temperature sensitivity of organic matter decomposition in two boreal forest soil profiles. Soil Biol Biochem 42:72–82
Kirschbaum MUF (1995) The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol Biochem 27:753–760
Kirschbaum MUF (2004) Soil respiration under prolonged soil warming: are rate reductions caused by acclimation or substrate loss? Glob Change Biol 10:1870–1877
Knorr W, Prentice IC, House JI, Holland EA (2005) Long-term sensitivity of soil carbon turnover to warming. Nature 433:298–301
Koch O, Tscherko D, Kandeler E (2007) Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Glob Biogeochem Cycles 21:GB4017
Larionova A, Yevdokimov I, Bykhovets S (2007) Temperature response of soil respiration is dependent on concentration of readily decomposable C. Biogeosciences 4:1073–1081
Leifeld J, Fuhrer J (2005) The temperature response of CO2 production from bulk soils and soil fractions is related to soil organic matter quality. Biogeochemistry 75:433–453
Li W, Dickinson RE, Fu R, Niu GY, Yang ZL, Canadell JG (2007) Future precipitation changes and their implications for tropical peatlands. Geophys Res Lett 34:L01403. doi:10.1029/2006GL028364
Liang J et al (2015) Methods for estimating temperature sensitivity of soil organic matter based on incubation data: a comparative evaluation. Soil Biol Biochem 80:127–135
Liao X, Inglett PW (2014) Dynamics of periphyton nitrogen fixation in short-hydroperiod wetlands revealed by high-resolution seasonal sampling. Hydrobiologia 722:263–277
Liski J, Ilvesniemi H, Mäkelä A, Westman CJ (2000) Temperature dependence of old soil organic matter. Ambio 29:56–57
Liu HS, Li LH, Ha XG, Huang JH, Sun JX, Wang HY (2006) Respiratory substrate availability plays a crucial role in the response of soil respiration to environmental factors. Appl Soil Ecol 32:284–292
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8:315–323
Lomander A, Kätterer T, Andrén O (1998) Carbon dioxide evolution from top- and subsoil as affected by moisture and constant and fluctuating temperature. Soil Biol Biochem 30:2017–2022. doi:10.1016/S0038-0717(98)00076-5
Luo YQ, Wan SQ, Hui DF, Wallace LL (2001) Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413:622–625
MacDonald NW, Zak DR, Pregitzer KS (1995) Temperature effects on kinetics of microbial respiration and net nitrogen and sulfur mineralization. Soil Sci Soc Am J 59:233–240. doi:10.2136/sssaj1995.03615995005900010036x
Matthews E, Fung I (1987) Methane emission from natural wetlands: global distribution, area, and environmental characteristics of sources. Glob Biogeochem Cycles 1:61–86
Medvedeff CA, Inglett KS, Inglett PW (2014) Evaluation of direct and indirect phosphorus limitation of methanogenic pathways in a calcareous subtropical wetland soil. Soil Biol Biochem 69:343–345
Megonigal JP, Schlesinger W (2002) Methane-limited methanotrophy in tidal freshwater swamps. Global Biogeochem Cycles 16:1088
Megonigal J, Mines M, Visscher P (2005) Anaerobic metabolism: linkages to trace gases and aerobic processes. Biogeochemistry 8:317–424
Melillo JM, Steudler PA, Aber JD et al (2002) Soil warming and carbon-cycle feedbacks to the climate system. Science 298:2173–2176
Melton JR et al (2013) Present state of global wetland extent and wetland methane modelling: conclusions from a model inter-comparison project (WETCHIMP). Biogeosciences 10:753–788. doi:10.5194/bg-10-753-2013
Mitsch WJ, Gosselink JG (2007) In: Wetlands, 4th edn. Wiley, Hoboken
Mitsch WJ, Nahlik A, Wolski P, Bernal B, Zhang L, Ramberg L (2010) Tropical wetlands: seasonal hydrologic pulsing, carbon sequestration, and methane emissions. Wetlands Ecol Manage 18:573–586
Mitsch WJ et al (2013) Wetlands, carbon, and climate change. Landscape Ecol 28:583–597
Moore PD (2001) Wetlands. New York, NY
Moore T, Dalva M (2001) Some controls on the release of dissolved organic carbon by plant tissues and soils. Soil Sci 166:38–47
Moore T, Knowles R (1990) Methane emissions from fen, bog and swamp peatlands in Quebec. Biogeochemistry 11:45–61
Neff JC, Hooper DU (2002) Vegetation and climate controls on potential CO2, DOC and DON production in northern latitude soils. Glob Change Biol 8:872–884
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. Methods of soil analysis Part 3, pp 961–1010
Newman S, Kumpf H, Laing J, Kennedy W (2001) Decomposition responses to phosphorus enrichment in an Everglades (USA) slough. Biogeochemistry 54:229–250
Nie M, Pendall E, Bell C, Gasch CK, Raut S, Tamang S, Wallenstein MD (2013) Positive climate feedbacks of soil microbial communities in a semi-arid grassland. Ecol Lett 16:234–241. doi:10.1111/ele.12034
Oechel WC, Vourlitis GL, Hastings SJ, Zulueta RC, Hinzman L, Kane D (2000) Acclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming. Nature 406:978–981
Osburn CL, Stedmon CA (2011) Linking the chemical and optical properties of dissolved organic matter in the Baltic-North Sea transition zone to differentiate three allochthonous inputs. Mar Chem 126:281–294. doi:10.1016/j.marchem.2011.06.007
Paré MC, Bedard-Haughn A (2013) Soil organic matter quality influences mineralization and GHG emissions in cryosols: a field-based study of sub-to high Arctic. Glob Change Biol 19:1126–1140
Penton CR, Newman S (2007) Enzyme activity responses to nutrient loading in subtropical wetlands. Biogeochemistry 84:83–98. doi:10.1007/s10533-007-9106-2
Potter B, Wimsatt J (2005) Determination of total organic carbon and specific UV absorbance at 254 nm in source water and drinking water. EPA Document: EPA
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/
Rey A, Jarvis P (2006) Modelling the effect of temperature on carbon mineralization rates across a network of European forest sites (FORCAST). Glob Change Biol 12:1894–1908
SAS software, Version (9.3) of the SAS System for Windows. Copyright © (2010) SAS Institute Inc., Cary, NC, USA
Schimel J (2001) 1.13-Biogeochemical models: implicit versus explicit microbiology. Global biogeochemical cycles in the climate system, pp 177–183
Schmidt MWI et al (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Schütz H, Seiler W, Conrad R (1990) Influence of soil temperature on methane emission from rice paddy fields. Biogeochemistry 11:77–95
Segers R (1998) Methane production and methane consumption: a review of processes underlying wetland methane fluxes. Biogeochemistry 41:23–51
Sihi D, Gerber S, Inglett PW, Inglett KS (2016) Comparing models of microbial-substrate interactions and their response to warming. Biogeosciences 13:1–20
Sinsabaugh RL, Linkins AE (1993) Statistical modeling of litter decomposition from integrated cellulase activity. Ecology 74:1594–1597. doi:10.2307/1940087
Sinsabaugh RL, Hill BH, Shah JJF (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798
Siskin M, Katritzky AR (1991) Reactivity of organic compounds in hot water: geochemical and technological implications. Science 254:231–237
Sistla SA, Schimel JP (2012) Stoichiometric flexibility as a regulator of carbon and nutrient cycling in terrestrial ecosystems under change. New Phytol 196:68–78. doi:10.1111/j.1469-8137.2012.04234.x
Sparling GP, Feltham CW, Reynolds J, West AW, Singleton P (1990) Estimation of soil microbial C by a fumigation-extraction method: use on soils of high organic matter content, and a reassessment of the kEC-factor. Soil Biology and Biochemistry 22(3):301–307
Spencer RG et al (2007) Diurnal variability in riverine dissolved organic matter composition determined by in situ optical measurement in the San Joaquin River (California, USA). Hydrol Process 21:3181–3189
Spencer RG, Hernes PJ, Ruf R, Baker A, Dyda RY, Stubbins A, Six J (2010) Temporal controls on dissolved organic matter and lignin biogeochemistry in a pristine tropical river, Democratic Republic of Congo. J Geophys Res Biogeosci 2005–2012:115
Stange RR Jr, Raph J, Peng J, Sims JJ, Midland SL, McDonald RE (2001) Acidolysis and hot water extraction provide new insight into the composition of the induced “lignin-like” material from squash fruit. Phytochemistry 57:1005–1011
Strack M (2008) Peatlands and climate change. IPS, International Peat Society
Sulman B, Desai A, Cook B, Saliendra N, Mackay D (2009) Contrasting carbon dioxide fluxes between a drying shrub wetland in Northern Wisconsin, USA, and nearby forests. Biogeosciences 6:1115–1126
Taggart M, Heitman JL, Shi W, Vepraskas M (2012) Temperature and water content effects on carbon mineralization for sapric soil material. Wetlands 32:939–944. doi:10.1007/s13157-012-0327-3
Teh YA, Silver WL (2006) Effects of soil structure destruction on methane production and carbon partitioning between methanogenic pathways in tropical rain forest soils. J Geophys Res Biogeosci 2005–2012:111
Ten Hulscher TE, Cornelissen G (1996) Effect of temperature on sorption equilibrium and sorption kinetics of organic micropollutants—a review. Chemosphere 32:609–626
Tfaily MM et al (2015) Utilization of PARAFAC-modeled excitation-emission matrix (EEM) fluorescence spectroscopy to identify biogeochemical processing of dissolved organic matter in a Northern Peatland. Photochem Photobiol 91:684–695
Todd-Brown KE, Hopkins FM, Kivlin SN, Talbot JM, Allison SD (2012) A framework for representing microbial decomposition in coupled climate models. Biogeochemistry 109:19–33
Torn MS, Vitousek PM, Trumbore SE (2005) The influence of nutrient availability on soil organic matter turnover estimated by incubations and radiocarbon modeling. Ecosystems 8:352–372
Tsutsuki K, Ponnamperuma FN (1987) Behavior of anaerobic decomposition products in submerged soils: Effects of organic material amendment, soil properties, and temperature. Soil Sci Plant Nutr 33:13–33
Tucker CL, Bell J, Pendall E, Ogle K (2013) Does declining carbon-use efficiency explain thermal acclimation of soil respiration with warming? Glob Change Biol 19:252–263
Turner BL, Romero TE (2009) Short-term changes in extractable inorganic nutrients during storage of tropical rain forest soils. Soil Sci Soc Am J 73:1972–1979
Twardowski MS, Boss E, Sullivan JM, Donaghay PL (2004) Modeling the spectral shape of absorption by chromophoric dissolved organic matter. Mar Chem 89:69–88
Updegraff K, Pastor J, Bridgham SD, Johnston CA (1995) Environmental and substrate controls over carbon and nitrogen mineralization in northern wetlands. Ecol Appl 5:151–163
USEPA (1993) Methods for chemical analysis of water and wastes. Environmental Monitoring Support Lab, Cincinnati
Van Hulzen J, Segers R, Van Bodegom P, Leffelaar P (1999) Temperature effects on soil methane production: an explanation for observed variability. Soil Biol Biochem 31:1919–1929
von Lüzow M, Kögel-Knabner I, Ekschmitt K, Flessa H, Guggenberger G, Matzner E et al (2007) Soil fractionation methods: relevance to functional pools and stabilization mechanisms. Soil Biol Biochem 39:2183–2207
Waddington J, Rotenberg P, Warren F (2001) Peat CO2 production in a natural and cutover peatland: implications for restoration. Biogeochemistry 54:115–130
Wagai R, Kishimoto-Mo AW, Yonemura S, Shirato Y, Hiradate S, Yagasaki Y (2013) Linking temperature sensitivity of soil organic matter decomposition to its molecular structure, accessibility, and microbial physiology. Glob Change Biol 19:1114–1125
Waldrop M, Firestone M (2004) Altered utilization patterns of young and old soil C by microorganisms caused by temperature shifts and N additions. Biogeochemistry 67:235–248
Waldrop MP, Wickland KP, White Iii R, Berhe AA, Harden JW, Romanovsky VE (2010) Molecular investigations into a globally important carbon pool: Permafrost-protected carbon in Alaskan soils. Glob Change Biol 16:2543–2554
Wallenstein M, Allison SD, Ernakovich J, Steinweg JM, Sinsabaugh R (2011) Controls on the temperature sensitivity of soil enzymes: a key driver of in situ enzyme activity rates. In: Soil enzymology. Springer, pp 245–258
Wang Q, Wang S (2007) Soil organic matter under different forest types in Southern China. Geoderma 142:349–356
Wardle DA (1998) Controls of temporal variability of the soil microbial biomass: a global-scale synthesis. Soil Biol Biochem 30:1627–1637
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708. doi:10.1021/es030360x
Weston NB, Joye SB (2005) Temperature-driven decoupling of key phases of organic matter degradation in marine sediments. Proc Natl Acad Sci USA 102:17036–17040. doi:10.1073/pnas.0508798102
White J, Reddy K (2000) Influence of phosphorus loading on organic nitrogen mineralization of Everglades soils. Soil Sci Soc Am J 64:1525–1534
Wieder WR, Bonan GB, Allison SD (2013) Global soil carbon projections are improved by modelling microbial processes. Nat Clim Change 3:909–912
Xu N, Saiers JE (2010) Temperature and hydrologic controls on dissolved organic matter mobilization and transport within a forest topsoil. Environ Sci Technol 44:5423–5429. doi:10.1021/es1002296
Yavitt JB, Lang GE (1990) Methane production in contrasting wetland sites: response to organic-chemical components of peat and to sulfate reduction. Geomicrobiology J 8:27–46
Yavitt J, Williams C, Wieder R (2005) Soil chemistry versus environmental controls on production of CH4 and CO2 in northern peatlands. Eur J Soil Sci 56:169–178
Yaws CL, Yang HC (1992) Henry’s law constant for compounds in water. In: Yaws CL (ed) Thermodynamic and physical property data. Gulf Publ. Co., Houston, pp 181–206
Yuste JC, Baldocchi DD, Gershenson A, Misson L, Wong S (2007) Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Glob Change Biol 13:2018–2035
Zhao L (2012) Spectral characteristics of dissolved organic matter released during the metabolic process of small medusa. Spectrosc Spect Anal 32:1584–1587
Zogg GP, Zak DR, Ringelberg DB, White DC, MacDonald NW, Pregitzer KS (1997) Compositional and functional shifts in microbial communities due to soil warming. Soil Sci Soc Am J 61:475–481
Acknowledgements
The authors would like to thank Matt Norton, Yu Wang, Biswanath Dari, and Swati Goswami for their laboratory and field assistance. We would like to give a special thanks to Dr. Mihai Giurcanu for assisting in statistical analysis. The project was supported by National Science Foundation (NSF) Grant DEB 0841596. We also thank two anonymous reviewers for their constructive comments which helped us significantly improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Edward Brzostek.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sihi, D., Inglett, P.W. & Inglett, K.S. Carbon quality and nutrient status drive the temperature sensitivity of organic matter decomposition in subtropical peat soils. Biogeochemistry 131, 103–119 (2016). https://doi.org/10.1007/s10533-016-0267-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-016-0267-8