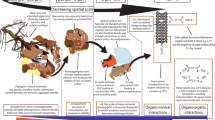
Abstract
Iron (Fe) minerals play an important role in stabilizing soil organic carbon (SOC). Fe-mediated SOC protection is mainly achieved through adsorption, co-precipitation, or aggregation. However, newly emerging evidence indicates that the electron transfer role of Fe exerts a crucial influence upon SOC turnover. In this review, we address the pathways of Fe mineral-associated soil organic carbon (Fe-SOC) formation and decomposition, and summarize the Fe-mediated biogeochemical, including redox reactions, and physical processes that control SOC cycling. The reduction of Fe can release SOC from Fe-SOC coprecipitates and Fe(III) cemented micro-aggregates, with the process also releasing CO2 from the metabolic coupling of SOC oxidation and Fe reduction. The abiotic oxidation of Fe(II) by oxidants can also oxidize SOC to produce CO2 due to reactive oxygen species production. Therefore, the functional roles of Fe on SOC sequestration may be a double-edged sword, and these processes are rarely explored concurrently. We conclude that the roles of Fe minerals in SOC stability depend on the properties of the Fe mineral, edaphic properties, and anthropogenic influence. We highlight knowledge gaps and promising directions of future research in redox-dynamic environments to optimize carbon storage in soil.
Graphical Abstract

Highlights
• The redox cycling of Fe plays a two-edged role on SOC stabilization and MAOC turnover.
• Microbial Fe(II) oxidation stabilizes SOC while chemical Fe(II) oxidation decomposes SOC.
• Microbial and photochemical Fe(III) reduction are coupled with SOC degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Soil is the largest carbon reservoir in the terrestrial biosphere, in which the soil organic carbon (SOC) pool estimated at 1500 Pg is decreasing due to soil respiration and soil erosion (Lal 2008). Much attention has been paid toward understanding mechanisms for SOC stabilization in soils (Mikutta and Kaiser 2011; Dungait et al. 2012; Crowther et al. 2016; Sanderman et al. 2017; Tamura et al. 2017), on account of that even small changes in the balance between SOC inputs and outputs can substantially affect the concentrations of atmospheric CO2 (Lal 2004; Smith et al. 2008). The mineral-associated soil organic carbon (MAOC) is considered as one of the main factors for SOC stabilization (Cotrufo et al. 2019). Meanwhile, Lehmann et al. (2020) proposed that the functional complexity can affect the stability of SOC at the lens of decomposers, such as the higher molecular diversity, higher spatial heterogeneity and greater temporal variability can reduce the decomposition of SOC by microorganisms.
Somewhat similar to the concept of functional complexity, Lützow et al. (2006) reviewed the mechanisms of SOC sequestration: (1) selective preservation, which depends on the SOC chemical properties; (2) spatial inaccessibility, which limits microbial access of SOC via occlusion within microaggregates and intercalation within phyllosilicates; and (3) interactions with mineral surfaces. The third mechanism could control long-term SOC persistence, especially with iron (Fe) mineral surfaces, while the other two mechanisms control short- and medium- term SOC stabilization (Kögel-Knabner et al. 2008). The association between SOC and soil minerals is identified as a non-negligible role (Chenu and Plante 2006; Throckmorton et al. 2015; Singh et al. 2018), and the minerals can be transformed by the prevalent changing climatic conditions, microorganisms, and shifts in the influx of electron donors and acceptors (Rasmussen et al. 2007; Melton et al. 2014; Mejia et al. 2016). However, the effect of mineral phase transformation on MAOC is unclear, especially for the formation and stability of Fe minerals associated SOC (Fe-SOC) during iron redox cycling processes.
Fe minerals, mainly including Fe oxides, (hydr)oxides and (oxy)hydroxides, are ubiquitous in soils and highly reactive to SOC (Cornell and Schwertmann 2003; Kappler and Straub 2005; Yang et al. 2017). The influence of Fe minerals on SOC stabilization has been evidenced by the positive correlations between Fe and SOC concentrations (Kaiser and Guggenberger 2007; Lalonde et al. 2012), and the inverse correlations between SOC turnover rates and Fe concentrations (Masiello et al. 2004). Fe mineral could serve as an efficient ‘rusty sink’ for trapping terrestrially-derived SOC in sediments, and it is a crucial factor in the SOC long-term storage with contribution to the global C cycling (Lalonde et al. 2012). Using EXAFS spectroscopy, Yang et al. (2017) have indicated that Fe is actively involved in the retention of SOC via Fe mineral adsorption and coprecipitation at high Fe/C ratios, whereas via Fe bridge at low Fe/C ratios. While Fe minerals can act as a SOC sorbent and provide structural roles for SOC protection, they are also involved in electron-transfer as electron donors or acceptors, which can drive SOC oxidation, depolymerization, and CO2 production (Chen et al. 2020).
This review aims to identify and highlight the pathways of Fe-SOC formation and decomposition, and how the SOC transformation processes are regulated by biogeochemical processes of Fe redox reactions. To better unravel the double-edged sword of the competing processes of carbon stabilization and degradation, our conclusion delivers recommendations for future research and development directions for Fe-SOC in redox-dynamic environments.
2 Pathways of Fe-SOC formation and decomposition
There are multiple pathways of Fe-SOC formation and decomposition (Fig. 1). The first pathway is the source of carbon supplying Fe-SOC pool. Biological carbon fixation converts CO2 into organic carbon through photosynthesis by plants or autotrophic CO2 fixation by soil microorganisms. Aboveground and belowground structural residues (e.g., root biomass) primarily supply the faster-cycling, particulate SOC pool (Cotrufo et al. 2015), with mineralization releasing nutrients and CO2, while a component is also important for macroaggregate development (Rasse et al. 2005). Recently review has concluded that the belowground C allocation by grasses (33%) is greater than crops (21%) (Pausch and Kuzyakov 2018). The low molecular weight plant C substrates (i.e., rhizodeposits) and microbial necromass can act as precursors for Fe-SOC formation (Sokolova 2020; Li et al. 2022). The second pathway is the association of Fe oxides with SOC. Fe minerals (i.e., ferrihydrite, goethite and hematite) can adsorb or co-precipitate SOC due to their large surface area and abundant hydroxyl groups (Kaiser and Guggenberger 2007; Chen et al. 2014; Kleber et al. 2015; Singh et al. 2017b; Churchman et al. 2020). The third pathway is the formation of soil aggregates via physicochemical and chemical processes, which provide physical protection of the Fe-SOC (Eusterhues et al. 2008; Zhang et al. 2015; Totsche et al. 2018; Krause et al. 2020), as shown below (Fig. 1):
2.1 The carbon source for the Fe-SOC pool
2.1.1 Plant
Plant-C inputs to soils include dissolved sugars, organic and amino acids, which can enter the mineral soil from both aboveground and belowground biomass inputs and rhizodeposition (Kuiters and Sarink 1986; Bolan et al. 2011). Aboveground and belowground structural residues primarily supply the faster-cycling, particulate SOC pool (Cotrufo et al. 2015), and can be associated with the formation of macroaggregates (Rasse et al. 2005). In contrast, low molecular weight carbon (LMWC) components of the plant-C source transfer to the slower-cycling Fe-SOC pool (i.e., in vivo microbial turnover vs. direct sorption). Traditional opinions suggested that Fe-SOC could be primarily composed of directly adsorbed plant compounds referred to “the direct sorption pathway” (Kramer et al. 2012). Others, however, showed that the majority of LMWC substrates in Fe-SOC were microbial derived, as the LMWC substrates underwent microbial anabolism, biosynthesis, and turnover. Microbial assimilates were then adsorbed to mineral surfaces - referred to as the “in vivo microbial turnover pathway” (Bradford et al. 2013; Kallenbach et al. 2016). Based on the argument, a recent study reconciled these two divergent ideas around Fe-SOC formation. This study proposes a concept of regional density of microbes, indicating that in areas of high microbial density, such as the rhizosphere, Fe-SOC is primarily formed through the “in vivo microbial turnover” pathway, while in areas of low microbial density, such as bulk soil, Fe-SOC is formed through the “direct sorption” pathway (Vidal et al. 2018; Sokol et al. 2019).
2.1.2 Microorganisms
Autotrophic microorganisms are widely present in different ecosystems. The pathways that autotrophic microorganisms assimilate CO2 include: (i) Calvin-Benson-Bassham (CBB) cycle; (ii) reducing tricarboxylic acid cycle; (iii) anaerobic acetyl-CoA; (iv) 3-hydroxypropionic acid; and (v) succinyl-CoA. The efficiency of CO2 assimilation by microorganisms affects the inputs and outputs of the SOC pool and other ecological processes. Part of the assimilated C returns to the atmosphere as CO2 and CH4 through soil respiration and methanogenesis, while a component is utilized in microbial biomass C with microbial necromass contributing to SOC (Miltner et al. 2012). Microbial-derived compounds are widely considered to be the primary components of stable SOC (Lundberg et al. 2001; Grandy and Neff 2008; Xu et al. 2018). Liang et al. (2017) further showed that the coupling of the soil microbial carbon pump and the entombing effect resulted in the persistence of majority of carbon in soils. The greater production of microbial residues should be transformed to more C compounds that are subsequently available for mineral-sorption (Cotrufo et al. 2013). With the decay of plant materials, soil microbes convert available C into microbial biomass, necromass or microbially processed compounds. The microbial residues accumulate on mineral-associated soil fractions (Kögel-Knabner et al. 2008; Cotrufo et al. 2013), and are considered to be relatively stable (Glaser et al. 2004) and accrue in the soil with iterative community turnover. Using 13C and 15N labeling, Kopittke et al. (2020) found that N-rich microbial metabolites of plant residues bind with mineral particle surfaces and stabilize SOC. The stabilization of rhizodeposits in soil has been shown to be a balance between microbial mineralization and Fe mineral associations, and branched filaments of organisms can promote aggregate formation and protect SOC from microbial access (Jeewani et al. 2020). A recent opinion proposed that the energy of SOC controls which fractions could be mineralized or stabilized, which was related to the net energy consumption during microbial decomposition of SOC (Gunina and Kuzyakov 2022). Microbial activities are controlled by the soil redox condition and subsequently affect the SOC composition. Generally, microbial decomposition of plant residues and SOC is slow under anoxic conditions, which is conductive to the enrich of plant-derived C in paddy soil, while oxic condition (e.g., in upland soil) favors the replenishment of microbial-derived C (Chen et al. 2021; Wei et al. 2021).
2.2 The associative mechanism of Fe mineral and SOC
The association of SOC with Fe minerals is an important mechanism to stabilize SOC against biodegradation (Singh et al. 2018). Fe minerals and SOC may form intricate associations via myriad interactions, and provide most of the reactive surface sites onto which SOC can be adsorbed (Yang et al. 2017). The adsorption of dissolved organic carbon (DOC) on Fe (hydr)oxides represents an important and well-documented process for SOC and contributes to SOC accumulation (Bolan et al. 2011). For example, the addition of Fe oxides such as goethite can decrease the decomposition rate of composts through increasing immobilization of SOC (Bolan et al. 2012). Similarly, increasing Fe contents of biosolids can slow down the rate of decomposition of biosolids, which is attributed to the adsorption or complexation of SOC by Fe cations (Bolan et al. 2013). Recently, Bao et al. (2021) systematically summarized the mechanisms showing the adsorption and co-precipitation of SOC on Fe oxides (Fig. 2).
Co-precipitation of SOC with Fe is ubiquitous in water and soils due to changes in pH or redox potential, which could explain the critical role of Fe oxides in C storage (Wagai and Mayer 2007). Natural environments tend to have periodically fluctuating redox conditions, and as such the interaction between SOC and Fe (hydr)oxides may not only involve organic coatings on mineral surfaces, but also form Fe-SOC co-precipitates in soil solutions containing large amounts of DOC and Fe(II) during the oxidation stage. For example, in temporarily waterlogged soils (e.g., paddy soils), where the molar C/Fe ratios of soil pore water are 0.2–6 (Katoh et al. 2004; Cheng et al. 2010), upon aeration Fe(II) is quickly oxidized to relatively insoluble Fe(III), which concurrently adsorbs SOC and ions. In contrast, after aeration a large amount of SOC was co-precipitated with Fe in the soil pore water with a high molar C/Fe ratio (10–30) (Riedel et al. 2013). Unlike the adsorption complexes, the co-precipitation of SOC with Fe can also alter the particle size and structural order of newly-formed Fe (oxy)hydroxides (Eusterhues et al. 2008; Mikutta and Kretzschmar 2011) and affect its reactivity (Eusterhues et al. 2014; Mikutta et al. 2014). Han et al. (2019) found that ferrihydrite-SOC associations in co-precipitates are more resistant to chemical reduction than the adsorption complexes. In addition, SOC composition was also found to be a crucial role controlling the SOC loadings and fractionation during adsorption and co-precipitation. Eusterhues et al. (2011) observed that SOC polysaccharide components were more enrichment in adsorption than in co-precipitation. However, for either straw-derived DOC that is rich in aromatic constituents, or SOC that is rich in carboxyl functional groups, less in aromatic and phenolic compounds, the co-precipitation could result in much higher SOC retention (Chen et al. 2014; Sodano et al. 2017).
2.3 The formation and stability of soil aggregates
Physical protection of SOC via micro-aggregation induced by Fe (hydr)oxides is another mechanism of SOC stabilization (Huang et al. 2016; Xue et al. 2019). Cationic bridges in soil are formed between ferric ion and microorganisms, and the bridge represents an organic mineral complex. The Fe (hydr)oxides are generally considered as one of the most important mineral phases involved in microaggregate formation (Totsche et al. 2018), and Fe-SOC serve as the nuclei for aggregate formation (Silva et al. 2015), and their oxides act as flocculants, binding fine particles to inorganic and/or organic molecules by physicochemical and chemical interactions involving cementing and gluing agents to form aggregates (Borggaard 1982), and promoting the stability of microaggregate occlusion in macroaggregates (Krause et al. 2020). The filamentous bacteria contributed to the development of aggregation via mycelium structures that promote the binding of soil particles to form microaggregates (Jeewani et al. 2020). Therefore, SOC adsorbed by minerals would be further protected by aggregation, which is the result of synergistic effects of biological, chemical, and physical mechanisms in SOC stabilization (Amézketa 1999; Filimonova et al. 2016; Jeewani et al. 2020).
3 Mechanisms of Fe cycling coupled with C stabilization and degradation
The biogeochemical process of Fe(II)/(III) is closely related to the fixation and mineralization of SOC in soil, and SOC protected by soil aggregates may also be released and decomposed by Fe cycling, especially for Fe-SOC, which is considered as a type of MAOC sensitive to environmental conditions.
3.1 Biogeochemical reactions of Fe(II) and Fe(III)
Generally, Fe(II) oxidation favors CO2 sequestration (Widdel et al. 1993), and dissimilatory Fe(III) reduction favors SOC mineralization (Weiss et al. 2005), except the Fenton reaction, which promotes SOC mineralization during oxidizing Fe(II). According to the order from top to bottom in flooded soil, we summarize these reactions under light, aerobic, microaerobic and anaerobic conditions in Figs. 3 and 4.
3.1.1 Phototrophic Fe(II) oxidation coupled with CO2 fixation
Oxidation of Fe(II) to Fe(III) by bacteria or by chemical oxidation has been previously thought to involve oxygen as the electron acceptor. However, in a hypoxic experiment, Fe(II) oxidation is probably driven by phototrophic microorganisms, which include Rhodopseudomonas palustris TIE-1, Rhodobacter sp. SW2, Chlorobium ferrooxidans (KoFox) and Thiodictyon sp. F4 (Melton et al. 2014). Photoferrotrophs can oxidize Fe(II) to poorly crystalline ferric (oxy)hydroxides using light energy, bicarbonate as the electron acceptors and carbon sources, and Fe(II) as the electron donor (Figs. 3 and 4). Widdel et al. (1993) described the first isolate of anoxygenic phototrophic Fe(II)-oxidizing bacteria that oxidized Fe(II) to brown Fe(III) and reduced CO2:
It is estimated that photoautotrophic Fe(II)-oxidizing bacteria can synthesize 48 billion tons of organic carbon per year in the ocean (Raiswell and Canfield 2012). The phototrophic Fe (II)-oxidizing bacteria are widely distributed on the land surface and the ocean (Kappler et al. 2005; Kappler and Straub 2005; Hedrich et al. 2011). Jiao et al. (2005) showed that 5.36 mg of biomass produced per mmol of Fe(II) which was oxidized by purple nonsulphur Fe(II)-oxidizing phototrophs - R. palustris TIE-1. This represents ~ 72% of the theoretical cell yield of 7.5 mg/mmol of Fe(II). Thus, within the photic zone, the Fe(II) oxidation coupled with CO2 assimilation process is an important pathway of SOC production.
3.1.2 Fenton reaction-induced SOC mineralization
Few microbial communities can produce oxidase that degrades lignin, and therefore soil abiotic Fe(II) oxidation is one of the important mechanisms that can facilitate the degradation of lignin and other phenolic compounds (Thevenot et al. 2010; Hall and Silver 2013, 2015; Wang et al. 2017b). The dissolved Fe(II) could be generated via biotic or abiotic reduction of Fe(III) under light or oxic conditions (Fig. 3), such as the direct bio-reduction of Fe(III) by cyanobacterial enzymes and the photochemical reduction of organic ligand bounded Fe(III) (Kranzler et al. 2011; Swanner et al. 2018). During the photochemical process, light drives electron transfer from ligand to Fe(III) and generates reactive oxygen species (ROS), including 1O2, O2·−, OH· and H2O2 (Pang et al. 2019; Lueder et al. 2020b). In oxic environments, Fe(III) could be reduced to Fe(II) by ROS, which is ubiquitous in surface waters and formed by atmospheric precipitation, photochemical reaction or biological production (Lueder et al. 2020a; Yu and Kuzyakov 2021). Thus, there is a Fe(II) pool under oxic conditions, and the Fe(II) can donate electrons to oxidants, such as O2, NO3−, or H2O2 (Melton et al. 2014). The Fenton reaction leads to the production of ROS, especially OH·, which can directly oxide SOC to small molecule compounds, increase the bioavailability of SOC, and even mineralize SOC into CO2 (Figs. 3 and 4) (Hall and Silver 2013; Patel et al. 2021). Chen et al. (2020) conducted experiments in soil slurries, and found an 8% increase in C mineralization, when MAOC was generated via Fe(II) oxidation without added SOC.
3.1.3 Microaerophilic Fe(II) oxidation coupled with CO2 assimilation
Microaerophilic Fe(II) oxidizers are lithotrophic bacteria that oxidize Fe(II) with O2 (Figs. 3 and 4) according to the following stoichiometric equation:
These microaerophilic Fe(II) oxidizing bacteria belong to the phylum Proteobacteria, including the marine genus Mariprofundus and the freshwater genera Leptothrix, Gallionella and Sideroxydans (Kucera and Wolfe 1957; Weiss et al. 2007; Fleming et al. 2011; Emerson et al. 2013). Growth of the microaerophilic Fe(II) oxidizers widely distributed in freshwater and marine environments exposed to O2 (Emerson and Moyer 1997; Edwards et al. 2003; Hegler et al. 2012) is severely inhibited under anoxic conditions, except for culture KS, which is chemolithoautotrophic nitrate-dependent Fe(II) oxidation enrichment culture and contains Sideroxydans spp. (Blöthe and Roden 2009). These chemolithoautotrophic microorganisms are widely distributed in marine and terrestrial wetland ecosystems (Weiss et al. 2007). Microaerophilic Fe(II)-oxidizing bacteria belong to the Gallionella and Leptothrix of β-proteobacteria, which can significantly increase cell production or ferrous Fe oxidation, thereby fixing CO2 (Bryan et al. 2012). NO3-dependent Fe(II) oxidating bacteria can oxidize Fe(II) and obtain energy from it to fix CO2 (Kappler and Straub 2005).
3.1.4 Dissimilatory Fe(III) reduction coupled with SOC mineralization
The main Fe(III) reduction processes include: photochemical Fe(III) reduction, O2•−-mediated Fe(III) reduction, and microbial Fe(III) reduction (Melton et al. 2014). Dissimilatory Fe(III) reduction is a form of microbial respiration, which can use Fe(III) as the terminal an electron acceptor and produce CO2 by oxidating electron donor (i.e., SOC) under anoxic conditions (Figs. 3 and 4) (Lipson et al. 2010). The electron donor and terminal electron acceptor availability influences anaerobic mineralization of SOC. Sutton-Grier et al. (2011) showed that soils amended with electron acceptors resulted in greater SOC mineralization in long term experiments, which increased with the free energy yield of electron acceptor pathway (Fe(III) respiration > SO42− reduction > methanogenesis). In soils from higher rainfall locations, microbial Fe reduction can account for up to 44% of anaerobic SOC oxidation (Dubinsky et al. 2010). This is consistent with the findings of Chen et al. (2020) that the Fe(III) was preferentially reduced, increasing the anaerobic mineralization of SOC by 32–71% under oxygen limited conditions in humid soil. Therefore, the coupling process of dissimilatory Fe(III) reduction and SOC mineralization plays a crucial role in C stabilization and degradation.
3.2 Biogeochemical reactions of Fe minerals
The SOC distributed in soil aggregates is stable slow-cycle carbon, but the Fe-SOC part in it is sensitive to environmental conditions. The reaction of Fe minerals under redox fluctuation conditions affects not only the stability of Fe-SOC but also the stability of soil aggregates, resulting in the SOC stabilization or degradation.
3.2.1 Effects on the formation and decomposition of Fe-SOC
Water saturated conditions in soil influence the concentration and distribution of high surface area minerals that are sorbents for C in soils (Wagai and Mayer 2007; Chen et al. 2017). Under aerobic condition, the change in morphology of Fe minerals can affect the stability of SOC, which may be partly due to Fe minerals transformed by Fe(II) oxidation forming Fe-SOC which is resistant to microbial mineralization (Mikutta et al. 2005; Rasmussen et al. 2006; Saidy et al. 2012). During the oxidation of Fe(II), the catalyzed production of hydroxyl radicals can enhance the oxidative activity of phenol (Sinsabaugh 2010; Hall and Silver 2013), resulting in simultaneous stabilization and decomposition of SOC.
Recent studies have focused on the process of dissimilatory Fe(III) reduction under anaerobic conditions, which leads to the dissolution of Fe minerals. Here, SOC is usually incompletely decomposed, resulting in the accumulation of SOC, especially short-chain fatty acids (Wu et al. 2015), and the dissimilatory Fe (III) reduction process can be coupled with the oxidation of short-chain fatty acids (Hori et al. 2010; Ding et al. 2015), thereby decreasing SOC content. Recent studies on the fate of Fe-SOC have demonstrated that Fe reduction can undermine the role of Fe minerals in stabilizing SOC (Pan et al. 2016; Adhikari et al. 2017; Zhao et al. 2017). During wetting and drying cycles, even in anoxic environments, Fe(II) can be oxidated through coupled biotic-abiotic reactions with oxidants such as NO2 and MnO2 (Hedrich et al. 2011; Picardal 2012; Roden 2012). Moreover, Liu et al. (2019) found that the contribution of nitrite to Fe(II) oxidation was higher than that of biological processes. The composition and diversity of microbial communities changed during the anaerobic nitrate reduction and Fe(II) oxidation at circumneutral pH (Li et al. 2016).
3.2.2 Effects on the formation and dissociation of soil aggregates
When the environmental conditions changed from aerobic to anaerobic during flooding, Fe oxides can be reduced to Fe(II), weakening the binding within the microaggregates in the process (De Campos et al. 2012). Soil aggregates are dissociated and exposed to fresh surfaces to form Fe mineral-aromatic C complexes, which are then protected by re-aggregation during the transition from anaerobic to aerobic conditions (Huang et al. 2018). Different forms of Fe minerals produced during redox fluctuations can influence aggregation. For example, short-range-ordered (SRO) Fe oxides have stronger cementing ability and may play an important role in the formation and stabilization of soil aggregates (Hou et al. 2007; Wang et al. 2016). However, Xue et al. (2019) found that in the > 0.25 mm aggregates, amorphous Fe oxides are the most important binder, while in 0.25–0.053 mm aggregates, amorphous and complex Fe oxides play a more prominent role in binding aggregates.
4 Key factors of Fe cycling coupled with C stabilization
The effects of Fe biogeochemical cycling on SOC sequestration are controlled by the characteristics of the Fe mineral, and environmental and soil conditions, which are relevant to the processes of Fe redox and formation of Fe-SOC (Table 1).
4.1 Fe mineral
There is a strong positive relationship between pedogenic Fe (Fe oxyhydroxide) content and C stocks when comparing disparate soils at regional and global scales (Kramer et al. 2012; Kleber et al. 2015). The significance of Fe/Al oxides in the persistence of SOC has partly been attributed to their physicochemical properties. The major features include:
4.1.1 Specific surface area
The association of SOC with soil minerals is generally attributed to the large specific surface area (SSA) of soil minerals (Kiem and Kögel-Knabner 2002; Singh et al. 2016), based on the positive relationship between soil mineral SSA and SOC storage (Singh et al. 2018, 2019; Churchman et al. 2020). Kennedy et al. (2002) found that 85% of variation in total SOC could be explained by mineral SSA in a black shale soil. Comparing ferrihydrite, iron sulfide, hematite and pyrite, the ferrihydrite has the highest carbon adsorption per mass, although its carbon adsorption per SSA is not the highest (Wang et al. 2019). The results may be due to the highest SSA of ferrihydrite among the four minerals, leading to its highest sorption capacity, which is consistent with the previous conclusion. However, other studies have found that the correlation between SSA and SOC in some soils is not very significant (Mayer and Xing 2001; Wiseman and Püttmann 2005), and it is still difficult to predict the potential of soil for SOC storage through SSA. This may be because SOC is distributed in heterogenous patches within the soil microstructure (Vogel et al. 2014; Steffens et al. 2017), limiting the direct relationship between SSA and SOC concentration. The general lack of agreement between SSA of Fe minerals and SOC storage requires further work, which can be resolved in future work using micro-spectroscopic techniques (Weng et al. 2021).
4.1.2 Fe mineral crystallinity
Compared to the ordered minerals (e.g., lepidocrocite, goethite and hematite), the SRO iron minerals such as ferrihydrite are considered particularly important for SOC stabilization due to the higher SSA and more surface hydroxyl groups (Chorover et al. 2004; Zhang et al. 2013; Wen et al. 2014; Xiao et al. 2015; Huang et al. 2018). Poorly crystalline Fe/Al oxides have been shown to be better predictors of stable SOC content in acid soils, compared to crystalline Fe/Al oxides (Kleber et al. 2005). The SRO iron can facilitate the rapid accumulation of plant-derived SOM, whereas crystalline iron tends to associate with microbial-derived SOM, the slower-cycling SOC (Hall et al. 2018). In addition, SOC association can slow down the transformation of poorly crystalline iron minerals by inhibiting the nucleation and the electron transfer, so that more SRO minerals can be retained to stabilize SOC (Hu et al. 2018; Lu et al. 2019).
4.1.3 Fe oxidation states
Fe minerals can directly transfer the electrons as a (semi)conductor, or indirectly mediate electron transfer process via the redox cycling between Fe(II) and Fe(III), resulting in the enhancement of SOC decomposition (Hu et al. 2021). Fe minerals can also promote the interspecies electron transfer between microbes and sequentially enhance the biodegradation of SOC and methane emission (Baek et al. 2018; Yin et al. 2018; Ren et al. 2020). The Fe oxidation states may affect their electron transport ability of Fe minerals (Li et al. 2019), and specifically, the conductivity of magnetite with Fe(II) and Fe(III) was much higher than most Fe minerals with Fe(III), such as ferrihydrite and goethite. Due to the coexistence of Fe(II) and Fe(III), magnetite can act as both an electron acceptor and donor and consequently promote energy metabolism for microbes under various conditions (Byrne et al. 2015). In addition, Fe(II) adsorbed on Fe mineral surface or defects could also promote the formation of secondary minerals, such as hematite and magnetite (Han et al. 2018; Notini et al. 2018), which could influence the SOC binding capacity via changing the electron transfer ability, surface charges, SSA and crystallinity of Fe minerals.
4.2 Impacts of natural and anthropogenic factors
4.2.1 Climate
Many studies have shown that climate change, including temperature and precipitation, is closely related to the SOC chemical diversity, and could induce variation of Fe-SOC in soils. For example, Doetterl et al. (2015) found that in humid regions with mild to warm temperatures, where (bio)chemical weathering rates are high, SOC is predominantly found in association with the mineral fraction. This may be due to the fact that in environments with humid climates and abundant primary minerals, poorly crystalline minerals (i.e., Fe/Al (oxy)hydroxides) increase with weathering process (Slessarev et al. 2021), and reduced Fe-bearing clays act as antibacterial agents (Wang et al. 2017a) to inhibit microbial activity, thus protecting SOC through physicochemical barriers. The humid environment is favorable to the formation of poorly crystalline iron minerals and binding to SOC, while higher precipitation might result in the microbial reduction of Fe and release of SOC in reducing environments (e.g., poor soil aeration) (Adhikari et al. 2016; Inagaki et al. 2019). The cold and dry conditions are also favorable for Fe-SOC (Mu et al. 2016), a possible explanation is that the colder and drier environments slow down the transformation of ferrihydrite and provide stronger protection for Fe-SOC, especially the aromatic SOC components (Gnanaprakash et al. 2007; Zhao et al. 2016).
It is worth noting that climate is not the dominant factor in the coupling of Fe cycling and carbon stabilization, and the common effects of geochemical factors cannot be ignored (Doetterl et al. 2015, 2018). For instance, temperature may not affect the formation or the stability of Fe-SOC associations (Nguyen et al. 2019), but depends on the properties of mineral and SOC. Overall, climate change alters Fe-SOC stability by affecting the weathering of Fe-bearing minerals, the formation and transformation of iron minerals, and microbial activity, which can also explain the fact that the decomposition of stable SOC is more sensitive to changes in temperature (Conant et al. 2008; Singh et al. 2017a).
4.2.2 Soil pH and Eh
Soil pH is always inversely correlated with redox potential (Eh), which is the result of coupling multi biogeochemical processes in soil (Yang et al. 2021). Soil pH affects Fe-OC bonding possibly through changes to the Fe speciation state and charge characteristics, and plays a key role in Fe cycling (Kappler and Straub 2005). In theory, under more acidic conditions Fe(II) has a slower oxidation rate, while under alkaline conditions Fe(II) has a faster oxidation rate. At acidic pH, Fe(II) can persist, even under oxic conditions (Cornell and Schwertmann 2003; Stumm and Morgan 2012). At neutral of alkaline pH, Fe(II) is only stable in anoxic environments and oxidized to Fe(III) minerals by molecular oxygen.
The structure and electro-affinity of DOC and Fe-containing minerals during the coprecipitation processes of Fe and DOC are directly affected by pH (Nierop et al. 2002; Jansen et al. 2003). The density of negative charge on the Fe hydroxides surfaces increases with the increase the solution pH, and can enhance the affinity for DOC (Jansen et al. 2003; Bolan et al. 2011). The decrease in pH enhanced the co-precipitation of DOC and Fe, which is consistent with the adsorption of DOC on Fe oxides (Du et al. 2018; Wang et al. 2021). DOC components with high molecular weight and abundant O-containing functional groups, including carboxyl and phenolic groups, have a stronger affinity for Fe (Kaiser et al. 2007; Wang et al. 2021). Newcomb et al. (2017) demonstrated that pH significantly changes the free energy of bonding. The interaction of the organic-mineral pairs with changes in pH, when the pH was tested below the pKa (pH = 4), the binding force between carboxylic acid and mica increased by an order of magnitude. This pH dependency has been reported for several organic-mineral systems, as stronger inner-sphere coordination generally occurs at low pH, while weaker outer-sphere complexation is observed at higher pH (Kleber et al. 2005; Persson and Axe 2005; Newcomb et al. 2017).
4.2.3 Soil texture
Giannetta et al. (2018) found that more thermally labile compounds were protected in the heavy fraction of soils in various ecosystems. Some studies showed that fine-sized residues often decompose faster than the coarse-sized ones, particularly in sandy and sandy loam soils (von Haden et al. 2019), suggesting that soil texture could control the residues decomposition and that clay minerals may favor the residue-derived C stabilization (Ye et al. 2017). The fine fractions of soils have higher SSA and be expected as active fraction for SOC adsorption, which contain many clay minerals and Fe/Al oxides. SOC could be stabilized by clays through weak electrostatic interactions and hydrogen bonds, precipitated by cations bridges, and associated with Al/Fe oxides (Stevenson 1994). Although the coarse fractions of soils, including sand and coarse silt, generally exhibit low SOC affinity, they can be coated by Fe oxides through electrostatic forces, Van der Waals interactions, and Fe-O-Si bonds (Scheidegger et al. 1993), thus increasing SOC adsorption and cation exchange capacity.
4.2.4 Anthropogenic activity
The application of N and P fertilizers can change a wide range of soil properties, with detrimental effects including acidification, aggregate destabilization, microbial community shift towards bacteria, and loss of oxidative enzyme activities, which could contribute to the change of SOC. Fertilizer N inputs have been shown to lower Fe-SOC through decreasing microbial growth and Ca-bound C, which is sensitive to soil pH, but at the same time increase total SOC and light fraction C content (Ye et al. 2018). Shen et al. (2018) also found that nutrient amendment did not affect total SOC content, but increased MAOC with decreasing soil aggregate stability, based on an experiment combining nitrogen and phosphorus inputs continuously for 15 years. The reduced aggregation and increased MAOC was attributed to the decrease of fungal biomass and increase of bacterial biomass (Shen et al. 2018).
Specific plant functional traits, such as biological nitrogen-fixation, are recognized as important drivers of C accumulation in grassland (Fornara and Tilman 2008; De Deyn et al. 2009, 2011) and cropland soils (Kallenbach et al. 2015; Frasier et al. 2016). Legumes enhanced the formation of organo-mineral complexes through increasing particulate organic matter nitrogen and microbial biomass nitrogen (Canarini et al. 2018). Organic acids (e.g., oxalic acid), components of root exudates, can release SOC from the protective mineral phases, and further stimulate the microbial reductive release of Fe-SOC under anoxic conditions (Ding et al. 2021). Hence, the crop type is an important anthropogenic activity affecting the stability of Fe-SOC.
5 Conclusions
The interaction between soil minerals, in particular the cycling of Fe minerals, and SOC has been widely recognized as a key driver for the stabilization of C in soil. Microbial Fe(II) oxidation is shown to favors the formation of amorphous Fe minerals thus promoting SOC adsorption and CO2 assimilation. Whereas chemical Fe(II) and microbial Fe(III) reduction oxidation facilitate the SOC mineralization via ROS or electron transfer, respectively. Photochemical Fe(III) reduction provides Fe(II) and ROS for the Fenton reaction, which indirectly promotes SOC mineralization. During Fe(II) oxidation, poorly crystalline Fe minerals with high reactivity are generated, which can facilitate SOC stabilization through the formation of Fe-SOC. On the contrary, the reductive dissolution of Fe minerals dissociates Fe-SOC and favors microbe access to degrade SOC. The coupled processes are largely controlled by redox conditions, the nature and properties of Fe minerals and edaphic properties. Thus, the cycling of Fe controls the two-edged of SOC stabilization and Fe-SOC turnover (Fig. 4).
6 Research needs and perspectives
The fate of Fe-SOC has been reviewed in light of the mechanisms which control these processes in soil. However, many knowledge gaps have been highlighted, not least being the ability to accurately quantify and visualize these organo-mineral assemblages (Weng et al. 2021). To gain a better understanding of the factors that control both the formation and composition of these organo-mineral associations, we have suggested some priority areas for future research:
-
(1)
The effects of Fe on SOC sequestration under oxic conditions have been of great interests, however, these have been rarely studied under anoixc conditions (i.e., paddy soils) or in the systems with fluctuating redox conditions. Some studies only focus on dissimilatory Fe reduction, and there is a lack of research on the extent and direction of other reaction processes under anaerobic conditions, such as ferrous oxidation on SOC stabilization processes. Therefore, understanding the Fe cycle, including biological aspects, under a range of conditions is required.
-
(2)
Based on the diversity and complexity of SOC components, different SOC components may have different binding mechanisms with Fe minerals and result in different stability of Fe-SOC. Therefore, it is essential to investigate the molecular mechanisms of Fe-SOC interactions under redox conditions using advanced characterization techniques (e.g., EXAFS, FT-ICR-MS, and NMR).
-
(3)
Most studies on the effect of Fe redox process on the accumulation of SOC are mainly qualitative, while Fe oxidation and reduction processes often occur simultaneously, and the intermediate processes governing these two major processes remain a “black box”. As much of the data to date is qualitative, we recommend greater quantification to clarify the contribution of each reaction process of the Fe cycle in relation to SOC sequestration. A kinetic modelling approach could provide additional valuable insights towards quantification.
-
(4)
Current studies on the effects of Fe minerals on SOC sequestration are mostly based on non-disturbed ecosystems. However, soils are commonly subjected to anthropogenic and natural disturbances. It will be crucial to gain a mechanistic understanding of which processes may result in greater stabilization via Fe-SOC, or indeed result in the dissociation of soil aggregates. This can then guide management practices to optimize soil C sequestration opportunities.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Adhikari D, Poulson SR, Sumaila S, Dynes JJ, McBeth JM, Yang Y (2016) Asynchronous reductive release of iron and organic carbon from hematite–humic acid complexes. Chem Geol 430:13–20
Adhikari D, Zhao Q, Das K, Mejia J, Huang R, Wang X, Poulson SR, Tang Y, Roden EE, Yang Y (2017) Dynamics of ferrihydrite-bound organic carbon during microbial Fe reduction. Geochim Cosmochim Acta 212:221–233
Amézketa E (1999) Soil aggregate stability: a review. J Sustain Agric 14(2–3):83–151
Baek G, Kim J, Kim J, Lee C (2018) Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 11(1):107
Bao Y, Bolan NS, Lai J, Wang Y, Jin X, Kirkham M, Wu X, Fang Z, Zhang Y, Wang H (2021) Interactions between organic matter and Fe (hydr) oxides and their influences on immobilization and remobilization of metal (loid) s: a review. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2021.1974766
Blöthe M, Roden EE (2009) Composition and activity of an autotrophic Fe (II)-oxidizing, nitrate-reducing enrichment culture. Appl Environ Microbiol 75(21):6937–6940
Bolan NS, Adriano DC, Kunhikrishnan A, James T, McDowell R, Senesi N (2011) Dissolved organic matter: biogeochemistry, dynamics, and environmental significance in soils. Adv Agron 110:1–75
Bolan NS, Kunhikrishnan A, Choppala G, Thangarajan R, Chung J (2012) Stabilization of carbon in composts and biochars in relation to carbon sequestration and soil fertility. Sci Total Environ 424:264–270
Bolan NS, Kunhikrishnan A, Naidu R (2013) Carbon storage in a heavy clay soil landfill site after biosolid application. Sci Total Environ 465:216–225
Borggaard O (1982) The influence of iron oxides on the surface area of soil. J Soil Sci 33(3):443–449
Bradford MA, Keiser AD, Davies CA, Mersmann CA, Strickland MS (2013) Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth. Biogeochemistry 113(1):271–281
Bryan C, Davis-Belmar C, Van Wyk N, Fraser M, Dew D, Rautenbach G, Harrison S (2012) The effect of CO2 availability on the growth, iron oxidation and CO2-fixation rates of pure cultures of Leptospirillum ferriphilum and Acidithiobacillus ferrooxidans. Biotechnol Bioeng 109(7):1693–1703
Byrne JM, Klueglein N, Pearce C, Rosso KM, Appel E, Kappler A (2015) Redox cycling of Fe(II) and Fe(III) in magnetite by Fe-metabolizing bacteria. Science 347(6229):1473–1476
Canarini A, Mariotte P, Ingram L, Merchant A, Dijkstra FA (2018) Mineral-associated soil carbon is resistant to drought but sensitive to legumes and microbial biomass in an Australian grassland. Ecosystems 21(2):349–359
Chen C, Dynes JJ, Wang J, Sparks DL (2014) Properties of Fe-organic matter associations via coprecipitation versus adsorption. Environ Sci Technol 48(23):13751–13759
Chen C, Hall SJ, Coward E, Thompson A (2020) Iron-mediated organic matter decomposition in humid soils can counteract protection. Nat Commun 11(1):1–13
Chen C, Kukkadapu RK, Lazareva O, Sparks DL (2017) Solid-phase Fe speciation along the vertical redox gradients in floodplains using XAS and Mossbauer spectroscopies. Environ Sci Technol 51(14):7903–7912
Chen X, Hu Y, Xia Y, Zheng S, Ma C, Rui Y, He H, Huang D, Zhang Z, Ge T (2021) Contrasting pathways of carbon sequestration in paddy and upland soils. Glob Chang Biol 27(11):2478–2490
Cheng L, Zhu J, Chen G, Zheng X, Oh NH, Rufty T, Richter D, Hu S (2010) Atmospheric CO2 enrichment facilitates cation release from soil. Ecol Lett 13(3):284–291
Chenu C, Plante A (2006) Clay-sized organo-mineral complexes in a cultivation chronosequence: revisiting the concept of the ‘primary organo-mineral complex’. Eur J Soil Sci 57(4):596–607
Chorover J, Amistadi MK, Chadwick OA (2004) Surface charge evolution of mineral-organic complexes during pedogenesis in Hawaiian basalt. Geochim Cosmochim Acta 68(23):4859–4876
Churchman GJ, Singh M, Schapel A, Sarkar B, Bolan N (2020) Clay minerals as the key to the sequestration of carbon in soils. Clay Clay Miner 68(2):135–143
Conant RT, Drijber RA, Haddix ML, Parton WJ, Paul EA, Plante AF, Six J, Steinweg JM (2008) Sensitivity of organic matter decomposition to warming varies with its quality. Glob Chang Biol 14(4):868–877
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences, and uses. Wiley-vch: Weinheim
Cotrufo MF, Ranalli MG, Haddix ML, Six J, Lugato E (2019) Soil carbon storage informed by particulate and mineral-associated organic matter. Nat Geosci 12(12):989–994
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, Wall DH, Parton WJ (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8(10):776–779
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The M icrobial E fficiency-M atrix S tabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Chang Biol 19(4):988–995
Crowther TW, Todd-Brown KE, Rowe CW, Wieder WR, Carey JC, Machmuller MB, Snoek B, Fang S, Zhou G, Allison SD (2016) Quantifying global soil carbon losses in response to warming. Nature 540(7631):104–108
De Campos AB, Huang C, Johnston CT (2012) Biogeochemistry of terrestrial soils as influenced by short-term flooding. Biogeochemistry 111(1):239–252
De Deyn GB, Quirk H, Yi Z, Oakley S, Ostle NJ, Bardgett RD (2009) Vegetation composition promotes carbon and nitrogen storage in model grassland communities of contrasting soil fertility. J Ecol 97(5):864–875
De Deyn GB, Shiel RS, Ostle NJ, McNamara NP, Oakley S, Young I, Freeman C, Fenner N, Quirk H, Bardgett RD (2011) Additional carbon sequestration benefits of grassland diversity restoration. J Appl Ecol 48(3):600–608
Ding L-J, Su J-Q, Xu H-J, Jia Z-J, Zhu Y-G (2015) Long-term nitrogen fertilization of paddy soil shifts iron-reducing microbial community revealed by RNA-13C-acetate probing coupled with pyrosequencing. ISME J 9(3):721–734
Ding Y, Ye Q, Liu M, Shi Z, Liang Y (2021) Reductive release of Fe mineral-associated organic matter accelerated by oxalic acid. Sci Total Environ 763:142937
Doetterl S, Berhe AA, Arnold C, Bodé S, Fiener P, Finke P, Fuchslueger L, Griepentrog M, Harden J, Nadeu E (2018) Links among warming, carbon and microbial dynamics mediated by soil mineral weathering. Nat Geosci 11(8):589–593
Doetterl S, Stevens A, Six J, Merckx R, Van Oost K, Casanova Pinto M, Casanova-Katny A, Muñoz C, Boudin M, Zagal Venegas E (2015) Soil carbon storage controlled by interactions between geochemistry and climate. Nat Geosci 8(10):780–783
Du Y, Ramirez CE, Jaffé R (2018) Fractionation of dissolved organic matter by co-precipitation with iron: effects of composition. Environ Process 5(1):5–21
Dubinsky EA, Silver WL, Firestone MK (2010) Tropical forest soil microbial communities couple iron and carbon biogeochemistry. Ecology 91(9):2604–2612
Dungait JA, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Chang Biol 18(6):1781–1796
Edwards KJ, Rogers DR, Wirsen CO, McCollom T (2003) Isolation and characterization of novel psychrophilic, neutrophilic, Fe-oxidizing, chemolithoautotrophic α-and γ-Proteobacteria from the deep sea. Appl Environ Microbiol 69(5):2906–2913
Emerson D, Field E, Chertkov O, Davenport K, Goodwin L, Munk C, Nolan M, Woyke T (2013) Comparative genomics of freshwater Fe-oxidizing bacteria: implications for physiology, ecology, and systematics. Front Microbiol 4:254
Emerson D, Moyer C (1997) Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol 63(12):4784–4792
Eusterhues K, Hädrich A, Neidhardt J, Küsel K, Keller T, Jandt K, Totsche K (2014) Reduction of ferrihydrite with adsorbed and coprecipitated organic matter: microbial reduction by Geobacter bremensis vs. abiotic reduction by Na-dithionite. Biogeosciences 11(18):4953–4966
Eusterhues K, Rennert T, Knicker H, Kogel-Knabner I, Totsche KU, Schwertmann U (2011) Fractionation of organic matter due to reaction with ferrihydrite: coprecipitation versus adsorption. Environ Sci Technol 45(2):527–533
Eusterhues K, Wagner FE, Häusler W, Hanzlik M, Knicker H, Totsche KU, Kögel-Knabner I, Schwertmann U (2008) Characterization of ferrihydrite-soil organic matter coprecipitates by X-ray diffraction and Mossbauer spectroscopy. Environ Sci Technol 42(21):7891–7897
Filimonova S, Kaufhold S, Wagner FE, Häusler W, Kögel-Knabner I (2016) The role of allophane nano-structure and Fe oxide speciation for hosting soil organic matter in an allophanic Andosol. Geochim Cosmochim Acta 180:284–302
Fleming EJ, Langdon AE, Martinez-Garcia M, Stepanauskas R, Poulton NJ, Masland EDP, Emerson D (2011) What's new is old: resolving the identity of Leptothrix ochracea using single cell genomics, pyrosequencing and FISH. PLoS One 6(3):e17769
Fornara D, Tilman D (2008) Plant functional composition influences rates of soil carbon and nitrogen accumulation. J Ecol 96(2):314–322
Frasier I, Noellemeyer E, Figuerola E, Erijman L, Permingeat H, Quiroga A (2016) High quality residues from cover crops favor changes in microbial community and enhance C and N sequestration. Glob Ecol Conserv 6:242–256
Giannetta B, Plaza C, Vischetti C, Cotrufo MF, Zaccone C (2018) Distribution and thermal stability of physically and chemically protected organic matter fractions in soils across different ecosystems. Biol Fertil Soils 54(5):671–681
Glaser B, Turrión M-B, Alef K (2004) Amino sugars and muramic acid—biomarkers for soil microbial community structure analysis. Soil Biol Biochem 36(3):399–407
Gnanaprakash G, Mahadevan S, Jayakumar T, Kalyanasundaram P, Philip J, Raj B (2007) Effect of initial pH and temperature of iron salt solutions on formation of magnetite nanoparticles. Mater Chem Phys 103(1):168–175
Grandy AS, Neff JC (2008) Molecular C dynamics downstream: the biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci Total Environ 404(2–3):297–307
Gunina A, Kuzyakov Y (2022) From energy to (soil organic) matter. Glob Chang Biol 28(7):2169–2182
Hall SJ, Berhe AA, Thompson A (2018) Order from disorder: do soil organic matter composition and turnover co-vary with iron phase crystallinity? Biogeochemistry 140(1):93–110
Hall SJ, Silver WL (2013) Iron oxidation stimulates organic matter decomposition in humid tropical forest soils. Glob Chang Biol 19(9):2804–2813
Hall SJ, Silver WL (2015) Reducing conditions, reactive metals, and their interactions can explain spatial patterns of surface soil carbon in a humid tropical forest. Biogeochemistry 125(2):149–165
Han L, Sun K, Keiluweit M, Yang Y, Yang Y, Jin J, Sun H, Wu F, Xing B (2019) Mobilization of ferrihydrite-associated organic carbon during Fe reduction: adsorption versus coprecipitation. Chem Geol 503:61–68
Han R, Liu T, Li F, Li X, Chen D, Wu Y (2018) Dependence of secondary mineral formation on Fe(II) production from Ferrihydrite reduction by Shewanella oneidensis MR-1. ACS Earth Space Chem 2(4):399–409
Hedrich S, Schlömann M, Johnson DB (2011) The iron-oxidizing proteobacteria. Microbiology 157(6):1551–1564
Hegler F, Lösekann-Behrens T, Hanselmann K, Behrens S, Kappler A (2012) Influence of seasonal and geochemical changes on the geomicrobiology of an iron carbonate mineral water spring. Appl Environ Microbiol 78(20):7185–7196
Hori T, Müller A, Igarashi Y, Conrad R, Friedrich MW (2010) Identification of iron-reducing microorganisms in anoxic rice paddy soil by 13C-acetate probing. ISME J 4(2):267–278
Hou T, Xu R, Zhao A (2007) Interaction between electric double layers of kaolinite and Fe/Al oxides in suspensions. Colloids Surf A Physicochem Eng Asp 297(1–3):91–94
Hu S, Liu T, Yang Y, Li F, Fang L (2021) Cysteine induced cascade electron transfer by forming a unique ternary complex with Fe(II) on goethite. Chem Geol 584:120561
Hu S, Lu Y, Peng L, Wang P, Zhu M, Dohnalkova AC, Chen H, Lin Z, Dang Z, Shi Z (2018) Coupled kinetics of ferrihydrite transformation and as(V) sequestration under the effect of humic acids: a mechanistic and quantitative study. Environ Sci Technol 52(20):11632–11641
Huang X, Jiang H, Li Y, Ma Y, Tang H, Ran W, Shen Q (2016) The role of poorly crystalline iron oxides in the stability of soil aggregate-associated organic carbon in a rice–wheat cropping system. Geoderma 279:1–10
Huang X, Tang H, Kang W, Yu G, Ran W, Hong J, Shen Q (2018) Redox interface-associated organo-mineral interactions: a mechanism for C sequestration under a rice-wheat cropping system. Soil Biol Biochem 120:12–23
Inagaki HK, Fontolan L, Romani S, Svoboda K (2019) Discrete attractor dynamics underlies persistent activity in the frontal cortex. Nature 566(7743):212–217
Jansen B, Nierop KG, Verstraten JM (2003) Mobility of Fe (II), Fe (III) and Al in acidic forest soils mediated by dissolved organic matter: influence of solution pH and metal/organic carbon ratios. Geoderma 113(3–4):323–340
Jeewani PH, Gunina A, Tao L, Zhu Z, Kuzyakov Y, Van Zwieten L, Guggenberger G, Shen C, Yu G, Singh BP (2020) Rusty sink of rhizodeposits and associated keystone microbiomes. Soil Biol Biochem 147:107840
Jiao Y, Kappler A, Croal LR, Newman DK (2005) Isolation and characterization of a genetically tractable photoautotrophic Fe (II)-oxidizing bacterium, Rhodopseudomonas palustris strain TIE-1. Appl Environ Microbiol 71(8):4487–4496
Kaiser K, Guggenberger G (2007) Sorptive stabilization of organic matter by microporous goethite: sorption into small pores vs. surface complexation. Eur J Soil Sci 58(1):45–59
Kaiser K, Mikutta R, Guggenberger G (2007) Increased stability of organic matter sorbed to ferrihydrite and goethite on aging. Soil Sci Soc Am J 71(3):711–719
Kallenbach C, Grandy AS, Frey S, Diefendorf A (2015) Microbial physiology and necromass regulate agricultural soil carbon accumulation. Soil Biol Biochem 91:279–290
Kallenbach CM, Frey SD, Grandy AS (2016) Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Commun 7(1):1–10
Kappler A, Pasquero C, Konhauser KO, Newman DK (2005) Deposition of banded iron formations by anoxygenic phototrophic Fe (II)-oxidizing bacteria. Geology 33(11):865–868
Kappler A, Straub KL (2005) Geomicrobiological cycling of iron. Rev Mineral Geochem 59(1):85–108
Katoh M, Murase J, Hayashi M, Matsuya K, Kimura M (2004) Nutrient leaching from the plow layer by water percolation and accumulation in the subsoil in an irrigated paddy field. Soil Sci Plant Nutr 50(5):721–729
Kennedy MJ, Pevear DR, Hill RJ (2002) Mineral surface control of organic carbon in black shale. Science 295(5555):657–660
Kiem R, Kögel-Knabner I (2002) Refractory organic carbon in particle-size fractions of arable soils II: organic carbon in relation to mineral surface area and iron oxides in fractions< 6 μm. Org Geochem 33(12):1699–1713
Kleber M, Eusterhues K, Keiluweit M, Mikutta C, Mikutta R, Nico PS (2015) Mineral–organic associations: formation, properties, and relevance in soil environments. Adv Agron 130:1–140
Kleber M, Mikutta R, Torn M, Jahn R (2005) Poorly crystalline mineral phases protect organic matter in acid subsoil horizons. Eur J Soil Sci 56(6):717–725
Kögel-Knabner I, Guggenberger G, Kleber M, Kandeler E, Kalbitz K, Scheu S, Eusterhues K, Leinweber P (2008) Organo-mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry. J Plant Nutr Soil Sci 171(1):61–82
Kopittke PM, Dalal RC, Hoeschen C, Li C, Menzies NW, Mueller CW (2020) Soil organic matter is stabilized by organo-mineral associations through two key processes: the role of the carbon to nitrogen ratio. Geoderma 357:113974
Kramer MG, Sanderman J, Chadwick OA, Chorover J, Vitousek PM (2012) Long-term carbon storage through retention of dissolved aromatic acids by reactive particles in soil. Glob Chang Biol 18(8):2594–2605
Kranzler C, Lis H, Shaked Y, Keren N (2011) The role of reduction in iron uptake processes in a unicellular, planktonic cyanobacterium. Environ Microbiol 13(11):2990–2999
Krause L, Klumpp E, Nofz I, Missong A, Amelung W, Siebers N (2020) Colloidal iron and organic carbon control soil aggregate formation and stability in arable Luvisols. Geoderma 374:114421
Kucera S, Wolfe R (1957) A selective enrichment method for Gallionella ferruginea. J Bacteriol 74(3):344–349
Kuiters A, Sarink H (1986) Leaching of phenolic compounds from leaf and needle litter of several deciduous and coniferous trees. Soil Biol Biochem 18(5):475–480
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304(5677):1623–1627
Lal R (2008) Carbon sequestration. Philos Trans R Soc Lond B Biol Sci 363(1492):815–830
Lalonde K, Mucci A, Ouellet A, Gélinas Y (2012) Preservation of organic matter in sediments promoted by iron. Nature 483(7388):198–200
Lehmann J, Hansel CM, Kaiser C, Kleber M, Maher K, Manzoni S, Nunan N, Reichstein M, Schimel JP, Torn MS (2020) Persistence of soil organic carbon caused by functional complexity. Nat Geosci 13(8):529–534
Li X, Huang J, Qu C, Chen W, Chen C, Cai P, Huang Q (2022) Diverse regulations on the accumulation of fungal and bacterial necromass in cropland soils. Geoderma 410:115675
Li X, Liu L, Wu Y, Liu T (2019) Determination of the redox potentials of solution and solid surface of Fe(II) associated with iron oxyhydroxides. ACS Earth Space Chem 3(5):711–717
Li X, Zhang W, Liu T, Chen L, Chen P, Li F (2016) Changes in the composition and diversity of microbial communities during anaerobic nitrate reduction and Fe (II) oxidation at circumneutral pH in paddy soil. Soil Biol Biochem 94:70–79
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol 2(8):1–6
Lipson DA, Jha M, Raab TK, Oechel WC (2010) Reduction of iron (III) and humic substances plays a major role in anaerobic respiration in an Arctic peat soil. J Geophys Res Biogeosci 115:G00I06. https://doi.org/10.1029/2009JG001147
Liu T, Chen D, Luo X, Li X, Li F (2019) Microbially mediated nitrate-reducing Fe (II) oxidation: quantification of chemodenitrification and biological reactions. Geochim Cosmochim Acta 256:97–115
Lu Y, Hu S, Wang Z, Ding Y, Lu G, Lin Z, Dang Z, Shi Z (2019) Ferrihydrite transformation under the impact of humic acid and Pb: kinetics, nanoscale mechanisms, and implications for C and Pb dynamics. Environ Sci Nano 6(3):747–762
Lueder U, Jorgensen BB, Kappler A, Schmidt C (2020a) Photochemistry of iron in aquatic environments. Environ Sci Process Impacts 22(1):12–24
Lueder U, Jorgensen BB, Kappler A, Schmidt C (2020b) Fe(III) Photoreduction producing Feaq(2+) in Oxic freshwater sediment. Environ Sci Technol 54(2):862–869
Lundberg P, Ekblad A, Nilsson M (2001) 13C NMR spectroscopy studies of forest soil microbial activity: glucose uptake and fatty acid biosynthesis. Soil Biol Biochem 33(4–5):621–632
Lützow M, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions–a review. Eur J Soil Sci 57(4):426–445
Masiello C, Chadwick O, Southon J, Torn M, Harden J (2004) Weathering controls on mechanisms of carbon storage in grassland soils. Glob Biogeochem Cycles 18(4):GB4023. https://doi.org/10.1029/2004GB002219
Mayer LM, Xing B (2001) Organic matter–surface area relationships in acid soils. Soil Sci Soc Am J 65(1):250–258
Mejia J, Roden EE, Ginder-Vogel M (2016) Influence of oxygen and nitrate on Fe (hydr) oxide mineral transformation and soil microbial communities during redox cycling. Environ Sci Technol 50(7):3580–3588
Melton ED, Swanner ED, Behrens S, Schmidt C, Kappler A (2014) The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat Rev Microbiol 12(12):797–808
Mikutta C, Kretzschmar R (2011) Spectroscopic evidence for ternary complex formation between arsenate and ferric iron complexes of humic substances. Environ Sci Technol 45(22):9550–9557
Mikutta R, Kaiser K (2011) Organic matter bound to mineral surfaces: resistance to chemical and biological oxidation. Soil Biol Biochem 43(8):1738–1741
Mikutta R, Kleber M, Jahn R (2005) Poorly crystalline minerals protect organic carbon in clay subfractions from acid subsoil horizons. Geoderma 128(1–2):106–115
Mikutta R, Lorenz D, Guggenberger G, Haumaier L, Freund A (2014) Properties and reactivity of Fe-organic matter associations formed by coprecipitation versus adsorption: clues from arsenate batch adsorption. Geochim Cosmochim Acta 144:258–276
Miltner A, Bombach P, Schmidt-Brücken B, Kästner M (2012) SOM genesis: microbial biomass as a significant source. Biogeochemistry 111(1):41–55
Mu C, Zhang T, Zhao Q, Guo H, Zhong W, Su H, Wu Q (2016) Soil organic carbon stabilization by iron in permafrost regions of the Qinghai-Tibet Plateau. Geophys Res Lett 43(19):10,286–210,294
Newcomb CJ, Qafoku NP, Grate JW, Bailey VL, De Yoreo JJ (2017) Developing a molecular picture of soil organic matter–mineral interactions by quantifying organo–mineral binding. Nat Commun 8(1):1–8
Nguyen ML, Goldfarb J, Plante A, Lau B, Hockaday W (2019) Sorption temperature and the stability of iron-bound soil organic matter. Geoderma 341:93–99
Nierop KG, Jansen B, Verstraten JM (2002) Dissolved organic matter, aluminium and iron interactions: precipitation induced by metal/carbon ratio, pH and competition. Sci Total Environ 300(1–3):201–211
Notini L, Latta DE, Neumann A, Pearce CI, Sassi M, N’Diaye AT, Rosso KM, Scherer MM (2018) The role of defects in Fe(II)-goethite electron transfer. Environ Sci Technol 52(5):2751–2759
Pan W, Kan J, Inamdar S, Chen C, Sparks D (2016) Dissimilatory microbial iron reduction release DOC (dissolved organic carbon) from carbon-ferrihydrite association. Soil Biol Biochem 103:232–240
Pang H, Zhang Q, Wang H, Cai D, Ma Y, Li L, Li K, Lu X, Chen H, Yang X, Chen J (2019) Photochemical aging of guaiacol by Fe(III)-oxalate complexes in atmospheric aqueous phase. Environ Sci Technol 53(1):127–136
Patel KF, Tejnecký V, Ohno T, Bailey VL, Sleighter RL, Hatcher PG (2021) Reactive oxygen species alter chemical composition and adsorptive fractionation of soil-derived organic matter. Geoderma 384:114805
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Glob Chang Biol 24(1):1–12
Persson P, Axe K (2005) Adsorption of oxalate and malonate at the water-goethite interface: molecular surface speciation from IR spectroscopy. Geochim Cosmochim Acta 69(3):541–552
Picardal F (2012) Abiotic and microbial interactions during anaerobic transformations of Fe (II) and NOx. Front Microbiol 3:112
Raiswell R, Canfield DE (2012) The iron biogeochemical cycle past and present. Geochem Perspect 1(1):1–2
Rasmussen C, Matsuyama N, Dahlgren RA, Southard RJ, Brauer N (2007) Soil genesis and mineral transformation across an environmental gradient on andesitic lahar. Soil Sci Soc Am J 71(1):225–237
Rasmussen C, Southard RJ, Horwath WR (2006) Mineral control of organic carbon mineralization in a range of temperate conifer forest soils. Glob Chang Biol 12(5):834–847
Rasse DP, Rumpel C, Dignac M-F (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269(1):341–356
Ren S, Usman M, Tsang DCW, O-Thong S, Angelidaki I, Zhu X, Zhang S, Luo G (2020) Hydrochar-facilitated anaerobic digestion: evidence for direct interspecies electron transfer mediated through surface oxygen-containing functional groups. Environ Sci Technol 54(9):5755–5766
Riedel T, Zak D, Biester H, Dittmar T (2013) Iron traps terrestrially derived dissolved organic matter at redox interfaces. Proc Natl Acad Sci 110(25):10101–10105
Roden EE (2012) Microbial iron-redox cycling in subsurface environments. Biochem Soc Trans 40(6):1249–1256
Saidy AR, Smernik R, Baldock J, Kaiser K, Sanderman J, Macdonald L (2012) Effects of clay mineralogy and hydrous iron oxides on labile organic carbon stabilisation. Geoderma 173:104–110
Sanderman J, Hengl T, Fiske GJ (2017) Soil carbon debt of 12,000 years of human land use. Proc Natl Acad Sci 114(36):9575–9580
Scheidegger A, Borkovec M, Sticher H (1993) Coating of silica sand with goethite: preparation and analytical identification. Geoderma 58(1–2):43–65
Shen D, Ye C, Hu Z, Chen X, Guo H, Li J, Du G, Adl S, Liu M (2018) Increased chemical stability but decreased physical protection of soil organic carbon in response to nutrient amendment in a Tibetan alpine meadow. Soil Biol Biochem 126:11–21
Silva LC, Doane TA, Corrêa RS, Valverde V, Pereira EI, Horwath WR (2015) Iron-mediated stabilization of soil carbon amplifies the benefits of ecological restoration in degraded lands. Ecol Appl 25(5):1226–1234
Singh M, Sarkar B, Biswas B, Bolan NS, Churchman GJ (2017a) Relationship between soil clay mineralogy and carbon protection capacity as influenced by temperature and moisture. Soil Biol Biochem 109:95–106
Singh M, Sarkar B, Biswas B, Churchman J, Bolan NS (2016) Adsorption-desorption behavior of dissolved organic carbon by soil clay fractions of varying mineralogy. Geoderma 280:47–56
Singh M, Sarkar B, Bolan NS, Ok YS, Churchman GJ (2019) Decomposition of soil organic matter as affected by clay types, pedogenic oxides and plant residue addition rates. J Hazard Mater 374:11–19
Singh M, Sarkar B, Hussain S, Ok YS, Bolan NS, Churchman GJ (2017b) Influence of physico-chemical properties of soil clay fractions on the retention of dissolved organic carbon. Environ Geochem Health 39(6):1335–1350
Singh M, Sarkar B, Sarkar S, Churchman J, Bolan N, Mandal S, Menon M, Purakayastha TJ, Beerling DJ (2018) Stabilization of soil organic carbon as influenced by clay mineralogy. Adv Agron 148:33–84
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42(3):391–404
Slessarev EW, Chadwick OA, Sokol NW, Nuccio EE, Pett-Ridge J (2021) Rock weathering controls the potential for soil carbon storage at a continental scale. Biogeochemistry 157(1):1–13
Smith P, Fang C, Dawson JJ, Moncrieff JB (2008) Impact of global warming on soil organic carbon. Adv Agron 97:1–43
Sodano M, Lerda C, Nisticò R, Martin M, Magnacca G, Celi L, Said-Pullicino D (2017) Dissolved organic carbon retention by coprecipitation during the oxidation of ferrous iron. Geoderma 307:19–29
Sokol NW, Sanderman J, Bradford MA (2019) Pathways of mineral-associated soil organic matter formation: integrating the role of plant carbon source, chemistry, and point of entry. Glob Chang Biol 25(1):12–24
Sokolova TA (2020) Low-molecular-weight organic acids in soils: sources, composition, concentrations, and functions: a review. Eurasian Soil Sci 53(5):580–594
Steffens M, Rogge DM, Mueller CW, Höschen C, Lugmeier J, Kölbl A, Kögel-Knabner I (2017) Identification of distinct functional microstructural domains controlling C storage in soil. Environ Sci Technol 51(21):12182–12189
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions. Wiley: New York
Stumm W, Morgan JJ (2012) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley: New York
Sutton-Grier AE, Keller JK, Koch R, Gilmour C, Megonigal JP (2011) Electron donors and acceptors influence anaerobic soil organic matter mineralization in tidal marshes. Soil Biol Biochem 43(7):1576–1583
Swanner ED, Maisch M, Wu W, Kappler A (2018) Oxic Fe(III) reduction could have generated Fe(II) in the photic zone of Precambrian seawater. Sci Rep 8(1):4238
Tamura M, Suseela V, Simpson M, Powell B, Tharayil N (2017) Plant litter chemistry alters the content and composition of organic carbon associated with soil mineral and aggregate fractions in invaded ecosystems. Glob Chang Biol 23(10):4002–4018
Thevenot M, Dignac M-F, Rumpel C (2010) Fate of lignins in soils: a review. Soil Biol Biochem 42(8):1200–1211
Throckmorton HM, Bird JA, Monte N, Doane T, Firestone MK, Horwath WR (2015) The soil matrix increases microbial C stabilization in temperate and tropical forest soils. Biogeochemistry 122(1):35–45
Totsche KU, Amelung W, Gerzabek MH, Guggenberger G, Klumpp E, Knief C, Lehndorff E, Mikutta R, Peth S, Prechtel A (2018) Microaggregates in soils. J Plant Nutr Soil Sci 181(1):104–136
Vidal A, Hirte J, Bender SF, Mayer J, Gattinger A, Höschen C, Schädler S, Iqbal TM, Mueller CW (2018) Linking 3D soil structure and plant-microbe-soil carbon transfer in the rhizosphere. Front Environ Sci 6:9
Vogel C, Mueller CW, Höschen C, Buegger F, Heister K, Schulz S, Schloter M, Kögel-Knabner I (2014) Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils. Nat Commun 5(1):1–7
von Haden AC, Kucharik CJ, Jackson RD, Marín-Spiotta E (2019) Litter quantity, litter chemistry, and soil texture control changes in soil organic carbon fractions under bioenergy cropping systems of the North Central US. Biogeochemistry 143(3):313–326
Wagai R, Mayer LM (2007) Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochim Cosmochim Acta 71(1):25–35
Wang P, Ding Y, Liang Y, Liu M, Lin X, Ye Q, Shi Z (2021) Linking molecular composition to proton and copper binding ability of fulvic acid: a theoretical modeling approach based on FT-ICR-MS analysis. Geochim Cosmochim Acta 312:279–298
Wang X, Dong H, Zeng Q, Xia Q, Zhang L, Zhou Z (2017a) Reduced Iron-containing clay minerals as antibacterial agents. Environ Sci Technol 51(13):7639–7647
Wang X, Yang Z, Liu X, Lin W, Yang Y, Liu Z, Zhao B, Su R (2016) Effects of different forms of Fe and Al oxides on soil aggregate stability in mid-subtropical mountainous area of southern China. Acta Ecol Sin 36(9):2588–2596
Wang Y, Wang H, He J-S, Feng X (2017b) Iron-mediated soil carbon response to water-table decline in an alpine wetland. Nat Commun 8(1):1–9
Wang Y, Zhang Z, Han L, Sun K, Jin J, Yang Y, Yang Y, Hao Z, Liu J, Xing B (2019) Preferential molecular fractionation of dissolved organic matter by iron minerals with different oxidation states. Chem Geol 520:69–76
Wei L, Ge T, Zhu Z, Luo Y, Yang Y, Xiao M, Yan Z, Li Y, Wu J, Kuzyakov Y (2021) Comparing carbon and nitrogen stocks in paddy and upland soils: accumulation, stabilization mechanisms, and environmental drivers. Geoderma 398:115121
Weiss JV, Emerson D, Megonigal JP (2005) Rhizosphere Iron (III) depostion and reduction in a Juncus effusus L.-dominated wetland. Soil Sci Soc Am J 69:1861-1870
Weiss JV, Rentz JA, Plaia T, Neubauer SC, Merrill-Floyd M, Lilburn T, Bradburne C, Megonigal JP, Emerson D (2007) Characterization of neutrophilic Fe (II)-oxidizing bacteria isolated from the rhizosphere of wetland plants and description of Ferritrophicum radicicola gen. nov. sp. nov., and Sideroxydans paludicola sp. nov. Geomicrobiol J 24(7–8):559–570
Wen Y, Li H, Xiao J, Wang C, Shen Q, Ran W, He X, Zhou Q, Yu G (2014) Insights into complexation of dissolved organic matter and Al (III) and nanominerals formation in soils under contrasting fertilizations using two-dimensional correlation spectroscopy and high resolution-transmission electron microscopy techniques. Chemosphere 111:441–449
Weng Z, Lehmann J, Van Zwieten L, Joseph S, Archanjo BS, Cowie B, Thomsen L, Tobin MJ, Vongsvivut J, Klein A (2021) Probing the nature of soil organic matter. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2021.1980346
Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B (1993) Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature 362(6423):834–836
Wiseman C, Püttmann W (2005) Soil organic carbon and its sorptive preservation in central Germany. Eur J Soil Sci 56(1):65–76
Wu J, Ge T, Hu Y (2015) A review on the coupling of bio-geochemical process for key elements and microbial regulation mechanisms in paddy rice ecosystems. Shengtai Xuebao 35(20):6626–6634
Xiao J, Wen Y, Li H, Hao J, Shen Q, Ran W, Mei X, He X, Yu G (2015) In situ visualisation and characterisation of the capacity of highly reactive minerals to preserve soil organic matter (SOM) in colloids at submicron scale. Chemosphere 138:225–232
Xu Y, Seshadri B, Sarkar B, Rumpel C, Sparks D, Bolan NS (2018) Microbial control of soil carbon turnover. In The future of soil carbon. Academic Press: 165–194
Xue B, Huang L, Huang Y, Yin Z, Li X, Lu J (2019) Effects of organic carbon and iron oxides on soil aggregate stability under different tillage systems in a rice–rape cropping system. Catena 177:1–12
Yang J, Liu J, Hu Y, Rumpel C, Bolan N, Sparks D (2017) Molecular-level understanding of malic acid retention mechanisms in ternary kaolinite-Fe (III)-malic acid systems: the importance of Fe speciation. Chem Geol 464:69–75
Yang Y, Yuan X, Chi W, Wang P, Hu S, Li F, Li X, Liu T, Sun Y, Qin H (2021) Modelling evaluation of key cadmium transformation processes in acid paddy soil under alternating redox conditions. Chem Geol 581:120409
Ye C, Bai T, Yang Y, Zhang H, Guo H, Li Z, Li H, Hu S (2017) Physical access for residue-mineral interactions controls organic carbon retention in an Oxisol soil. Sci Rep 7(1):1–9
Ye C, Chen D, Hall SJ, Pan S, Yan X, Bai T, Guo H, Zhang Y, Bai Y, Hu S (2018) Reconciling multiple impacts of nitrogen enrichment on soil carbon: plant, microbial and geochemical controls. Ecol Lett 21(8):1162–1173
Yin Q, Yang S, Wang Z, Xing L, Wu G (2018) Clarifying electron transfer and metagenomic analysis of microbial community in the methane production process with the addition of ferroferric oxide. Chem Eng J 333:216–225
Yu G-H, Kuzyakov Y (2021) Fenton chemistry and reactive oxygen species in soil: abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth Sci Rev 214:103525
Zhang H, Ding W, Luo J, Bolan N, Yu H (2015) The dynamics of glucose-derived 13C incorporation into aggregates of a sandy loam soil following two-decade compost or inorganic fertilizer amendments. Soil Tillage Res 148:14–19
Zhang J, Zhang L, Wang P, Huang Q, Yu G, Li D, Shen Q, Ran W (2013) The role of non-crystalline Fe in the increase of SOC after long-term organic manure application to the red soil of s outhern C hina. Eur J Soil Sci 64(6):797–804
Zhao Q, Adhikari D, Huang R, Patel A, Wang X, Tang Y, Obrist D, Roden EE, Yang Y (2017) Coupled dynamics of iron and iron-bound organic carbon in forest soils during anaerobic reduction. Chem Geol 464:118–126
Zhao Q, Poulson SR, Obrist D, Sumaila S, Dynes JJ, McBeth JM, Yang Y (2016) Iron-bound organic carbon in forest soils: quantification and characterization. Biogeosciences 13(16):4777–4788
Funding
This study was support by the National Natural Science Foundation of China (32001141, 42107338), the Postdoctoral Research Foundation of China (2020 M682636), the GDAS’ Project of Science and Technology Development (2020GDASYL-20200103081, 2021GDASYL-20210103044, 2020GDASYL-20200103080) and the Scientific Research Foundation of Guilin University of Technology (GUTQDJJ2018055).
Author information
Authors and Affiliations
Contributions
XXS and PW: Conceptualization, Literature collection and analysis, Visualization, Writing. LVZ, NB, HLW and XML: Review and Editing. KC, YY and MLW: Literature collection and analysis. TXL: Supervision, Conceptualization, Review and Editing. FBL: Supervision, Conceptualization. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
All authors declare that they are consent for publication in the journal of Carbon Research.
Competing interests
Lukas Van Zwieten, Nanthi Bolan, Hailong Wang, Tongxu Liu and Fangbai Li are editorial board members for Carbon Research and were not involved in the editorial review, or the decision to publish this article. All authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, X., Wang, P., Van Zwieten, L. et al. Towards a better understanding of the role of Fe cycling in soil for carbon stabilization and degradation. carbon res 1, 5 (2022). https://doi.org/10.1007/s44246-022-00008-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-022-00008-2