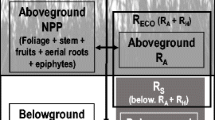
Abstract
Accurate prediction of future atmospheric CO2 concentrations is essential for evaluating climate change impacts on ecosystems and human societies. One major source of uncertainty in model predictions is the extent to which global warming will increase atmospheric CO2 concentrations through enhanced microbial decomposition of soil organic carbon. Recent advances in microbial ecology could help reduce this uncertainty, but current global models do not represent direct microbial control over decomposition. Instead, all of the coupled climate models reviewed in the most recent Intergovernmental Panel on Climate Change (IPCC) report assume that decomposition is a first-order decay process, proportional to the size of the soil carbon pool. Here we argue for the development of a new generation of models that link decomposition directly to the size and activity of microbial communities in coupled global models. This process begins with the formulation and validation of fine-scale models that capture fundamental microbial mechanisms without excessive mathematical complexity. These mechanistic models must then be scaled up through an aggregation process and validated at ecosystem to global scales prior to incorporation into global climate models (GCMs). Parameterizing microbial models at the global scale is challenging because some microbial properties such as in situ extracellular enzyme activities are very difficult to measure directly. New parameter fitting procedures may therefore be needed to infer the values of important microbial variables. Validating decomposition models at the global scale is also a challenge, and has not yet been accomplished with the land carbon models embedded in current GCMs. Fortunately new global datasets on soil carbon stocks and fluxes offer promising opportunities to validate both existing land carbon models and new microbial models. If challenges in scaling, parameterization, and validation can be overcome, a new generation of microbially-based decomposition models could substantially improve predictions of carbon–climate feedbacks in the Earth system. Development of new models structures would also reduce any bias due to the assumption of first-order decomposition across all of the models currently referenced in reports of the IPCC.

Similar content being viewed by others
References
Ågren GI, Bosatta E (1996a) Quality: a bridge between theory and experiment in soil organic matter studies. Oikos 76:522–528
Ågren GI, Bosatta E (1996b) Theoretical ecosystem ecology: understanding element cycles. Cambridge University Press, Cambridge
Ågren G, Wetterstedt JÅM (2007) What determines the temperature response of soil organic matter decomposition? Soil Biol Biochem 39:1794–1798
Ågren G, Bosatta E, Magill AH (2001) Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 128:94–98
Akaike H (1974) A new look at the statistical model evaluation. IEEE Trans Autom Control 19:716–723
Allison SD (2005) Cheaters, diffusion, and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol Lett 8:626–635
Allison SD (2006) Soil minerals and humic acids alter enzyme stability: implications for ecosystem processes. Biogeochemistry 81:361–373
Allison SD, Martiny JBH (2008) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci USA 105(Suppl 1):11512–11519
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340
Andrews JA, Matamala R, Westover KM, Schlesinger WH (2000) Temperature effects on the diversity of soil heterotrophs and the 13C of soil-respired CO2. Soil Biol Biochem 32:699–706
Bardgett RD, Freeman C, Ostle NJ (2008) Microbial contributions to climate change through carbon cycle feedbacks. ISME J 2:805–814
Bond-Lamberty B, Thomson A (2010) Temperature-associated increases in the global soil respiration record. Nature 464:579–582
Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, Mohan JE, Reynolds JF, Treseder KK, Wallenstein MD (2008) Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett 11:1316–1327. doi:10.1111/j.1461-0248.2008.01251.x
Burns RG (1978) Soil enzymes. Academic Press, New York
Burns RG (1982) Enzyme activity in soil: location and a possible role in microbial ecology. Soil Biol Biochem 14:423–427
Cadule P, Friedlingstein P, Bopp L, Sitch S, Jones CD, Ciais P, Piao SL, Peylin P (2010) Benchmarking coupled climate-carbon models against long-term atmospheric CO2 measurements. Glob Biogeochem Cycles 24:GB2016. doi:2010.1029/2009GB003556
Chapin FS III, McFarland J, McGuire AD, Euskirchen ES, Ruess RW, Kielland K (2009) The changing global carbon cycle: linking plant–soil carbon dynamics to global consequences. J Ecol 97:840–850
Cleveland CC, Liptzen D (2007) C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Harguindeguy NP, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan T, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskai NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Cox P (2001) Description of the “TRIFFID” dynamic global vegetation model. Technical note 24. Hadley Centre, Met Office
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187
Craine JM, Fierer N, McLauchlan KK (2010) Widespread coupling between the rate and temperature sensitivity of organic matter decay. Nat Geosci 3:854–857
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Davidson EA, Belk E, Boone RD (1998) Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob Change Biol 4:217–227
Davidson EA, Janssens IA, Luo Y (2006) On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Glob Change Biol 12:154–164
Devêvre OC, Horwath WR (2000) Decomposition of rice straw and microbial carbon use efficiency under different soil temperatures and moistures. Soil Biol Biochem 32:1773–1785
Dumbrell AJ, Ashton PD, Aziz N, Feng G, Nelson M, Dytham C, Fitter AH, Helgason T (2011) Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol 190:794–804
Fierer N, Craine JM, McLauchlan K, Schimel JP (2005) Litter quality and the temperature sensitivity of decomposition. Ecology 86:320–326
Fischer G, Nachtergaele F, Prieler S, van Velthuizen HT, Verelst L, Wiberg D (2008) Global agro-ecological zones assessment for agriculture (GAEZ 2008). IIASA and FOA, Laxenburg and Rome
Foley JA, Prentice IC, Ramankutty N, Levis S, Pollard D, Sitch S, Haxeltine A (1996) An integrated biosphere model of land surface processes, terrestrial carbon balance, and vegetation dynamics. Glob Biogeochem Cycles 10:603–628
Fontaine S, Barot S (2005) Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Ecol Lett 8:1075–1087
Friedlingstein P, Prentice IC (2010) Carbon-climate feedbacks: a review of model and observation based estimates. Curr Opin Environ Sustain 2:251–257
Friedlingstein P, Fung I, Holland E, John J, Brasseur G, Erickson D, Schimel D (1995) On the contribution of CO2 fertilization to the missing biospheric sink. Glob Biogeochem Cycles 9:541–556. doi:10.1029/95gb02381
Friedlingstein P, Cox P, Betts R, Bopp L, Von Bloh W, Brovkin V, Cadule P, Doney S, Eby M, Fung I, Bala G, John J, Jones C, Joos F, Kato T, Kawamiya M, Knorr W, Lindsay K, Matthews HD, Raddatz T, Rayner P, Reick C, Roeckner E, Schnitzler KG, Schnur R, Strassmann K, Weaver AJ, Yoshikawa C, Zeng N (2006) Climate-carbon cycle feedback analysis: results from the C4MIP model intercomparison. J Clim 19:3337–3353
German DP, Chacon SS, Allison SD (2011) Substrate concentration and enzyme allocation can affect rates of microbial decomposition. Ecology 92:1471–1480
Gershenson A, Bader NE, Cheng W (2009) Effects of substrate availability on the temperature sensitivity of soil organic matter decomposition. Glob Change Biol 15:176–183. doi:10.1111/j.1365-2486.2008.01827.x
Hanson CA, Allison SD, Bradford MA, Wallenstein MD, Treseder KK (2008) Fungal taxa target different carbon sources in forest soil. Ecosystems 11:1157–1167
Hawkes CV, Kivlin SN, Rocca JD, Huguet V, Thomsen MA, Suttle KB (2011) Fungal community responses to precipitation. Glob Change Biol 17:1637–1645. doi:10.1111/j.1365-2486.2010.02327.x
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, Oxford
IPCC (2007) Working group I contribution to the IPCC fourth assessment report. Climate change 2007: the physical science basis
Ito A, Oikawa T (2002) A simulation model of the carbon cycle in land ecosystems (Sim-CYCLE): a description based on dry-matter production theory and plot-scale validation. Ecol Model 151:143–176
Jenkinson DS (1976) The effects of biocidal treatments on metabolism in soil—IV. The decomposition of fumigated organisms in soil. Soil Biol Biochem 8:203–208
Jenkinson DS, Rayner JH (1977) The turnover of soil organic matter in some of the Rothamsted classical experiments. Soil Sci 123:298–305
Jobbágy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–436
Karhu K, Fritze H, Hämäläinen K, Vanhala P, Junger H, Oinonen M, Sonninen E, Tuomi M, Spetz P, Kitunen V, Liski J (2010) Temperature sensitivity of soil carbon fractions in boreal forest soil. Ecology 91:370–376
Kleber M, Nico PS, Plante A, Filley T, Kramer M, Swanston C, Sollins P (2011) Old and stable soil organic matter is not necessarily chemically recalcitrant: implications for modeling concepts and temperature sensitivity. Glob Change Biol 17:1097–1107
Knorr W (2000) Annual and interannual CO2 exchanges of the terrestrial biosphere: process-based simulations and uncertainties. Glob Ecol Biogeogr 9:225–252. doi:10.1046/j.1365-2699.2000.00159.x
Knutti R, Allen MR, Friedlingstein P, Gregory JM, Hegerl GC, Meehl GA, Meinshausen M, Murphy JM, Plattner GK, Raper SCB, Stocker TF, Stott PA, Teng H, Wigley TML (2008) A review of uncertainties in global temperature projections over the twenty-first century. J Clim 21:2651–2663. doi:10.1175/2007JCLI2119.1
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162
Krinner G, Viovy N, de Noblet-Ducoudré N, Ogée J, Polcher J, Friedlingstein P, Ciais P, Sitch S, Prentice IC (2005) A dynamic global vegetation model for studies of the coupled atmosphere-biosphere system. Glob Biogeochem Cycles 19:GB1015. doi:1010.1029/2003gb002199
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Lawrence CR, Neff JC, Schimel JP (2009) Does adding microbial mechanisms of decomposition improve soil organic matter models? A comparison of four models using data from a pulsed rewetting experiment. Soil Biol Biochem 41:1923–1934
Liang C, Cheng G, Wixon D, Balser T (2011) An Absorbing Markov Chain approach to understanding the microbial role in soil carbon stabilization. Biogeochemistry. doi:10.1007/s10533-010-9525-3
Lipson DA, Schmidt SK (2004) Seasonal changes in an alpine soil bacterial community in the Colorado Rocky Mountains. Appl Environ Microbiol 70:2867–2879
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8:315–323
Luo Y (2007) Terrestrial carbon-cycle feedback to climate warming. Annu Rev Ecol Evol Syst 38:683–712
Malcolm GM, López-Gutiérrez JC, Koide RT, Eissenstat DM (2008) Acclimation to temperature and temperature sensitivity of metabolism by ectomycorrhizal fungi. Glob Change Biol 14:1169–1180
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80:89–106. doi:10.1890/09-0179.1
Matthews HD, Eby M, Ewen T, Friedlingstein P, Hawkins BJ (2007) What determines the magnitude of carbon cycle-climate feedbacks? Glob Biogeochem Cycles 21:GB2012. doi:2010.1029/2006gb002733
McGuire KL, Treseder KK (2010) Microbial communities and their relevance for ecosystem models: decomposition as a case study. Soil Biol Biochem 42:529–535
Meir P, Cox P, Grace J (2006) The influence of terrestrial ecosystems on climate. Trends Ecol Evol 21:254–260
Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E (1953) Equations of state calculations by fast computing machines. J Chem Phys 21:1087–1092
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174
Morales P, Sykes MT, Prentice IC, Smith P, Smith B, Bugmann H, Zierl B, Friedlingstein P, Viovy N, Sabaté S, Sánchez A, Pla E, Gracia CA, Sitch S, Arneth A, Ogee J (2005) Comparing and evaluating process-based ecosystem model predictions of carbon and water fluxes in major European forest biomes. Glob Change Biol 11:2211–2233
Ostle NJ, Smith P, Fisher R, Ian Woodward F, Fisher JB, Smith JU, Galbraith D, Levy P, Meir P, McNamara NP, Bardgett RD (2009) Integrating plant-soil interactions into global carbon cycle models. J Ecol 97:851–863
Pansu M, Sarmiento L, Rujano MA, Ablan M, Acevedo D, Bottner P (2010) Modeling organic transformations by microorganisms of soils in six contrasting ecosystems: validation of the MOMOS model. Glob Biogeochem Cycles 24:GB1008. doi:1010.1029/2009GB003527
Parton WJ, Stewart JWB, Cole CV (1988) Dynamics of C, N, P, and S in grassland soils—a model. Biogeochemistry 5:109–131
Parton WJ, Scurlock JMO, Ojima DS, Gilmanov TG, Scholes RJ, Schimel DS, Kirchner T, Menaut JC, Seastedt T, Moya EG, Kamnalrut A, Kinyamario JI (1993) Observations and modeling of biomass and soil organic matter dynamics for the grassland biome worldwide. Glob Biogeochem Cycles 7:785–809
Potter CS, Randerson JT, Field CB, Matson PA, Vitousek PM, Mooney HA, Klooster SA (1993) Terrestrial ecosystem production: a process model based on global satellite and surface data. Glob Biogeochem Cycles 7:811–841
Raich JW, Russell AE, Kitayama K, Parton WJ, Vitousek PM (2006) Temperature influences carbon accumulation in moist tropical forests. Ecology 87:76–87
Randerson JT, Thompson MV, Malmstrom CM, Field CB, Fung IY (1996) Substrate limitations for heterotrophs: implications for models that estimate the seasonal cycle of atmospheric CO2. Glob Biogeochem Cycles 10:585–602. doi:10.1029/96gb01981
Randerson JT, Hoffman FM, Thornton PE, Mahowald NM, Lindsay K, Lee Y-H, Nevison CD, Doney SC, Bonan G, Stökli R, Covey C, Running SW, Fung IY (2009) Systematic assessment of terrestrial biogeochemistry in coupled climate-carbon models. Glob Change Biol 15:2462–2484
Ricciuto DM, Davis KJ, Keller K (2008) A Bayesian calibration of a simple carbon cycle model: the role of observations in estimating and reducing uncertainty. Glob Biogeochem Cycles 22:GB2030. doi:2010.1029/2006gb002908
Saleska SR, Shaw MR, Fischer ML, Dunne JA, Still CJ, Holman ML, Harte J (2002) Plant community composition mediates both large transient decline and predicted long-term recovery of soil carbon under climate warming. Glob Biogeochem Cycles 16:1055. doi:1010.1029/2001GB001573
Schimel J (1995) Ecosystem consequences of microbial diversity and community structure. In: Chapin FS III, Körner C (eds) Arctic and alpine biodiversity. Ecological studies, vol 113. Springer-Verlag, Berlin, pp 239–254
Schimel J (2001) Biogeochemical models: implicit versus explicit microbiology. In: Schulze ED, Harrison SP, Heimann M et al (eds) Global biogeochemical cycles in the climate system. Academic Press, San Diego, pp 177–183
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Schlesinger WH (1997) Biogeochemistry: an analysis of global change. Academic Press, San Diego
Schlesinger WH (2004) Better living through biogeochemistry. Ecology 85:2402–2407
Schwalm CR, Williams CA, Schaefer K, Anderson R, Arain MA, Baker I, Barr A, Black TA, Chen GS, Chen JM, Ciais P, Davis KJ, Desai A, Dietze M, Dragoni D, Fischer ML, Flanagan LB, Grant R, Gu LH, Hollinger D, Izaurralde RC, Kucharik C, Lafleur P, Law BE, Li LH, Li ZP, Liu SG, Lokupitiya E, Luo YQ, Ma SY, Margolis H, Matamala R, McCaughey H, Monson RK, Oechel WC, Peng CH, Poulter B, Price DT, Riciutto DM, Riley W, Sahoo AK, Sprintsin M, Sun JF, Tian HQ, Tonitto C, Verbeeck H, Verma SB (2010) A model-data intercomparison of CO2 exchange across North America: results from the North American Carbon Program site synthesis. J Geophys Res Biogeosci 115:G00H05. doi:10.1029/2009jg001229
Sinsabaugh RL (1994) Enzymic analysis of microbial pattern and process. Biol Fertil Soils 17:69–74
Sinsabaugh RL, Antibus RK, Linkins AE (1991) An enzymic approach to the analysis of microbial activity during plant litter decomposition. Agric Ecosyst Environ 34:43–54
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sitch S, Smith B, Prentice IC, Arneth A, Bondeau A, Cramer W, Kaplan JO, Levis S, Lucht W, Sykes MT, Thonicke K, Venevsky S (2003) Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob Change Biol 9:161–185. doi:10.1046/j.1365-2486.2003.00569.x
Sitch S, Huntingford C, Gedney N, Levy PE, Lomas M, Piao SL, Betts R, Ciais P, Cox P, Friedlingstein P, Jones CD, Prentice IC, Woodward FI (2008) Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon cycle feedbacks using five Dynamic Global Vegetation Models (DGVMs). Glob Change Biol 14:2015–2039. doi:10.1111/j.1365-2486.2008.01626.x
Sivia D, Skilling J (2006) Data analysis: a Bayesian tutorial. Oxford University Press, New York
Skilling J (2006) Nested sampling for general Bayesian computation. Bayesian Anal 1:833–860
Smith P, Smith JU, Powlson DS, McGill WB, Arah JRM, Chertov OG, Coleman K, Franko U, Frolking S, Jenkinson DS, Jensen LS, Kelly RH, Klein-Gunnewiek H, Komarov AS, Li C, Molina JAE, Mueller T, Parton WJ, Thornley JHM, Whitmore AP (1997) A comparison of the performance of nine soil organic matter models using datasets from seven long-term experiments. Geoderma 81:153–225
Sokolov AP, Kicklighter DW, Melillo JM, Felzer BS, Schlosser CA, Cronin TW (2008) Consequences of considering carbon-nitrogen interactions on the feedbacks between climate and the terrestrial carbon cycle. J Clim 21:3776–3796. doi:10.1175/2008jcli2038.1
Steinweg JM, Plante AF, Conant RT, Paul EA, Tanaka DL (2008) Patterns of substrate utilization during long-term incubations at different temperatures. Soil Biol Biochem 40:2722–2728
Strickland MS, Lauber C, Fierer N, Bradford MA (2009) Testing the functional significance of microbial community composition. Ecology 90:441–451
Sutton R, Sposito G (2005) Molecular structure in soil humic substances: the new view. Environ Sci Technol 39:9009–9015
Suzuki T, Ichii K (2010) Evaluation of a terrestrial carbon cycle submodel in an Earth system model using networks of eddy covariance observations. Tellus B Chem Phys Meteorol 62:729–742. doi:10.1111/j.1600-0889.2010.00478.x
Thornton PE, Rosenbloom NA (2005) Ecosystem model spin-up: estimating steady state conditions in a coupled terrestrial carbon and nitrogen cycle model. Ecol Model 189:25–48
Thornton PE, Lamarque JF, Rosenbloom NA, Mahowald NM (2007) Influence of carbon-nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Glob Biogeochem Cycles 21:GB4018. doi:4010.1029/2006GB002868
Trasar-Cepeda C, Gil-Sotres F, Leirós MC (2007) Thermodynamic parameters of enzymes in grassland soils from Galicia, NW Spain. Soil Biol Biochem 39:311–319
Treseder KK, Balser TC, Bradford MA, Brodie EL, Dubinsky EA, Eviner VT, Hofmockel KS, Lennon JT, Levine UY, MacGregor BJ, Pett-Ridge J, Waldrop MP (2011a) Integrating microbial ecology into ecosystem models: challenges and priorities. Biogeochemistry. doi:10.1007/s10533-011-9636-5
Treseder KK, Kivlin SN, Hawkes CV (2011b) Evolutionary trade-offs among decomposers determine responses to nitrogen enrichment. Ecol Lett. doi:10.1111/j.1461-0248.2011.01650.x
Tringe SG, von Mering C, Kobayashi A, Salamov AA, Chen K, Chang HW, Podar M, Short JM, Mathur EJ, Detter JC, Bork P, Hugenholtz P, Rubin EM (2005) Comparative metagenomics of microbial communities. Science 308:554–557
Trumbore S (2000) Age of soil organic matter and soil respiration: radiocarbon constraints on belowground C dynamics. Ecol Appl 10:399–411
van Ginkel JH, Gorissen A, Polci D (2000) Elevated atmospheric carbon dioxide concentration: effects of increased carbon input in a Lolium perenne soil on microorganisms and decomposition. Soil Biol Biochem 32:449–456
Vetter YA, Denning JW, Jumars PA, Krieger-Brockett BB (1998) A predictive model of bacterial foraging by means of freely released extracellular enzymes. Microb Ecol 36:75–92
von Lützow M, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur J Soil Sci 57:426–445
Vrugt JA, ter Braak CJF, Diks CGH, Higdon D, Robinson BA, Hyman JM (2009) Accelerating Markov chain Monte Carlo simulation by differential evolution with self-adaptive randomized subspace sampling. Int J Nonlinear Sci Numer Simul 10:273–290
Waldrop MP, Balser TC, Firestone MK (2000) Linking microbial community composition to function in a tropical soil. Soil Biol Biochem 32:1837–1846
Wallenstein MD, Hall EK (2011) A trait-based framework for predicting when and where microbial adaptation to climate change will affect ecosystem functioning. Biogeochemistry. doi:10.1007/s10533-011-9641-8
Wallenstein MD, Weintraub MN (2008) Emerging tools for measuring and modeling in situ activity of soil extracellular enzymes. Soil Biol Biochem 40:2098–2106
Wang YP, Trudinger CM, Enting IG (2009) A review of applications of model-data fusion to studies of terrestrial carbon fluxes at different scales. Agric For Meteorol 149:1829–1842. doi:10.1016/j.agrformet.2009.07.009
Wetterstedt JÅM, Ågren GI (2011) Quality or decomposer efficiency—which is most important in the temperature response of litter decomposition? A modelling study using the GLUE methodology. Biogeosciences 8:477–487. doi:10.5194/bg-8-477-2011
Williams M, Rastetter EB, Fernandes DN, Goulden ML, Shaver GR, Johnson LC (1997) Predicting gross primary productivity in terrestrial ecosystems. Ecol Appl 7:882–894
Wutzler T, Reichstein M (2008) Colimitation of decomposition by substrate and decomposers—a comparison of model formulations. Biogeosciences 5:749–759
Zeng N, Mariotti A, Wetzel P (2005) Terrestrial mechanisms of interannual CO2 variability. Glob Biogeochem Cycles 19:GB1016. doi:1010.1029/2004GB002273
Acknowledgments
This research was funded by a grant from the Advancing Theory in Biology program of the US National Science Foundation to SDA. We thank two anonymous reviewers whose thoughtful comments improved the manuscript substantially.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Todd-Brown, K.E.O., Hopkins, F.M., Kivlin, S.N. et al. A framework for representing microbial decomposition in coupled climate models. Biogeochemistry 109, 19–33 (2012). https://doi.org/10.1007/s10533-011-9635-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-011-9635-6