Abstract
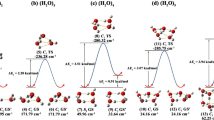
Density functional theory (DFT) calculations are performed to study the hydrogen-bonding in the DMSO-water and DMF-water complexes. Quantitative molecular electrostatic potential (MESP) and atoms-in-molecules (AIM) analysis are applied to quantify the relative complexation of DMSO and DMF with water molecules. The interaction energy of DMSO with water molecules was higher than in DMF-water complexes. The existence of cooperativity effect helps in the strong complex formation. A linear dependence was observed between the hydrogen bond energies EHB, and the total electron densities in the BCP’s of microsolvated complexes which supports the existence of cooperativity effect for the complexation process. Due to the stronger DMSO/DMF and water interaction, the water molecules in the formed complexes have a different structure than the isolated water clusters. NCI analysis shows that the steric area is more pronounced in DMF-water complex than the DMSO-water complex which accounts for the low stability of DMF-water complexes compared to the DMSO-water complex.

NCI analysis shows that the steric area is more pronounced in DMF-water complex than the DMSO-water complex which accounts for the low stability of DMF-water complexes compared to the DMSO-water complex.





Similar content being viewed by others
References
Carpriotti K, Capriotti JA (2012) J Clin Asethet Dermatol 5:24
Li Q, An X, Gong B, Cheng J (2007) J PhysChem A 111:10166
Zhang C, Ren Z, Liu L, Yin Z (2013) Mol Simulat 39:875
Chen X, Zhang Y, Liu C, Zhang Y, Zhou X, Zhou T, Mao Y, Kan B, Wei Y-Q, Li J (2010) Drug Deliv 17:385
McAlphine AS, McEwan AG, Bailey S (1998) J Mol Biol 275:613
Varnal T (1996) Struct Chem 7:111
Venkataramanan NS (2012) Int J Quantum Chem 112:2599
Clark T, Murray JS, Lane P, Politzer P (2008) J Mol Model 14:689
Leszczynski J, Shukla MK (eds) (2010) Practical aspects of computational chemistry. Springer, Dordrecht
Del Bene JE (1975) J Chem Phys 62:1314
Fraser GT, Suenram RD, Lovas FJ (1988) J Mol Struct 189:165
Jasie PG, Sevens WJ (1986) J Chem Phys 84:3271
Fu A, Du D, Zhou Z (2003) J Mol Struct (THEOCHEM) 623:315
Parreira RLT, Caramori GF, Morgon NH, Galembeck SE (2012) Int J Quantum Chem 112:1401
Blanco S, López JC, Lesarri A, Alonso JL (2006) J Am Chem Soc 128:12111
Liang W, Li H, Hu X, Han S (2004) J Phys Chem A 108:10219
Vasudevan V, Mushrif SH (2015) J Mol Liq 206:338
Frisch MJ, Trucks GW, Schlegel HB et al. (2009) Gaussian 09, revision D.01. Gaussian Inc, Wallingford
Truhlar DG, Zhao Y (2008) Theor Chem Acc 120:215
Sedlak R, Janowski T, Pitoňák M, Řezáč J, Pulay P, Hobza P (2013) J Chem Theory Comput 9:3364
Bulat FA, Toro-Labbė A, Brinck T, Murray JS, Politzer P (2010) J Mol Model 16:1679
Ji J, Zeng Y, Zhang X, Zheng S, Meng L (2013) J Mol Model 19:4887
Murray JS, Lane P, Politzer P (2009) J Mol Model 15:723
Murray JS, Lane P, Clark T, Politzer P (2007) J Mol Model 13:1033
O’Hair RAJ, Williams CM, Clark T (2010) J Mol Model 16:559
Murray JS, Lane P, Clark T, Riley KE, Politzer P (2012) J Mol Model 18:541
Politzer P, Murray JS, Clark T (2015) J Mol Model 21:52
Biegler-König F, Schönbohm J, Bayles D (2001) J Comp Chem 22:545
Venkataramanan NS, Suvitha A (2015) J Inc Phenom Macrocylc Chem 83:387
Houston S, Venkataramanan NS, Suvitha A, Wheate NJ (2016) Aust J Chem. doi:10.1071/CH16067
Venkatesan P, Rajakannan V, Venkataramanan NS, Ilangovan A, Sundius T, Thamotharan S (2016) J Mol Struct 119:259
Esrafili MD, Fatehi P, Solimannejad M (2013) Comput Theor Chem 1022:115
Esrafili MD, Esmailpour P, Mohammadian-Sabet F, Solimannejad M (2014) Int J Quantum Chem 114:295
Alkorta I, Elguero J, Yáňez M, Mó O (2014) Phys Chem Chem Phys 16:4305
Remya K, Suresh CH (2014) J Comput Chem 35:910
Saha S, Sastry GN (2015) J Phys Chem B 119:11121
Angelina EL, Peruchena NM (2011) J Phys Chem A 115:4701
Politzer P, Murray JS, Clark T (2013) Phys Chem Chem Phys 15:11178
Bader RF (1990) Atoms in molecules: a quantum theory. Clarendon Press, Oxford
Venkataramanan NS, Suvitha A, Mizuseki H, Kawazoe Y (2015) Int J Quantum Chem 115:1515
Duarte DJR, Angelina EL, Peruchena NM (2014) J Mol Model 20:2510
Koch U, Popelier PLA (1995) J Phys Chem 99:9747
Popelier PLA (1998) J Phys Chem A 102:1873
Ziólkowski M, Grabowski SJ, Leszczynski J (2006) J Phys Chem A 110:6514
Parthasarathi P, Subramanian V, Sathyamurthy N (2008) Syn React Inorg Met 38:18
Contreras-Garcia J, Johnson ER, Keinan S, Chaudret R, Piquemal J-P, Beratan DN, Yang W (2011) J Chem Theory Comput 7:625
Saleh G, Gatti C, Lo Presti L, Contreras-Garcia J (2012) Chem Eur J 18:15523
Li Q-Z, Xu W-R, Li R, Liu X-F, Li W-Z, Cheng J-B (2012) Spectrochim Acta Part A 97:600
Acknowledgments
The author thanks the SASTRA University for constant encouragement and for providing necessary infrastructure and a generous time slot on the high performance computing cluster. The author also register his thanks to CDAC-pune for providing computational resource through the PARAM Yuva II system.
Funding
The author thanks the SERB-DST, India for funding through a project (EMR-II-SB/S1/PC-047/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 974 kb)
Rights and permissions
About this article
Cite this article
Venkataramanan, N.S. Cooperativity of intermolecular hydrogen bonds in microsolvated DMSO and DMF clusters: a DFT, AIM, and NCI analysis. J Mol Model 22, 151 (2016). https://doi.org/10.1007/s00894-016-3022-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3022-0