Abstract
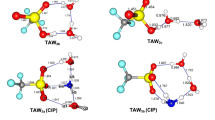
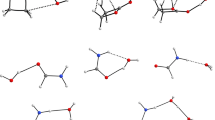
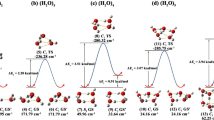
In this work, the possible molecular association structure in isopropanol-water solution has been analyzed by density functional theory (DFT) calculations. The properties of the isopropanol-water clusters with different number of water molecules, including optimal structure, intermolecular hydrogen bonding, binding energy and theoretical Raman spectra, are particularly investigated in the gas phase with B3LYP/6–31 + G (d, p) basis set. According to the simulated optimal structure, we found that the influence of hydration in the molecular configuration of isopropanol is mainly reflected in the O–H bond, which increases with the augment of water molecules. Meanwhile, in terms of the most stable structure, isopropanol-(H2O)5 will show the transition from a two-dimensional planar ring structure to a three-dimensional cage structure. In addition, the position and intensity of intermolecular hydrogen bonds interaction between the isopropanol and water molecules have been investigated by atoms in molecule (AIM) analysis and reduced density gradient (RDG) methods. The theoretical Raman spectra of isopropanol-(H2O)n (n = 1–5) clusters show the redshifts of the O–H bond tensile peak, which indicates that the O–H tensile strength is weakened and the hydrogen bonds interaction is strengthened with the increase in water molecules.






Similar content being viewed by others
References
Romero-Montalvo E, Dilabio GA (2021) Computational study of hydrogen bond interactions in water cluster-organic molecule complexes. J Phys Chem A 125:3369–3377. https://doi.org/10.1021/acs.jpca.1c01377
Zhong J, Kumar M, Francisco JS, Zeng XC (2018) Insight into chemistry on cloud/aerosol water surfaces. Acc Chem Res 51:1229–1237. https://doi.org/10.1021/acs.accounts.8b00051
Zhong J, Kumar M, Zhu CQ, Francisco JS, Zeng XC (2017) Surprising stability of larger criegee intermediates on aqueous interfaces. Angew Chemie - Int Ed 56:7740–7744. https://doi.org/10.1002/anie.201702722
Shi Y, Zhang Z, Jiang W, Wang Z (2017) Theoretical study on electronic and vibrational properties of hydrogen bonds in glycine-water clusters. Chem Phys Lett 684:53–59. https://doi.org/10.1016/j.cplett.2017.06.039
Jalili S, Akhavan M (2007) Study of hydrogen-bonded clusters of 2-methoxyphenol-water. Theor Chem Acc 118:947–957. https://doi.org/10.1007/s00214-007-0378-3
Saenger W (1979) Circular hydrogen bonds. Nature 279:343–344. https://doi.org/10.1038/279343a0
Xantheas SS, Dunning TH (1993) Ab initio studies of cyclic water clusters (H2O)n, n=1–6. I. Optimal structures and vibrational spectra. J Chem Phys 99:8774–8792. https://doi.org/10.1063/1.465599
Guevara-Vela JM, Romero-Montalvo E, Mora Gómez VA, Chávez-Calvillo R, García-Revilla M, Francisco E, Pendás ÁM, Rocha-Rinza T (2016) Hydrogen bond cooperativity and anticooperativity within the water hexamer. Phys Chem Chem Phys 18:19557–19566. https://doi.org/10.1039/c6cp00763e
Xantheas SS, Dunning TH (1993) The structure of the water trimer from ab initio calculations. J Chem Phys 98:8037–8040. https://doi.org/10.1063/1.464558
Farrell AE, Plevin RJ, Turner BT, Jones AD, Michael OH, Kammen DM (2006) Ethanol can contribute to energy and environmental goals. Science 311:506–508. https://doi.org/10.1126/science.1121416
Raina G, Kulkarni GU, Rao CNR (2001) Surface enrichment in alcohol−water mixtures. J Phys Chem A 105:10204–10207. https://doi.org/10.1021/jp011190i
Mejía SM, Flórez E, Mondragón F (2012) An orbital and electron density analysis of weak interactions in ethanol-water, methanol-water, ethanol and methanol small clusters. J Chem Phys. https://doi.org/10.1063/1.3701563
Li F, Men Z, Li S, Wang S, Li Z, Sun C (2018) Study of hydrogen bonding in ethanol-water binary solutions by Raman spectroscopy. Spectrochim. Acta - Part A Mol. Biomol Spectrosc 189:621–624. https://doi.org/10.1016/j.saa.2017.08.077
Gereben O, Pusztai L (2017) Cluster formation and percolation in ethanol-water mixtures. Chem Phys 496:1–8. https://doi.org/10.1016/j.chemphys.2017.09.003
Das S, Naskar B, Ghosh S (2014) Influence of temperature and organic solvents (isopropanol and 1,4-dioxane) on the micellization of cationic gemini surfactant (14–4–14). Soft Matter 10:2863–2875. https://doi.org/10.1039/c3sm52938j
Iriondo A, Tainta M, Saldias J, Arriba M, Ochoa B, Goñi FM, Martinez-Lage P, Abad-García B (2019) Isopropanol extraction for cerebrospinal fluid lipidomic profiling analysis. Talanta 195:619–627. https://doi.org/10.1016/j.talanta.2018.11.101
Papaspyrides CD, Tingas SG (1998) Comparison of isopropanol and isooctane as food simulants in plasticizer migration tests. Food Addit Contam 15:681–689. https://doi.org/10.1080/02652039809374698
Thongson C, Davidson PM, Mahakarnchanakul W, Weiss J (2004) Antimicrobial activity of ultrasound-assisted solvent-extracted spices. Lett Appl Microbiol 39:01–406. https://doi.org/10.1111/j.1472-765X.2004.01605.x
Medina-Valtierra J, Frausto-Reyes C, Camarillo-Martínez G, Ramírez-Ortiz JA (2009) Complete oxidation of isopropanol over Cu4O3 (paramelaconite) coating deposited on fiberglass by CVD. Appl Catal A Gen 356:36–42. https://doi.org/10.1016/j.apcata.2008.12.014
Wei Y, Zou W, Shen CH, Yang JG (2020) Basic flavor types and component characteristics of Chinese traditional liquors: A review. J Food Sci 85:4096–4107. https://doi.org/10.1111/1750-3841.15536
Aher A, Cai Y, Majumder M, Bhattacharyya D (2017) Synthesis of graphene oxide membranes and their behavior in water and isopropanol. Carbon N Y 116:145–153. https://doi.org/10.1016/j.carbon.2017.01.086
Devi DA, Smitha B, Sridhar S, Aminabhavi TM (2005) Pervaporation separation of isopropanol/water mixtures through crosslinked chitosan membranes. J Memb Sci 262:91–99. https://doi.org/10.1016/j.memsci.2005.03.051
Yang B, Cao X, Wang S, Sun C (2020) Exploring molecular association of isopropanol-water binary solution by Raman spectroscopy. Optik (Stuttg) 204:163544. https://doi.org/10.1016/j.ijleo.2019.163544
McGregor J, Li R, Zeitler JA, D’Agostino C, Collins JHP, Mantle MD, Manyar H, Holbrey JD, Falkowska M, Youngs TGA, Hardacre C, Stitt EH, Gladden LF (2015) Structure and dynamics of aqueous 2-propanol: A THz-TDS, NMR and neutron diffraction study. Phys Chem Chem Phys 17:30481–30491. https://doi.org/10.1039/c5cp01132a
Pothoczki S, Pusztai L, Bakó I (2019) Molecular dynamics simulation studies of the temperature-dependent structure and dynamics of isopropanol-water liquid mixtures at low alcohol content. J Phys Chem B 123:7599–7610. https://doi.org/10.1021/acs.jpcb.9b05631
Han C, Yao Y, Lv S, Wu Y, Lu A, Yan C, Liu Y, Luo X, Ni X (2018) Study on the components of isopropanol aqueous solution. Optik (Stuttg) 155:307–314. https://doi.org/10.1016/j.ijleo.2017.10.164
Evangelisti L, Gou Q, Feng G, Caminati W, Mead GJ, Finneran IA, Carroll PB, Blake GA (2017) Conformational equilibrium and internal dynamics in the iso-propanol-water dimer. Phys Chem Chem Phys 19:568–573. https://doi.org/10.1039/c6cp06315b
T. Lu, Molclus program, Version 1.9.5, http://www.keinsci.com/research/molclus.html.
J.J.P. Stewart, MOPAC2016, http://OpenMOPAC.net.
Temelso B, Archer KA, Shields GC (2011) Benchmark structures and binding energies of small water clusters with anharmonicity corrections. J Phys Chem A 115:12034–12046. https://doi.org/10.1021/jp2069489
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Bader RFW (1990) Atoms in molecules. A quantum theory. Oxford University Press, New York, NY
Lu T, Chen FW (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506. https://doi.org/10.1021/ja100936w
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. https://www.tapbiosystems.com/tap/products/index.htm
Carbonniere P, Barone V (2004) Performances of different density functionals in the computation of vibrational spectra beyond the harmonic approximation. Chem Phys Lett 399:226–229. https://doi.org/10.1016/j.cplett.2004.10.020
Carbonniere P, Lucca T, Pouchan C, Rega N, Barone V (2005) Vibrational computations beyond the harmonic approximation: performances of the B3LYP density functional for semirigid molecules. J Comput Chem 26:384–388. https://doi.org/10.1002/jcc.20170
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, and Fox DJ (2010) Gaussian 09, Revision C.01. Gaussian, Inc., Wallingford CT
White RP, Mayne HR (1998) An investigation of two approaches to basin hopping minimization for atomic and molecular clusters. Chem Phys Lett 289:463–468. https://doi.org/10.1016/S0009-2614(98)00431-X
Takeuchi H (2008) Development of an efficient geometry optimization method for water clusters. J Chem Inf Model 48:2226–2233. https://doi.org/10.1021/ci800238w
Lu QL, Luo QQ, Huang SG, De Li Y, Wan JG (2016) Analysis of the structures and properties of (GaSb)n (n = 4–9) clusters through density functional theory. J Phys Chem A 120:4560–4564. https://doi.org/10.1021/acs.jpca.6b05529
Llanio-Trujillo JL, Marques JMC, Pereira FB (2013) New insights on lithium-cation microsolvation by solvents forming hydrogen-bonds: Water versus methanol. Comput Theor Chem 1021:124–134. https://doi.org/10.1016/j.comptc.2013.06.043
Pérez C, Muckle MT, Zaleski DP, Seifert NA, Temelso B, Shields GC, Kisiel Z, Pate BH (2012) Structures of cage, prism, and book isomers of water hexamer from broadband rotational spectroscopy. Science 336:897–901. https://doi.org/10.1126/science.1220574
Akman F, Issaoui N, Kazachenko AS (2020) Intermolecular hydrogen bond interactions in the thiourea/water complexes (Thio-(H2O)n) (n = 1,…, 5): X-ray, DFT, NBO, AIM, and RDG analyses. J Mol Model 26:161. https://doi.org/10.1007/s00894-020-04423-3
Espinosa E, Souhassou M, Lachekar H, Lecomte C (1999) Topological analysis of the electron density in hydrogen bonds. Acta Crystallogr Sect B Struct Sci 55:563–572. https://doi.org/10.1107/S0108768199002128
Grabowski SJ (2001) An estimation of strength of intramolecular hydrogen bonds - Ab initio and AIM studies. J Mol Struct 562:137–143. https://doi.org/10.1016/S0022-2860(00)00863-2
Popelier A, Bader W (1992) The existence of an intramolecular C-H···O hydrogen bond in creatine and carbamoyl sarcosine. Chem Phys Lett 189:542–548. https://doi.org/10.1016/0009-2614(92)85247-8
Acknowledgements
This work was supported by the National Key Research and Development Program of China [Grand Number 2018YFD0400402]; and the Key Research and Development Program of Jiangsu Province [Grand Number BE2020756].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, S., Zhu, C., Chen, G. et al. A theoretical study on intermolecular hydrogen bonds of isopropanol-water clusters. Theor Chem Acc 141, 6 (2022). https://doi.org/10.1007/s00214-022-02865-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-022-02865-x