Abstract
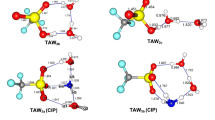
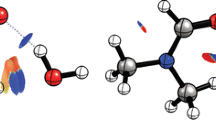
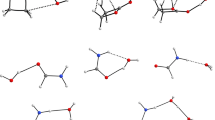
Micro-hydrated trimethylamine oxide (TMAO) has been investigated using a range-separated-hybrid functional including empirical dispersion correction. Electrophilic and nucleophilic sites on TMAO and water clusters have been identified using the molecular electrostatic potential (MESP). The nature of the chemical bonding in the different isomers of the micro-hydrated complexes has been investigated with the topological analysis of the electron density (QTAIM) method. For complexes containing one to four water molecules, the strongest intermolecular interactions consist in hydrogen bonding between the oxygen atom of the TMAO and hydrogen atoms of water molecules. From five water molecules, interactions between water molecules become the main source of stabilization of the most stable isomer. From four stationary points corresponding to the 1:1 (TMAO:H2O) complex, we determined the minimum distances between water molecules and central TMAO allowing the latter molecule to be encapsulated within a water clathrate-type cage. Optimization of TMAO encapsulated within two water cages (512 and 51262) suggests that only in the case of the 512 62 water cage the insertion of TMAO, the preservation of the hydrogen bonding between water molecules is energetically favorable. The interaction energy between one inserted TMAO and the 512 62 water cage was calculated to be around 150 kJ/mol with respect to the ground state of two partners. This result suggests that a thorough investigation of mono-hydrated complexes may be particularly relevant to identify the most suitable water cage for encapsulating a given solute.









Similar content being viewed by others
References
Franks F (1965) Hydrophobic hydration and the effect of hydrogen bonding solutes on the structure of water. Ann. N. Y. Acad. Sci. 125(2):277–289
Stillinger FH (1973) Structure in aqueous solutions of nonpolar solutes from the standpoint of scaled-particle theory. In The Physical Chemistry of Aqueous System (pp. 43–60). Springer, Boston, MA
Djikaev YS, Ruckenstein E (2016) Recent developments in the theoretical, simulational, and experimental studies of the role of water hydrogen bonding in hydrophobic phenomena. Adv. Colloid Interf. Sci. 235:23–45
Hajari T, Bandyopadhyay S (2017) Water structure around hydrophobic amino acid side chain analogs using different water models. J. Chem.Phys. 146(22):225104
Chandler D (2005) Interfaces and the driving force of hydrophobic assembly. Nature 437(7059):640
Remsing RC, Weeks JD (2013) Dissecting hydrophobic hydration and association. J. Phys. Chem.B 117(49):15479–15491
Ohto T, Hunger J, Backus EH, Mizukami W, Bonn M, Nagata Y (2017) Trimethylamine-N-oxide: its hydration structure, surface activity, and biological function, viewed by vibrational spectroscopy and molecular dynamics simulations. Phys. Chem. Chem. Phys. 19(10):6909–6920
Imoto S, Forbert H, Marx D (2018) Aqueous TMAO solutions as seen by theoretical THz spectroscopy: hydrophilic versus hydrophobic water. Phys. Chem. Chem. Phys. 20(9):6146–6158
Stirnemann G, Duboué-Dijon E, Laage D (2017) Ab initio simulations of water dynamics in aqueous TMAO solutions: temperature and concentration effects. J. Phys. Chem.B 121(49):11189–11197
Esser A, Belsare S, Marx D, Head-Gordon T (2017) Mode specific THz spectra of solvated amino acids using the AMOEBA polarizable force field. Phys. Chem. Chem. Phys. 19(7):5579–5590
Munroe KL, Magers DH, Hammer NI (2011) Raman spectroscopic signatures of noncovalent interactions between trimethylamine N-oxide (TMAO) and water. J. Phys. Chem.B 115(23):7699–7707
Murray JS, Politzer P (2011) The electrostatic potential: an overview. Wiley Interdisciplinary Reviews: Computational Molecular Science 1(2):153–163
Politzer P, Murray JS, Clark T (2015) Mathematical modeling and physical reality in noncovalent interactions. J. Mol. Model. 21(3):52
Politzer P, Murray JS (2018) The Hellmann-Feynman theorem: a perspective. J. Mol. Model. 24(9):266
Silvi B, Savin A (1994) Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371(6499):683
Savin A, Nesper R, Wengert S, Fässler TF (1997) ELF: the electron localization function. Angew. Chem. International Ed. 36(17):1808–1832
Silvi B (2015) The relevance of the ELF topological approach to the Lewis, Kossel, and Langmuir bond model. In The Chemical Bond II (pp. 213–247). Springer, Cham
Gillespie RJ, Robinson EA (2007) Gilbert N. Lewis and the chemical bond: the electron pair and the octet rule from 1916 to the present day. J. Comput. Chem. 28(1):87–97
Bader RF, Streitwieser A, Neuhaus A, Laidig KE, Speers P (1996) Electron delocalization and the Fermi hole. J. Am. Chem. Soc. 118(21):4959–4965
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09 (Gaussian, Inc., Wallingford CT, 2009)
Vydrov OA, Scuseria GE (2006) Assessment of a long-range corrected hybrid functional. J. Chem.Phys. 125(23):234109
Vydrov OA, Heyd J, Krukau AV, Scuseria GE (2006) Importance of short-range versus long-range Hartree-Fock exchange for the performance of hybrid density functionals. J. Chem.Phys. 125(7):074106
Vydrov OA, Scuseria GE, Perdew JP (2007) Tests of functionals for systems with fractional electron number. J. Chem.Phys. 126(15):154109
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32(7):1456–1465
Noury S, Krokidis X, Fuster F, Silvi B (1999) Computational tools for the electron localization function topological analysis. Comput. Chem. 23(6):597–604
AIMAll (Version 17.11.14), Todd A. Keith, TK Gristmill Software, Overland Park KS, USA, 2017 (aim.tkgristmill.com)
Sun N, Li Z, Qiu N, Yu X, Zhang X, Li Y, Yang L, Luo K, Huang Q, Du S (2017) Ab initio studies on the clathrate hydrates of some nitrogen-and sulfur-containing gases. J. Phys. Chem.A 121(13):2620–2626
Wang K, Li W, Li S (2014) Generalized energy-based fragmentation CCSD (T)-F12a method and application to the relative energies of water clusters (H2O)20. J. Chem. Theory Comput. 10(4):1546–1553
Arunan E et al (2011) Definition of the hydrogen bond (IUPAC recommendations 2011). Pure Appl. Chem. 83(8):1637–1641
Pérez C, Neill JL, Muckle MT, Zaleski DP, Peña I, Lopez JC, Alonso JL, Pate BH (2015) Water–water and water–solute interactions in microsolvated organic complexes. Angew. Chem. International Ed. 127(3):993–996
Riffet V, Frison G, Bouchoux G (2018) Quantum-chemical modeling of the first steps of the Strecker synthesis: from the gas-phase to water solvation. J. Phys. Chem.A 122(6):1643–1657
Fanourgakis GS, Apra E, Xantheas SS (2004) High-level ab initio calculations for the four low-lying families of minima of (H2O)20. I. Estimates of MP2/CBS binding energies and comparison with empirical potentials. J. Chem. Phys. 121(6):2655–2663
Xantheas SS (2000) Cooperativity and hydrogen bonding network in water clusters. Chem. Phys. 258(2–3):225–231
A discussion on this point, as well as "limit cases" are presented in the following article: Guevara-Vela, J. M. et al. (2016). Hydrogen bond cooperativity and anticooperativity within the water hexamer. Phys. Chem. Chem. Phys., 18(29), 19557–19566
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection QUITEL 2018 (44th Congress of Theoretical Chemists of Latin Expression)
Electronic supplementary material
ESM 1
(DOCX 10499 kb)
Rights and permissions
About this article
Cite this article
Derbali, I., Zins, EL. & Alikhani, M.E. What is the hydrophobic interaction contribution to the stabilization of micro-hydrated complexes of trimethylamine oxide (TMAO)? A joint DFT-D, QTAIM, and MESP study. J Mol Model 25, 363 (2019). https://doi.org/10.1007/s00894-019-4217-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4217-y