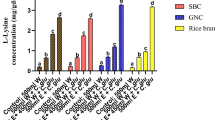
Abstract
The production of ε-poly-l-lysine (ε-PL) from cassava bagasse hydrolysate (CBH) by Streptomyces albulus US3-18 was investigated in this study. With 30 g/L glucose from CBH, 1.30 g/L ε-PL and 10.68 g/L biomass were obtained in shake flask fermentation. Interestingly, the two values were increased by 14.0% and 21.5%, respectively, compared to the control (1.14 g/L and 8.79 g/L). Simultaneously, the activities of four key enzymes of ε-PL synthesis during CBH fermentation were enhanced to varying degrees. In batch fermentation of 5-L bioreactor, 3.39 g/L ε-PL and 10.17 g/L DCW were harvested with 40 g/L glucose from CBH. The combination of fed-batch fermentation with two-stage pH strategy significantly increased ε-PL titer and biomass to 37.41 g/L and 41.0 g/L, respectively. Moreover, eleven volatile components were detected in CBH by GC–MS, and 6-pentyl-α-pyrone (6PP) was first identified as the most abundant volatile ingredient. The results in CBH fermentation demonstrated that S. albulus US3-18 exhibited high tolerance to these volatile byproducts. Using ICP–MS, the calcium concentration in CBH was determined as 195.0 mg/(kg hydrolyzate), and cobalt, copper, lead, chromium, mercury and arsenic were not detected. By adding 0.05 g/L CaCl2 to M3G medium, ε-PL yield was improved by 28.0%, indicating calcium was one of the factors for the enhanced ε-PL production. The study provides a reference for the efficient production of ε-PL from low-cost agricultural residues.
Graphical abstract







Similar content being viewed by others
References
Shima S, Sakai H (1977) Polylysine produced by Streptomyces. Agric Biol Chem 41:1807–1809. https://doi.org/10.1271/bbb1961.41.1807
Shih I, Shen M, Van Y (2006) Microbial synthesis of poly(ε-lysine) and its various applications. Bioresour Technol 97(9):1148–1159. https://doi.org/10.1016/j.biortech.2004.08.012
Chen S, Huang S, Li Y, Zhou C (2021) Recent advances in epsilon-poly-l-lysine and l-lysine-based dendrimer synthesis, modification, and biomedical applications. Front Chem 9:659304. https://doi.org/10.3389/fchem.2021.659304
Xu Z, Xu Z, Feng X, Xu D, Liang J, Xu H (2016) Recent advances in the biotechnological production of microbial poly(ε-l-lysine) and understanding of its biosynthetic mechanism. Appl Microbiol Biotechnol 100:6619–6630. https://doi.org/10.1007/s00253-016-7677-3
Wang L, Zhang C, Zhang J, Rao Z, Xu X, Mao Z, Chen X (2021) Epsilon-poly-l-lysine: recent advances in biomanufacturing and applications. Front Bioeng Biotechnol 9:748976. https://doi.org/10.3389/fbioe.2021.748976
Shima S, Sakai H (1981) Poly-l-lysine produced by Streptomyces. Part II. Taxonomy and fermentation studies. Agric Biol Chem 45(11):2497–2502. https://doi.org/10.1080/00021369.1981.10864929
Zhou YP, Ren XD, Wang L, Chen XS, Mao ZG, Tang L (2015) Enhancement of ε-poly-lysine production in ε-poly-lysine-tolerant Streptomyces sp. by genome shuffling. Bioproc Biosyst Eng 38:1705–1713. https://doi.org/10.1007/s00449-015-1410-y
Wang L, Chen X, Wu G, Zeng X, Ren X, Li S, Tang L, Mao Z (2016) Genome shuffling and gentamicin resistance to improve epsilon-poly-l-lysine productivity of Streptomyces albulus W-156. Appl Biochem Biotechnol 180:1601–1617. https://doi.org/10.1007/s12010-016-2190-9
Wang L, Chen X, Wu G, Li S, Zeng X, Ren X, Tang L, Mao Z (2017) Enhanced ε-poly-l-lysine production by inducing double antibiotic-resistant mutations in Streptomyces albulus. Bioprocess Biosyst Eng 40:271–283. https://doi.org/10.1007/s00449-016-1695-5
Liu Y, Chen X, Zhao J, Li Q, Mao Z (2019) Improvement of ε-poly-l-lysine production of Streptomyces albulus by continuous introduction of streptomycin resistance. Process Biochem 82:10–18. https://doi.org/10.1016/j.procbio.2019.04.006
Xiang J, Yang Y, Dabbour M, Mintah BK, Zhang Z, Dai C, He R, Huang G, Ma H (2020) Metabolomic and genomic profiles of Streptomyces albulus with a higher ε-polylysine production through ARTP mutagenesis. Biochem Eng J 162:107720. https://doi.org/10.1016/j.bej.2020.107720
Xu D, Wang R, Xu Z, Xu Z, Li S, Wang M, Feng X, Xu H (2019) Discovery of a short-chain ε-poly-l-lysine and its highly efficient production via synthetase swap strategy. J Agric Food Chem 67:1453–1462. https://doi.org/10.1021/acs.jafc.8b06019
Wang A, Tian W, Cheng L, Xu Y, Wang X, Qin J, Yu B (2020) Enhanced ε-poly-l-lysine production by the synergistic effect of ε-poly-l-lysine synthetase overexpression and citrate in Streptomyces albulus. Front Bioeng Biotechnol 8:288. https://doi.org/10.3389/fbioe.2020.00288
Yamanaka K, Hamano Y, Oikawa T (2020) Enhancement of metabolic flux toward ε-poly-l-lysine biosynthesis by targeted inactivation of concomitant polyene macrolide biosynthesis in Streptomyces albulus. J Biosci Bioeng 129:558–564. https://doi.org/10.1016/j.jbiosc.2019.12.002
Purev E, Kondo T, Takemoto D, Niones JT, Ojika M (2020) Identification of ε-poly-l-lysine as an antimicrobial product from an Epichloë endophyte and isolation of fungal ε-PL synthetase gene. Molecules 25:1032. https://doi.org/10.3390/molecules25051032
Li W, Lv J, Dong T, Li X, Li X, Tan Z, Jia S (2021) Effects of amino acids and overexpression of dapA gene on the production of ε-poly-l-lysine by Streptomyces diastatochromogenes strains. Curr Microbiol 78:2640–2647. https://doi.org/10.1007/s00284-021-02510-z
Hiraki J, Hatakeyama M, Morita H, Izumi Y (1998) Improved epsilonpoly-l-lysine production of an S-(2-aminoethyl)-l-cysteine resistant mutant of Streptomyces albulus. Seibutsu-Kogaku Kais (Tokyo) 76:487–493
Kahar P, Iwata T, Hiraki J, Park EY, Okabe M (2001) Enhancement of ε-polylysine production by Streptomyces albulus strain 410 using pH control. J Biosci Bioeng 91:190–194. https://doi.org/10.1263/jbb.91.190
Ren XD, Chen XS, Zeng X, Wang L, Tang L, Mao ZG (2015) Acidic pH shock induced overproduction of ε-poly-l-lysine in fed-batch fermentation by Streptomyces sp. M-Z18 from agro-industrial by-products. Bioproc Biosyst Eng 38:1113–1125. https://doi.org/10.1007/s00449-015-1354-2
Wang L, Li S, Zhao J, Liu Y, Chen X, Tang L, Mao Z (2019) Efficiently activated ε-poly-l-lysine production by multiple antibiotic-resistance mutations and acidic pH shock optimization in Streptomyces albulus. MicrobiologyOpen 8(5):e728. https://doi.org/10.1002/mbo3.728
Xu Z, Bo F, Xia J, Sun Z, Li S, Feng X, Xu H (2015) Effects of oxygen-vectors on the synthesis of epsilon-poly-lysine and the metabolic characterization of Streptomyces albulus PD-1. Biochem Eng J 94:58–64. https://doi.org/10.1016/j.bej.2014.11.009
Bankar SB, Singhal RS (2011) Improved poly-ε-lysine biosynthesis using Streptomyces noursei NRRL 5126 by controlling dissolved oxygen during fermentation. J Microbiol Biotechnol 21:652–658. https://doi.org/10.4014/jmb.1103.02007
Liu SR, Yang XJ, Sun DF (2021) Enhanced production of ε-poly-l-lysine by immobilized Streptomyces ahygroscopicus through repeated-batch or fed-batch fermentation with in situ product removal. Bioprocess Biosyst Eng 44:2109–2120. https://doi.org/10.1007/s00449-021-02587-7
Wang C, Chen X, Jiang Y, Li N, Zhu P, Xu H (2022) Facile and green synthesis of reduced graphene oxide/loofah sponge for Streptomyces albulus immobilization and ε-poly-l-lysine production. Bioresour Technol 349:126534. https://doi.org/10.1016/j.biortech.2021.126534
Ren XD, Xu YJ, Zeng X, Chen XS, Tang L, Mao ZG (2015) Microparticle-enhanced production of ε-poly-l-lysine in fed-batch fermentation. RSC Adv 5:82138–82143. https://doi.org/10.1039/c5ra14319e
Chen XS, Li S, Liao LJ, Ren XD, Li F, Tang L, Zhang JH, Mao ZG (2011) Production of ε-poly-l-lysine using a novel two-stage pH control strategy by Streptomyces sp. M-Z18 from glycerol. Bioprocess Biosyst Eng 34:561–567. https://doi.org/10.1007/s00449-010-0505-8
Pandey A, Soccol CR, Nigam P, Soccol VT, Vandenberghe LPS, Mohan R (2000) Biotechnological potential of agro-industrial residues. II: cassava bagasse. Bioresour Technol 74:81–87. https://doi.org/10.1016/S0960-8524(99)00143-1
Zhang S, Qu C, Huang X, Suo Y, Liao Z, Wang J (2016) Enhanced isopropanol and n-butanol production by supplying exogenous acetic acid via co-culturing two clostridium strains from cassava bagasse hydrolysate. J Ind Microbiol Biotechnol 43:915–925. https://doi.org/10.1007/s10295-016-1775-1
Lu C, Zhao J, Yang ST, Wei D (2012) Fed-batch fermentation for n-butanol production from cassava bagasse hydrolysate in a fibrous bed bioreactor with continuous gas stripping. Bioresour Technol 104:380–387. https://doi.org/10.1016/j.biortech.2011.10.089
Escaramboni B, Fernández Núñez EG, Carvalho AFA, de Oliva NP (2018) Ethanol biosynthesis by fast hydrolysis of cassava bagasse using fungal amylases produced in optimized conditions. Ind Crop Prod 112:368–377. https://doi.org/10.1016/j.indcrop.2017.12.004
Huang J, Du Y, Bao T, Lin M, Wang J, Yang ST (2019) Production of n-butanol from cassava bagasse hydrolysate by engineered Clostridium tyrobutyricum overexpressing adhE2: kinetics and cost analysis. Bioresour Technol 292:121969. https://doi.org/10.1016/j.biortech.2019.121969
Shi X, Chen Y, Ren H, Liu D, Zhao T, Zhao N, Ying H (2014) Economically enhanced succinic acid fermentation from cassava bagasse hydrolysate using Corynebacterium glutamicum immobilized in porous polyurethane filler. Bioresour Technol 174:190–197. https://doi.org/10.1016/j.biortech.2014.09.137
Chen H, Chen B, Su Z, Wang K, Wang B, Wang Y, Si Z, Wu Y, Cai D, Qin P (2020) Efficient lactic acid production from cassava bagasse by mixed culture of Bacillus coagulans and lactobacillus rhamnosus using stepwise pH controlled simultaneous saccharification and co-fermentation. Ind Crop Prod 146:112175. https://doi.org/10.1016/j.indcrop.2020.112175
Chen J, Liu X, Wei D, Chen G (2015) High yields of fatty acid and neutral lipid production from cassava bagasse hydrolysate (CBH) by heterotrophic Chlorella protothecoides. Bioresour Technol 191:281–290. https://doi.org/10.1016/j.biortech.2015.04.116
Liu L, Chen J, Lim PE, Wei D (2018) Enhanced single cell oil production by mixed culture of Chlorella pyrenoidosa and Rhodotorula glutinis using cassava bagasse hydrolysate as carbon source. Bioresour Technol 255:140–148. https://doi.org/10.1016/j.biortech.2018.01.114
Chen J, Wang Y, Guo X, Rao D, Zhou W, Zheng P, Sun J, Ma Y (2020) Efficient bioproduction of 5-aminolevulinic acid, a promising biostimulant and nutrient, from renewable bioresources by engineered Corynebacterium glutamicum. Biotechnol Biofuels 13:41. https://doi.org/10.1186/s13068-020-01685-0
Srianta I, Kusdiyantini E, Zubaidah E, Ristiarini S, Nugerahani I, Alvin A, Iswanto N, Zhang BB (2021) Utilization of agro-industrial by-products in Monascus fermentation: a review. Bioresour Bioprocess 8:129. https://doi.org/10.1186/s40643-021-00473-4
Zeng X, Chen XS, Ren XD, Liu QR, Wang L, Sun QX, Tang L, Mao ZG (2014) Insights into the role of glucose and glycerol as a mixed carbon source in the improvement of ε-poly-l-lysine productivity. Appl Biochem Biotechnol 173:2211–2224. https://doi.org/10.1007/s12010-014-1026-8
Rebière L, Clark AC, Schmidtke LM, Prenzler PD, Scollary GR (2010) A robust method for quantification of volatile compounds within and between vintages using headspace-solid-phase micro-extraction coupled with GC-MS–Application on Semillon wines. Anal Chim Acta 660:149–157. https://doi.org/10.1016/j.aca.2009.10.029
Gounden T, Moodley R, Jonnalagadda SB (2018) Elemental analysis and nutritional value of edible Trifolium (clover) species. J Environ Sci Heal B 53(8):487–492. https://doi.org/10.1080/03601234.2018.1462923
Xia J, Xu Z, Xu H, Liang J, Li S, Feng X (2014) Economical production of poly(ε-l-lysine) and poly(l-diaminopropionic acid) using cane molasses and hydrolysate of streptomyces cells by Streptomyces albulus PD-1. Bioresour Technol 164:241–247. https://doi.org/10.1016/j.biortech.2014.04.078
Diaz CAC, Bennett RK, Papoutsakis ET, Antoniewicz MR (2019) Deletion of four genes in Escherichia coli enables preferential consumption of xylose and secretion of glucose. Metab Eng 52:168–177. https://doi.org/10.1016/j.ymben.2018.12.003
TranNgoc K, Pham N, Lee C, Jang SH (2019) Cloning, Expression and characterization of a psychrophilic glucose 6-phosphate dehydrogenase from Sphingomonas sp. PAMC 26621. Int J Mol Sci 20:1362. https://doi.org/10.3390/ijms20061362
Li S, Wang N, Du ZJ, Chen GJ (2018) Intergeneric hybridization between Streptomyces albulus and Bacillus subtilis facilitates production of ε-poly-l-lysine from corn starch residues. Biotechnol Bioprocess Eng 23:580–587. https://doi.org/10.1007/s12257-018-0253-1
El-Hasan A, Walker F, Schöne J, Buchenauer H (2007) Antagonistic effect of 6-pentyl-alpha-pyrone produced by Trichoderma harzianum toward Fusarium moniliforme. J Plant Dis Protect 114:62–68. https://doi.org/10.1007/bf03356205
Serrano Carreón L, Balderas Ruíz K, Galindo E, Rito Palomares M (2002) Production and biotransformation of 6-pentyl-α-pyrone by Trichoderma harzianum in two-phase culture systems. Appl Microbiol Biotechnol 58:170–174. https://doi.org/10.1007/s00253-001-0874-7
Lu ZY, Zhong JJ (2020) Effect of furfural addition on validamycin-A production in fermentation of Streptomyces hygroscopicus 5008. Process Biochem 92:43–48. https://doi.org/10.1016/j.procbio.2020.03.002
Wu S, Lu HY, Chen QH, Xie HC, Jiao YC (2021) Anthocyanin extract from Lycium ruthenicum enhanced production of biomass and polysaccharides during submerged fermentation of Agaricus bitorquis (Quél.) Sacc. Chaidam. Biotechnol Bioprocess Eng 44:2303–2313. https://doi.org/10.1007/s00449-021-02605-8
Wang C, Ren X, Yu C, Wang J, Wang L, Zhuge X, Liu X (2020) Physiological and transcriptional responses of Streptomyces albulus to acid stress in the biosynthesis of ε-poly-l-lysine. Front Microbiol 11:1379. https://doi.org/10.3389/fmicb.2020.01379
Xu D, Yao H, Cao C, Xu Z, Li S, Xu Z, Zhou J, Feng X, Xu H (2018) Enhancement of ε-poly-l-lysine production by overexpressing the ammonium transporter gene in Streptomyces albulus PD-1. Bioprocess Biosyst Eng 41:1337–1345. https://doi.org/10.1007/s00449-018-1961-9
Chen XS, Ren XD, Zeng X, Zhao FL, Tang L, Zhang HJ, Zhang JH, Mao ZG (2013) Enhancement of ε-poly-l-lysine production coupled with precursor l-lysine feeding in glucose–glycerol co-fermentation by Streptomyces sp. M-Z18. Bioprocess Biosyst Eng 36:1843–1849. https://doi.org/10.1007/s00449-013-0958-7
Zhang Y, Bai J, Wu C, Wang Y, Ju X, Qi X, Li L, Ji L, Fu J (2021) Efficient production of ε-poly-l-lysine using cassava starch and fish meal by Streptomyces albulus FQC-24. Prep Biochem Biotechnol. https://doi.org/10.1080/10826068.2021.1969577
Ottenheim C, Nawrath M, Wu JC (2018) Microbial mutagenesis by atmospheric and room-temperature plasma (ARTP): the latest development. Bioresour Bioprocess 5:12. https://doi.org/10.1186/s40643-018-0200-1
Funding
This work was supported by the National Natural Science Foundation of China (No. 21676173), the Agricultural Infrastructure Project of Suzhou Science and Technology Development Plan (No. SNG2017053), the Research Fund Project of Suzhou University of Science and Technology (No. XKZ201411), and the Natural Science Foundation of Jiangsu Province (No. BK20210864).
Author information
Authors and Affiliations
Contributions
Jiaolong Fu and Cong Li contributed equally to this work. Jiaolong Fu: Conceptualization, Writing—review & editing, Funding acquisition; Cong Li: Methodology, Investigation, Validation, Formal analysis, Writing—original draft; Xin Ju: Conceptualization, Writing—review & editing; Jing Bai: Writing—review & editing; Yunfeng Zhou: Validation; Yi Zhang: Data curation; Yue Wang: Writing—original draft; Zilong Sun: Data curation; Cuiying Hu: Methodology; Liangzhi Li: Writing—review & editing; Lilian Ji: Supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, J., Li, C., Ju, X. et al. Efficient production of ε-poly-l-lysine from cassava bagasse hydrolysate used as carbon source by Streptomyces albulus US3-18. Bioprocess Biosyst Eng 45, 1407–1419 (2022). https://doi.org/10.1007/s00449-022-02755-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02755-3