Abstract
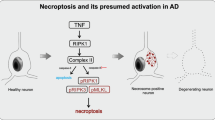
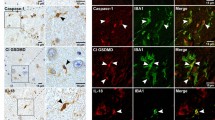
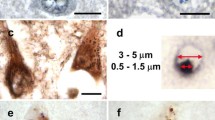
Alzheimer’s disease (AD) is characterized by a specific pattern of neuropathological changes, including extracellular amyloid β (Aβ) deposits, intracellular neurofibrillary tangles (NFTs), granulovacuolar degeneration (GVD) representing cytoplasmic vacuolar lesions, synapse dysfunction and neuronal loss. Necroptosis, a programmed form of necrosis characterized by the assembly of the necrosome complex composed of phosphorylated proteins, i.e. receptor-interacting serine/threonine-protein kinase 1 and 3 (pRIPK1 and pRIPK3) and mixed lineage kinase domain-like protein (pMLKL), has recently been shown to be involved in AD. However, it is not yet clear whether necrosome assembly takes place in brain regions showing AD-related neuronal loss and whether it is associated with AD-related neuropathological changes. Here, we analyzed brains of AD, pathologically defined preclinical AD (p-preAD) and non-AD control cases to determine the neuropathological characteristics and distribution pattern of the necrosome components. We demonstrated that all three activated necrosome components can be detected in GVD lesions (GVDn+, i.e. GVD with activated necrosome) in neurons, that they colocalize with classical GVD markers, such as pTDP-43 and CK1δ, and similarly to these markers detect GVD lesions. GVDn + neurons inversely correlated with neuronal density in the early affected CA1 region of the hippocampus and in the late affected frontal cortex layer III. Additionally, AD-related GVD lesions were associated with AD-defining parameters, showing the strongest correlation and partial colocalization with NFT pathology. Therefore, we conclude that the presence of the necrosome in GVD plays a role in AD, possibly by representing an AD-specific form of necroptosis-related neuron death. Hence, necroptosis-related neuron loss could be an interesting therapeutic target for treating AD.









Similar content being viewed by others
References
Association A (2017) 2017 Alzheimer’s disease facts and figures. Alzheimer’s Dement 13:325–373. https://doi.org/10.1016/j.jalz.2017.02.001
Ball MJ (1977) Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. Acta Neuropathol 37:111–118. https://doi.org/10.1007/BF00692056
Ball MJ (1978) Topographic distribution of neurofibrillary tangles and granulovacuolar degeneration in hippocampal cortex of aging and demented patients. A quantitative study. Acta Neuropathol 42:73–80. https://doi.org/10.1007/BF00690970
Balusu S, Brkic M, Libert C, Vandenbroucke RE (2016) The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: more than just a barrier. Neural Regen Res 11:534. https://doi.org/10.4103/1673-5374.180372
Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM (1989) Accumulation of abnormally phosphorylated τ precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res 477:90–99. https://doi.org/10.1016/0006-8993(89)91396-6
Barnes J, Bartlett JW, van de Pol LA, Loy CT, Scahill RI, Frost C, Thompson P, Fox NC (2009) A meta-analysis of hippocampal atrophy rates in Alzheimer’s disease. Neurobiol Aging 30:1711–1723. https://doi.org/10.1016/j.neurobiolaging.2008.01.010
Braak F, Braak H, Mandelkow E-M (1994) A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol 87:554–567. https://doi.org/10.1007/BF00293315
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. https://doi.org/10.1007/s00401-006-0127-z
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. https://doi.org/10.1007/BF00308809
Caccamo A, Branca C, Piras IS, Ferreira E, Huentelman MJ, Liang WS, Readhead B, Dudley JT, Spangenberg EE, Green KN, Belfiore R, Winslow W, Oddo S (2017) Necroptosis activation in Alzheimer’s disease. Nat Neurosci 20:1236–1246. https://doi.org/10.1038/nn.4608
Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16:55–65. https://doi.org/10.1038/ncb2883
Chen X, Li W, Ren J, Huang D, He W, Song Y, Yang C, Li W, Zheng X, Chen P, Han J (2013) Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res 24:105. https://doi.org/10.1038/cr.2013.171
Cho Y, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK-M (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137:1112–1123. https://doi.org/10.1016/j.cell.2009.05.037
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. https://doi.org/10.1007/s00401-014-1349-0
Dickson DW, Ksiezak-Reding H, Davies P, Yen S-H (1987) A monoclonal antibody that recognizes a phosphorylated epitope in Alzheimer neurofibrillary tangles, neurofilaments and tau proteins immunostains granulovacuolar degeneration. Acta Neuropathol 73:254–258. https://doi.org/10.1007/BF00686619
Duyckaerts C, Delaère P, Hauw JJ (1992) Alzheimer’s disease and neuroanatomy: hypotheses and proposals. In: Boller F, Forette F, Khachatarian Z, Poncet M, Christen Y (eds) Heterogeneity of Alzheimer’s disease, Springer, Berlin, pp 144–155. https://doi.org/10.1007/978-3-642-46776-9_15
Eskelinen E-L, Saftig P (2009) Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta (BBA) Mol Cell Res 1793:664–673. https://doi.org/10.1016/j.bbamcr.2008.07.014
Funk KE, Mrak RE, Kuret J (2011) Granulovacuolar degeneration (GVD) bodies of Alzheimer’s disease (AD) resemble late-stage autophagic organelles. Neuropathol Appl Neurobiol 37:295–306. https://doi.org/10.1111/j.1365-2990.2010.01135.x
Ganz AB, Beker N, Hulsman M, Sikkes S, Bank NB, Scheltens P, Smit AB, Rozemuller AJM, Hoozemans JJM, Holstege H (2018) Neuropathology and cognitive performance in self-reported cognitively healthy centenarians. Acta Neuropathol Commun 6:64. https://doi.org/10.1186/s40478-018-0558-5
Giacobini E, Gold G (2013) Alzheimer disease therapy—moving from amyloid-β to tau. Nat Rev Neurol 9:677. https://doi.org/10.1038/nrneurol.2013.223
Goodpaster T, Randolph-Habecker J (2014) A Flexible mouse-on-mouse immunohistochemical staining technique adaptable to biotin-free reagents, immunofluorescence, and multiple antibody staining. J Histochem Cytochem 62:197–204. https://doi.org/10.1369/0022155413511620
Grootjans S, Vanden Berghe T, Vandenabeele P (2017) Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ 24:1184–1195. https://doi.org/10.1038/cdd.2017.65
He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137:1100–1111. https://doi.org/10.1016/j.cell.2009.05.021
Hecht M, Kramer LM, von Arnim CAF, Otto M, Thal DR (2018) Capillary cerebral amyloid angiopathy in Alzheimer's disease: association with allocortical/hippocampal microinfarcts and cognitive decline. Acta Neuropathol 135:681-694. https://doi.org/10.1007/s00401-018-1834-y
Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, Pierotti C, Garnier J-M, Dobson RCJ, Webb AI (2014) Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci 111:15072–15077. https://doi.org/10.1073/pnas.1408987111
Hirano A, Dembitzer HM, Kurland LT, Zimmerman HM (1968) The fine structure of some intraganglionic alterations: neurofibrillary tangles, granulovacuolar bodies and “rod-like” structures as seen in Guam amyotrophic lateral sclerosis and parkinsonism-dementia complex. J Neuropathol Exp Neurol 27:167–182. https://doi.org/10.1097/00005072-196804000-00001
Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr SU, Culliford D, Perry VH (2009) Systemic inflammation and disease progression in Alzheimer disease. Neurology 73:768–774. https://doi.org/10.1212/WNL.0b013e3181b6bb95
Hoozemans JJM, van Haastert ES, Nijholt DAT, Rozemuller AJM, Eikelenboom P, Scheper W (2009) The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am J Pathol 174:1241–1251. https://doi.org/10.2353/ajpath.2009.080814
Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL (1984) Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225:1168–1170. https://doi.org/10.1126/science.6474172
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E (2012) National institute on aging–Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement 8:1–13. https://doi.org/10.1016/j.jalz.2011.10.007
Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y, Hitomi J, Zhu H, Chen H, Mayo L (2016) RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 353:603–608. https://doi.org/10.1126/science.aaf6803
Jing Z, Caltagarone J, Bowser R (2009) Altered subcellular distribution of c-Abl in Alzheimer’s disease. J Alzheimer’s Dis 17:409–422. https://doi.org/10.3233/JAD-2009-1062
Kadokura A, Yamazaki T, Kakuda S, Makioka K, Lemere CA, Fujita Y, Takatama M, Okamoto K (2009) Phosphorylation-dependent TDP-43 antibody detects intraneuronal dot-like structures showing morphological characters of granulovacuolar degeneration. Neurosci Lett 463:87–92. https://doi.org/10.1016/j.neulet.2009.06.024
Kannanayakal TJ, Tao H, Vandre DD, Kuret J (2006) Casein kinase-1 isoforms differentially associate with neurofibrillary and granulovacuolar degeneration lesions. Acta Neuropathol 111:413–421. https://doi.org/10.1007/s00401-006-0049-9
Köhler C (2016) Granulovacuolar degeneration: a neurodegenerative change that accompanies tau pathology. Acta Neuropathol 132:339–359. https://doi.org/10.1007/s00401-016-1562-0
Köhler C, Dinekov M, Götz J (2014) Granulovacuolar degeneration and unfolded protein response in mouse models of tauopathy and Aβ amyloidosis. Neurobiol Dis 71:169–179. https://doi.org/10.1016/j.nbd.2014.07.006
Kumar S, Wirths O, Stüber K, Wunderlich P, Koch P, Theil S, Rezaei-Ghaleh N, Zweckstetter M, Bayer TA, Brüstle O, Thal DR, Walter J (2016) Phosphorylation of the amyloid β-peptide at Ser26 stabilizes oligomeric assembly and increases neurotoxicity. Acta Neuropathol 131:525–537. https://doi.org/10.1007/s00401-016-1546-0
Lagalwar S, Guillozet-Bongaarts AL, Berry RW, Binder LI (2006) Formation of phospho-SAPK/JNK granules in the hippocampus is an early event in Alzheimer disease. J Neuropathol Exp Neurol 65:455–464. https://doi.org/10.1097/01.jnen.0000229236.98124.d8
Leroy K, Boutajangout A, Authelet M, Woodgett JR, Anderton BH, Brion J-P (2002) The active form of glycogen synthase kinase-3β is associated with granulovacuolar degeneration in neurons in Alzheimer’s disease. Acta Neuropathol 103:91–99
Lewis J, Dickson DW, Lin W-L, Chisholm L, Corral A, Jones G, Yen S-H, Sahara N, Skipper L, Yager D (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293:1487–1491. https://doi.org/10.1126/science.1058189
Liu S, Liu H, Johnston A, Hanna-Addams S, Reynoso E, Xiang Y, Wang Z (2017) MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc Natl Acad Sci 114:E7450–E7459. https://doi.org/10.1073/pnas.1707531114
Lockshin RA, Zakeri Z (2001) Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol cell Biol 2:545. https://doi.org/10.1038/35080097
Lund H, Gustafsson E, Svensson A, Nilsson M, Berg M, Sunnemark D, von Euler G (2014) MARK4 and MARK3 associate with early tau phosphorylation in Alzheimer’s disease granulovacuolar degeneration bodies. Acta Neuropathol Commun 2:22. https://doi.org/10.1186/2051-5960-2-22
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, Van Belle G, Berg L (1991) The consortium to establish a registry for Alzheimer’s disease (CERAD): Part II Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479. https://doi.org/10.1212/WNL.41.4.479
Morris JC, Heyman A, Mohs RC, Hughes JP, Van Belle G, Fillenbaum G, Mellits ED, Clark C (1989) The consortium to establish a registry for Alzheimer’s disease (CERAD): I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. https://doi.org/10.1212/WNL.39.9.1159
Morsch R, Simon W, Coleman PD (1999) Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol 58:188–197. https://doi.org/10.1097/00005072-199902000-00008
Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang J-G, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI (2013) The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39:443–453. https://doi.org/10.1016/j.immuni.2013.06.018
Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del TK (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71:362–381. https://doi.org/10.1097/NEN.0b013e31825018f7
Nishikawa T, Takahashi T, Nakamori M, Hosomi N, Maruyama H, Miyazaki Y, Izumi Y, Matsumoto M (2016) The identification of raft-derived tau-associated vesicles that are incorporated into immature tangles and paired helical filaments. Neuropathol Appl Neurobiol 42:639–653. https://doi.org/10.1111/nan.12288
Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg H (2015) Activation of necroptosis in multiple sclerosis. Cell Rep 10:1836–1849. https://doi.org/10.1016/j.celrep.2015.02.051
Ofengeim D, Mazzitelli S, Ito Y, DeWitt JP, Mifflin L, Zou C, et al. (2017) RIPK1 mediates a disease-associated microglial response in Alzheimer's disease. Proc Natl Acad Sci U S A 114:E8788-E8797. https://doi.org/10.1073/pnas.1714175114
Ohm TG, Müller H, Braak H, Bohl J (1995) Close-meshed prevalence rates of different stages as a tool to uncover the rate of Alzheimer’s disease-related neurofibrillary changes. Neuroscience 64:209–217. https://doi.org/10.1016/0306-4522(95)90397-P
Okamoto K, Hirai S, Iizuka T, Yanagisawa T, Watanabe M (1991) Reexamination of granulovacuolar degeneration. Acta Neuropathol 82:340–345. https://doi.org/10.1007/BF00296544
Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E, Galluzzi S, Marizzoni M, Frisoni GB (2016) Brain atrophy in Alzheimer’s disease and aging. Ageing Res Rev 30:25–48. https://doi.org/10.1016/j.arr.2016.01.002
Re DB, Le Verche V, Yu C, Amoroso MW, Politi KA, Phani S, Ikiz B, Hoffmann L, Koolen M, Nagata T (2014) Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron 81:1001–1008. https://doi.org/10.1016/j.neuron.2014.01.011
Rijal Upadhaya A, Kosterin I, Kumar S, Von Arnim CAF, Yamaguchi H, Fändrich M, Walter J, Thal DR (2014) Biochemical stages of amyloid-β peptide aggregation and accumulation in the human brain and their association with symptomatic and pathologically preclinical Alzheimer’s disease. Brain 137:887–903. https://doi.org/10.1093/brain/awt362
Riku Y, Duyckaerts C, Boluda S, Plu I, Le Ber I, Millecamps S, et al. (2019) Increased prevalence of granulovacuolar degeneration in C9orf72 mutation. Acta Neuropathol 138:783-793. https://doi.org/10.1007/s00401-019-02028-6
Ros U, Peña-Blanco A, Hänggi K, Kunzendorf U, Krautwald S, Wong WWL, García-Sáez AJ (2017) Necroptosis execution is mediated by plasma membrane nanopores independent of calcium. Cell Rep 19:175–187. https://doi.org/10.1016/j.celrep.2017.03.024
Rossi S, Motta C, Studer V, Barbieri F, Buttari F, Bergami A, Sancesario G, Bernardini S, De Angelis G, Martino G (2014) Tumor necrosis factor is elevated in progressive multiple sclerosis and causes excitotoxic neurodegeneration. Mult Scler J 20:304–312. https://doi.org/10.1177/1352458513498128
Sassin I, Schultz C, Thal DR, Rüb U, Arai K, Braak E, Braak H (2000) Evolution of Alzheimer’s disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol 100:259–269. https://doi.org/10.1007/s004019900178
Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27:1372–1384. https://doi.org/10.1016/j.neurobiolaging.2005.09.012
Schwab C, DeMaggio AJ, Ghoshal N, Binder LI, Kuret J, McGeer PL (2000) Casein kinase 1 delta is associated with pathological accumulation of tau in several neurodegenerative diseases. Neurobiol Aging 21:503–510. https://doi.org/10.1016/S0197-4580(00)00110-X
Selznick LA, Holtzman DM, Han BH, Gökden M, Srinivasan AN, Johnson EM Jr, Roth KA (1999) In situ immunodetection of neuronal caspase-3 activation in Alzheimer disease. J Neuropathol Exp Neurol 58:1020–1026. https://doi.org/10.1097/00005072-199909000-00012
Simchowicz T (1911) Histopathologische Studien über die senile Demenz. In: F. Nissl, and A. Alzheimer (eds) Histologie und histopathologische Arbeiten über die Großhirnrinde, Fischer, Jena, pp 267–444
Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Brück W, Jellinger K, Lassmann H (1999) Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease: evidence for apoptotic cell death. Am J Pathol 155:1459–1466. https://doi.org/10.1016/S0002-9440(10)65460-0
Stadelmann C, Lassmann H (2000) Detection of apoptosis in tissue sections. Cell Tissue Res 301:19–31. https://doi.org/10.1007/s004410000203
Su JH, Kesslak PJ, Head E, Cotman CW (2002) Caspase-cleaved amyloid precursor protein and activated caspase-3 are co-localized in the granules of granulovacuolar degeneration in Alzheimer’s disease and Down’s syndrome brain. Acta Neuropathol 104:1–6. https://doi.org/10.1007/s00401-002-0548-2
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148:213–227. https://doi.org/10.1016/j.cell.2011.11.031
Susaki EA, Tainaka K, Perrin D, Yukinaga H, Kuno A, Ueda HR (2015) Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat Protoc 10:1709. https://doi.org/10.1038/nprot.2015.085
Terry RD, DeTeresa R, Hansen LA (1987) Neocortical cell counts in normal human adult aging. Ann Neurol Off J Am Neurol Assoc Child Neurol Soc 21:530–539. https://doi.org/10.1002/ana.410210603
Thal DR, Holzer M, Rüb U, Waldmann G, Günzel S, Zedlick D, Schober R (2000) Alzheimer-related τ-pathology in the perforant path target zone and in the hippocampal stratum oriens and radiatum correlates with onset and degree of dementia. Exp Neurol 163:98–110. https://doi.org/10.1006/exnr.2000.7380
Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800. https://doi.org/10.1212/WNL.58.12.1791
Thal DR, Rüb U, Schultz C, Sassin I, Ghebremedhin E, Del Tredici K, Braak E, Braak H (2000) Sequence of Aβ-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol 59:733–748. https://doi.org/10.1093/jnen/59.8.733
Thal DR, Del Tredici K, Ludolph AC, Hoozemans JJM, Rozemuller AJ, Braak H, Knippschild U (2011) Stages of granulovacuolar degeneration: their relation to Alzheimer’s disease and chronic stress response. Acta Neuropathol 122:577–589. https://doi.org/10.1007/s00401-011-0871-6
Tomlinson BE, Kitchener D (1972) Granulovacuolar degeneration of hippocampal pyramidal cells. J Pathol 106:165–185. https://doi.org/10.1002/path.1711060305
Virard F, Cousty S, Cambus J-P, Valentin A, Kémoun P, Clément F (2015) Cold atmospheric plasma induces a predominantly necrotic cell death via the microenvironment. PLoS ONE 10:e0133120. https://doi.org/10.1371/journal.pone.0133120
Wang Y, Martinez-Vicente M, Krüger U, Kaushik S, Wong E, Mandelkow E-M, Cuervo AM, Mandelkow E (2009) Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet 18:4153–4170. https://doi.org/10.1093/hmg/ddp367
Wegner KW, Saleh D, Degterev A (2017) Complex pathologic roles of RIPK1 and RIPK3: moving beyond necroptosis. Trends Pharmacol Sci 38:202–225. https://doi.org/10.1016/j.tips.2016.12.005
West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 344:769–772. https://doi.org/10.1016/S0140-6736(94)92338-8
Wiersma VI, van Ziel AM, Vazquez-Sanchez S, Nolle A, Berenjeno-Correa E, Bonaterra-Pastra A, et al. (2019) Granulovacuolar degeneration bodies are neuron-selective lysosomal structures induced by intracellular tau pathology. Acta Neuropathol 138:943-970. https://doi.org/10.1007/s00401-019-02046-4
Yamazaki Y, Matsubara T, Takahashi T, Kurashige T, Dohi E, Hiji M, Nagano Y, Yamawaki T, Matsumoto M (2011) Granulovacuolar degenerations appear in relation to hippocampal phosphorylated tau accumulation in various neurodegenerative disorders. PLoS ONE 6:e26996. https://doi.org/10.1371/journal.pone.0026996
Zarow C, Vinters HV, Ellis WG, Weiner MW, Mungas D, White L, Chui HC (2005) Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Ann Neurol Off J Am Neurol Assoc Child Neurol Soc 57:896–903. https://doi.org/10.1002/ana.20503
Zhang D-W, Shao J, Lin J, Zhang N, Lu B-J, Lin S-C, Dong M-Q, Han J (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325:332–336. https://doi.org/10.1126/science.1172308
Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu Z-G (2012) Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci 109:5322–5327. https://doi.org/10.1073/pnas.1200012109
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12:723. https://doi.org/10.1038/nrn3114
Acknowledgements
The authors gratefully acknowledge the assistance of Mrs. Alicja Ronisz. They also thank Mathias De Decker (Laboratory for Neurobiology, VIB-KU Leuven, Belgium) for providing SH-SY5Y cells. Also, we thank the VIB Imaging Core Facility in Leuven for expert assistance and overall technical support in super-resolution imaging of cleared tissue using spinning disk confocal microscopy. The study was supported by: Fonds Wetenschappelijk Onderzoek (FWO) G0F8516N, 1S46219N, (DRT, RV); C1-internal funds from KU Leuven C14-17-107 (DRT); Vlaamse Impulsfinanciering voor Netwerken voor Dementie-onderzoek (IWT 135043) (RV, BDS, DRT) and a Methusalem grant of the Flemish Government and the KU Leuven to BDS. EVS is funded by an SB PhD Fellowship of FWO-Vlaanderen (1S46219N). SB received a post-doc fellowship from FWO-Vlaaderen (12P5919N).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
DRT received consultant honorary from GE Healthcare (UK) and Covance Laboratories (UK), speaker honorary from Novartis Pharma AG (Switzerland), travel reimbursement from GE Healthcare (UK) and UCB (Belgium) and collaborated with Novartis Pharma AG (Switzerland), Probiodrug (Germany), GE Healthcare (UK), and Janssen Pharmaceutical Companies (Belgium). BDS collaborated with Janssen Pharmaceutical companies (Belgium), Abbvie (USA) and received consulting fees from Eisai (Japan). None related to the work in this paper. RV’s institution has clinical trial agreements (RV as PI) with AbbVie, Genentech, Novartis, and Roche, material transfer agreements (RV as PI) with Avid a subsidiary of EliLilly, and consultancy agreements (RV as PI) with Prevail Therapeutics and Rodin Therapeutics. CAFvA received honoraria from serving on the scientific advisory board of Nutricia GmbH, Roche, Dr. Willmar Schwabe GmbH and Honkong University Research council and has received funding for travel and speaker honoraria from Nutricia GmbH, Lilly Deutschland GmbH, Desitin Arzneimittel GmbH, Biogen, Roche and Dr. Willmar Schwabe GmbH &Co. KG.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koper, M.J., Van Schoor, E., Ospitalieri, S. et al. Necrosome complex detected in granulovacuolar degeneration is associated with neuronal loss in Alzheimer’s disease. Acta Neuropathol 139, 463–484 (2020). https://doi.org/10.1007/s00401-019-02103-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-019-02103-y